Cornelia de Lange syndrome (CdLS) is a rare congenital developmental disorder with multisystemic involvement. The clinical presentation is highly variable, but the classic phenotype, characterized by distinctive craniofacial features, pre- and postnatal growth retardation, extremity reduction defects, hirsutism and intellectual disability can be distinguished from the nonclassic phenotype, which is generally milder and more difficult to diagnose. In addition, the clinical features overlap with those of other neurodevelopmental disorders, so the use of consensus clinical criteria and artificial intelligence tools may be helpful in confirming the diagnosis.
Pathogenic variants in NIPBL, which encodes a protein related to the cohesin complex, have been identified in more than 60% of patients, and pathogenic variants in other genes related to this complex in another 15%: SMC1A, SMC3, RAD21, and HDAC8. Technical advances in large-scale sequencing have allowed the description of additional genes (BRD4, ANKRD11, MAU2), but the lack of molecular diagnosis in 15% of individuals and the substantial clinical heterogeneity of the syndrome suggest that other genes and mechanisms may be involved.
Although there is no curative treatment, there are symptomatic/palliative treatments that paediatricians should be aware of. The main medical complication in classic SCdL is gastro-esophageal reflux (GER), which should be treated early.
El síndrome de Cornelia de Lange (SCdL) es un raro trastorno congénito del desarrollo de afectación multisistémica. Las manifestaciones clínicas son muy variables, pero se distingue entre un fenotipo clásico, caracterizado por unos rasgos craneofaciales distintivos, retraso del crecimiento pre y postnatal, defectos por reducción de las extremidades, hirsutismo y discapacidad intelectual y un fenotipo no clásico, generalmente más leve y más difícil de diagnosticar. Además, las características clínicas se superponen con las de otros desordenes del neurodesarrollo, por lo que la utilización de criterios clínicos consensuados y herramientas de inteligencia artificial pueden ser útiles para confirmar el diagnóstico.
En más del 60% de los pacientes se han identificado variantes patogénicas en el gen NIPBL, que codifica una proteína relacionada con el complejo de la cohesina, y en otro 15% en 4 genes también asociados a este complejo: SMC1A, SMC3, RAD21 y HDAC8. Los progresos técnicos en las técnicas de secuenciación masiva han permitido describir otros genes relacionados (BRD4, ANKRD11 y MAU2), pero la ausencia de diagnóstico molecular en el 15% de los casos y la gran heterogeneidad clínica del síndrome, sugiere la existencia de otros genes y mecanismos relacionados.
Aunque no haya un tratamiento curativo, sí hay tratamientos sintomáticos/paliativos que deben ser conocidos por el pediatra. La principal complicación médica en el SCdL clásico es el reflujo gastroesofágico (RGE), que debe ser tratado de forma precoz y contundente, ya que es una de las causas más frecuentes de fallecimiento en estos pacientes.
Cornelia de Lange syndrome (CdLS) is a rare disease named after the Dutch paediatrician Cornelia de Lange, who in 1933 published the cases of two unrelated female infants who presented with very similar phenotypes.1 She called the syndrome typus degenerativus amstelodamensis, in honour of the city of Amsterdam where she worked. Before that, in 1916, Brachmann had published the case of a patient with growth retardation, hirsutism and bilateral monodactyly that has since been recognised as the first documented case of CdLS,2 which is why some publications refer to this disease as Brachmann-de Lange syndrome.
At present, the prevalence of CdLS is estimated at 1 case per 10 000 – 30 000 live birth births in the general population.3 This includes cases with atypical presentations of CdLS that were not diagnosed in previous decades when the genetic tests currently used were not yet available.
Clinical formsClassic Cornelia de Lange syndromeClassic CdLS, recognisable at birth, has a characteristic phenotype consisting of distinctive craniofacial features, intrauterine and postnatal growth retardation, reduction deformities (especially the upper extremities), hirsutism and psychomotor delay/intellectual disability. Additional abnormalities in more than one system are also common: gastro-oesophageal reflux (the most frequent and important medical complication in patients with CdLS), sensorineural hearing loss, congenital heart disease and renal and genital anomalies, among others.3,4
The distinctive craniofacial features include microcephaly with brachycephaly, synophrys, thick arched eyebrows, long eyelashes, short nose with a concave nasal ridge and anteverted nares, a long philtrum and thin upper lip and retrognathia3,4 (Fig. 1 and Table 1).
Facial features and limb abnormalities in patients with CdLS and pathogenic variants in different causative genes. The figure also shows the analysis made with the Face2Gene software and the scores calculated using the criteria proposed by Kline et al.3
Clinical manifestations of CdLS organised by category, defined features, HPO (Human Phenotype Ontology) ID and frequency.
| Category | HPO ID | HPO name | Frequency | Category | HPO ID | HPO name | Frequency |
|---|---|---|---|---|---|---|---|
| Growth | HP:0004322 | Short stature | Very frequent | Skeletal system | HP:0000248 | Brachycephaly | Very frequent |
| HP:0001508 | Failure to thrive | Frequent | HP:0000347 | Micrognathia | Very frequent | ||
| HP:0001511 | Intrauterine growth retardation | Frequent | HP:0000470 | Short neck | Very frequent | ||
| HP:0008850 | Severe postnatal growth retardation | Frequent | HP:0001770 | Toe syndactyly 2-3 | Very frequent | ||
| HP:0001956 | Truncal obesity | Occasional | HP:0001773 | Short foot | Very frequent | ||
| Head and neck | HP:0000218 | High palate | Very frequent | HP:0002750 | Delayed skeletal maturation | Very frequent | |
| HP:0000233 | Thin vermilion border | Very frequent | HP:0002983 | Micromelia | Very frequent | ||
| HP:0000343 | Long philtrum | Very frequent | HP:0009623 | Proximal placement of thumb | Very frequent | ||
| HP:0000463 | Anteverted nares | Very frequent | HP:0010034 | Short 1st metacarpal | Very frequent | ||
| HP:0000684 | Delayed eruption of teeth | Very frequent | HP:0200055 | Small hand | Very frequent | ||
| HP:0000687 | Widely spaced teeth | Very frequent | HP:0001387 | Joint stiffness | Frequent | ||
| HP:0002714 | Downturned corners of mouth | Very frequent | HP:0002974 | Radioulnar synostosis | Frequent | ||
| HP:0003196 | Short nose | Very frequent | HP:0003042 | Elbow dislocation | Frequent | ||
| HP:0005280 | Depressed nasal bridge | Very frequent | HP:0004209 | Clinodactyly of the 5th finger | Frequent | ||
| HP:0000498 | Blepharitis | Frequent | HP:0000767 | Pectus excavatum | Occasional | ||
| HP:0000175 | Cleft palate | Occasional | HP:0001385 | Hip dysplasia | Occasional | ||
| HP:0000453 | Choanal atresia | Occasional | HP:0002827 | Hip dislocation | Occasional | ||
| HP:0010880 | Increased nuchal translucency | Occasional | HP:0040071 | Abnormal morphology of ulna | Occasional | ||
| Auditory system | HP:0000413 | Atresia of the external auditory canal | Very frequent | HP:0012165 | Oligodactyly | Occasional | |
| HP:0000368 | Low-set, posteriorly rotated ears | Frequent | Muscular system | HP:0001276 | Hypertonia | Very frequent | |
| HP:0000405 | Conductive hearing impairment | Frequent | HP:0000776 | Congenital diaphragmatic hernia | Occasional | ||
| HP:0000407 | Sensorineural hearing impairment | Frequent | HP:0001252 | Hypotonia | Occasional | ||
| HP:0000400 | Macrotia | Occasional | Extremities | HP:0001883 | Talipes | Occasional | |
| Visual system | HP:0000482 | Microcornea | Frequent | Skin, hair and nails | HP:0000294 | Low anterior hairline | Very frequent |
| HP:0000508 | Ptosis | Frequent | HP:0000527 | Long eyelashes | Very frequent | ||
| HP:0000545 | Myopia | Frequent | HP:0000574 | Thick eyebrow | Very frequent | ||
| HP:0000667 | Phthisis bulbi | Frequent | HP:0000664 | Synophrys | Very frequent | ||
| HP:0000486 | Strabismus | Occasional | HP:0002162 | Low posterior hairline | Very frequent | ||
| HP:0000501 | Glaucoma | Occasional | HP:0002230 | Generalised hirsutism | Very frequent | ||
| HP:0000518 | Cataract | Occasional | HP:0002553 | Highly arched eyebrow | Very frequent | ||
| HP:0000639 | Nystagmus | Occasional | HP:0007665 | Curly eyelashes | Very frequent | ||
| Cardiovascular system | HP:0001629 | Ventricular septal defect | Occasional | HP:0000965 | Cutis marmorata | Frequent | |
| HP:0001631 | Atrial septal defect | Occasional | HP:0007598 | Bilateral single transverse palmar creases | Frequent | ||
| HP:0030680 | Abnormal cardiovascular system morphology | Occasional | Nervous system | HP:0000252 | Microcephaly | Very frequent | |
| Breast | HP:0002557 | Hypoplastic nipples | Frequent | HP:0001249 | Intellectual disability | Very frequent | |
| Gastrointestinal system | HP:0002020 | Gastro-esophageal reflux | Very frequent | HP:0010300 | Abnormally low-pitched voice | Very frequent | |
| HP:0008872 | Feeding difficulties in infancy | Frequent | HP:0010864 | Intellectual disability, severe | Very frequent | ||
| HP:0002021 | Pyloric stenosis | Occasional | HP:0000722 | Compulsive behaviours | Frequent | ||
| HP:0002566 | Intestinal malrotation | Occasional | HP:0000739 | Anxiety | Frequent | ||
| HP:0002580 | Volvulus | Occasional | HP:0002167 | Abnormality of speech or vocalization | Frequent | ||
| Endocrine system | HP:0000823 | Delayed puberty | Occasional | HP:0002360 | Sleep abnormality | Frequent | |
| Genitourinary system | HP:0000003 | Multicystic kidney dysplasia | Frequent | HP:0007018 | Attention-deficit hyperactivity disorder | Frequent | |
| HP:0000028 | Cryptorchidism | Frequent | HP:0000717 | Autism | Occasional | ||
| HP:0000047 | Hypospadias | Frequent | HP:0001250 | Seizure | Occasional | ||
| HP:0000059 | Hypoplastic labia majora | Frequent | HP:0002119 | Ventriculomegaly | Occasional | ||
| HP:0000076 | Vesicoureteral reflux | Frequent | HP:0002120 | Cerebral cortical atrophy | Occasional | ||
| HP:0008736 | Hypoplasia of penis | Frequent | HP:0007360 | Aplasia/hypoplasia of the cerebellum | Occasional | ||
| HP:0000083 | Renal insufficiency | Occasional | HP:0009830 | Peripheral neuropathy | Occasional | ||
| HP:0000130 | Abnormality of the uterus | Occasional | Prenatal development and birth | HP:0001622 | Premature birth | Frequent | |
| HP:0000786 | Primary amenorrhoea | Occasional | HP:0001557 | Prenatal movement abnormality | Occasional |
Frequency categories in Human Phenotype Ontology (HPO, https://hpo.jax.org/app/) system: Very frequent 99–80%; Frequent 79–30%; Occasional 29–5%.
Growth retardation is very frequent and starts antenatally. Patients exhibit generalised and symmetrical microsomia that results in low weight, short stature and microcephaly persisting through adulthood.5,6 This has prompted the development of specific growth charts for this syndrome.7 Although growth hormone levels are usually normal, some patients with low levels have been managed with growth hormone replacement therapy.8,9
Psychomotor delay and/or intellectual disability are present in most patients with classic CdLS and are usually severe.10 They are usually associated with absent or delayed expressive language development and autism spectrum and other behavioural disorders. From a neurologic standpoint, affected patients may have epilepsy, motor disorders (stereotypical movements) and abnormalities in sensory processing (hypoalgesia or heat or cold intolerance), hypotonia or sleep disturbances. Recently, evidence has emerged of autonomic nervous system involvement with small fibre neuropathy, in some cases associated with asymmetries in the sympathetic responses in the lower extremities.11
Limb abnormalities are present in 80% of cases. Between 25% and 30% of patients have severe malformations, chiefly reduction deformities in the upper extremities, such as monodactyly or oligodactyly, while the rest usually have small hands or feet that may be associated with minor malformations, such as proximally placed thumbs or clinodactyly. These anomalies are usually bilateral and asymmetrical12 (Fig. 1, Table 1).
Gastro-esophageal reflux (GER) is the main medical disorder in classic CdLS, affecting 93.3% of patients.13,14 Patients must be assessed for GOR from an early age, especially if they exhibit feeding problems, by means of oesophageal pH impedance or manometry or, if not available, a barium swallow. When not managed appropriately (see the “Treatment and Follow-up” section below), GOR can cause complications such as oesophagitis, pneumonitis/aspiration pneumonia and behavioural changes (irritability, self-injury, etc.) that are frequently treated inappropriately with psychotropic drugs.
Other possible gastrointestinal abnormalities, which are much less frequent, include intestinal malrotation (2%–3% of cases), which must always be considered in patients with CdLS presenting in the emergency department with acute abdominal pain, pyloric stenosis (7%) or congenital diaphragmatic hernia.15
In the sensory area, the most frequent complication is sensorineural hearing loss, found in 80% of patients, of who nearly half have severe bilateral hearing loss.16 When it comes to vision, palpebral ptosis, usually bilateral, and myopia (>50% cases) are frequent, as is nystagmus (40%). Less frequent ophthalmological features include dacryostenosis, strabismus, microcornea, glaucoma or optic nerve coloboma.17
As regards the cardiovascular system, approximately one third of patients have congenital heart anomalies, most frequently atrial or ventricular septal defect, pulmonary valve stenosis, tetralogy of Fallot, hypoplastic left heart syndrome and bicuspid aortic valve.18
A recent study demonstrated that some patients may develop early cardiomyopathy that can be detected through speckle-tracking echocardiography before the development of symptoms or other echocardiographic or laboratory abnormalities.19
In the genitourinary system, cryptorchidism is frequent in male patients (75%), bicornate uterus in female patients (25%) and hypoplasia of genitalia in patients of any sex (50%). Renal anomalies are rare, and the most frequent one is vesicoureteral reflux20 (10%) (Table 1).
A degree of endocrine dysregulation has also been described, including abnormal levels of prolactin and delayed puberty. Abnormal results in the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) have been associated with early development of insulin resistance in some patients.9 Low-levels of lean mass and decreased bone density have also been reported.21
Other, less frequent identified abnormalities include cleft palate, dental anomalies, cutis marmorata and low-pitched cry.3
Natural historyIn patients with classic CdLS, the diagnosis is usually made at birth and therefore appropriate treatment can be initiated early, especially as regards nutrition, which frequently requires placement of a nasogastric tube or even performance of gastrostomy to be maintained for the first few months or even years of life. It is important to take into account that in these patients, anthropometric measurements at birth and during growth and development tend to be at percentiles below the normal range, and therefore paediatricians should not insist on overfeeding, which would only worsen the GOR experienced by most patients with CdLS. Therefore, we recommend using specific growth charts for this syndrome (available at https://www.corneliadelange.es/). The most frequent complications throughout the lifespan are pneumonia/pneumonitis due to aspiration of stomach acid (from GER), which is one of the leading causes of early mortality14 (31% of deaths), bowel obstruction (due to volvulus in cases of intestinal malrotation) (19%), complications from severe congenital anomalies (congenital diaphragmatic hernia or heart defects) (15%). Other causes of early mortality were neurologic complications, accidental injury (8%) and sepsis (4%).
Non-classic Cornelia de Lange syndromeBeyond classic CdLS, advances in genetic diagnosis have allowed the diagnosis of additional non-classic CdLS phenotypes, usually milder, that may be difficult to identify for paediatricians. Due to the substantial phenotypic heterogeneity of these atypical forms, some authors prefer to use the term Cornelia de Lange spectrum instead of syndrome.
In general, patients with atypical forms tend to have a more nonspecific phenotype (less pronounced features) compared to patients with classic phenotypes, and the most salient features may be synophrys and the facial gestalt (Fig. 1).
Differential diagnosisThe differential diagnosis of CdLS can be complex due to the variability of its clinical manifestations and the overlap with other genetic neurodevelopmental disorders, such as Coffin-Siris syndrome, Rubinstein-Taybi syndrome, KBG syndrome, CHOPS syndrome or Wiedemann-Steiner syndrome.22–26 In 2018, a consensus group of international experts proposed clinical diagnostic criteria that paediatricians could apply easily when they encounter patients with suspected CdLS3 (Table 2). These criteria allow differentiation between classic and non-classic phenotypes and of CdLS and diseases with similar presentations. It is based on assigning a specific value to a series of cardinal features and other suggestive features and, based on the obtained score, classification as classic CdLS (score ≥ 11 points, with at least 3 cardinal features present) and non-classic CdLS (score of 9–10 points, with at least 2 cardinal features present). In addition, a score is between 4 and 8 points warrants performance of molecular testing for CdLS to make the diagnosis (Table 2).
Clinical features of Cornelia de Lange syndrome.
| [0,1-2]Main features (2 points each) | |
| Synophrys and/or thick eyebrows | HP:0000664 and/or HP:0000574 |
| Short nose, concave nasal ridge and/or upturned nasal tip | HP:0003196, HP:0011120 and/or HP:0000463 |
| Long and/or smooth philtrum | HP:0000343 and/or HP:0000319 |
| Thin upper lip vermilion and/or downturned corners of mouth | HP:0000219 and/or HP:0002714 |
| Hand oligodactyly and/or adactyly | HP:0001180 and/or HP:0009776 |
| Congenital diaphragmatic hernia | HP:0000776 |
| [0,1-2]Suggestive features (1 point each) | |
| Global developmental delay and/or intellectual disability | HP:0001263 and/or HP:0001249 |
| Prenatal growth retardation | HP:0001511 |
| Postnatal growth retardation | HP:0008897 |
| Microcephaly (prenatally and/or postnatally) | HP:0000252 |
| Small hands and/or feet | HP:0200055 and/or HP:0001773 |
| Short 5th finger | HP:0009237 |
| Hirsutism | HP:0001007 |
Interpretation of score:
≥11 points, of which at least 3 are main classic CdLS.
9 or 10 points, of which at least 2 are main: non-classic CdLS.
4–8 points, of which at least 1 is main molecular testing for CdLS indicated.
<4 points: insufficient to indicate molecular testing for CdLS.
In addition, in recent years artificial intelligence (AI) tools have been developed that facilitate the clinical diagnosis of rare diseases through the analysis of facial morphology. Latorre et al.27 tested the Face2Gene application (https://www.face2gene.com) using frontal facial photographs of individuals with CdLS. Their results showed that the software offered a high sensitivity in the facial recognition of CdLS, with a success rate of 83.7% defining success as CdLS being the disorder listed as the first prediction (Fig. 1).
In short, at the time of diagnosis (Fig. 2) it is key to take a thorough history with a standardized history-taking approach, collecting clinical information using Human Phenotype Ontology (HPO) codes (Table 1). In addition, AI tools for facial phenotyping may become increasingly useful (Fig. 1). However, the definitive clinical diagnosis, and the classification as classic or non-classic CdLS should be based on the criteria defined by Kline et al.3 (Table 2).
Molecular diagnosisCornelia de Lange syndrome can be diagnosed by the identification of a pathogenic variant in one of the 5 causative genes associated with the cohesin complex (NIPBL, SMC1A, SMC3, RAD21, HDAC8) and, with less certainty, in 3 recently described additional genes (BRD4, ANKRD11, MAU2).28,29 The cohesin complex consists of a central ring formed by proteins SMC1A, SMC3 and RAD21 and a series of regulatory proteins such as NIPBL, the main protein involved in loading cohesins on DNA, and HDAC8, a SMC3 deacetylase that allows the release of the cohesin complex from chromatin3 (Fig. 3).
Location of the proteins that cause Cornelia de Lange syndrome: NIPBL and BRD4 are responsible for nucleosome clearance in DNA so that proteins SMC1A, SMC3, RAD21 and STAG2 can form the cohesin ring. MAU2 forms a heterodimeric complex with NIPBL to help load cohesin on chromatin. Then, protein HDAC8 deacetylates SMC3, allowing the release of the cohesin ring. Protein ANKRD11 is involved in the transcription activation process.
The cohesin complex is a multifunctional complex involved in many biological processes, such as chromosome segregation, genomic stabilization and organization and, above all, regulation of gene expression. Most individuals with CdLS (more than 60%) have a pathogenic variant of NIPBL, a gene that encodes a protein that interacts with chromatin remodelers and transcription factors.30 This is the reason that CdLS is classified as a transcriptomopathy, that is, a disease caused by the global dysregulation of gene expression throughout the organism, which explains the multisystemic involvement and the phenotypic variability of this syndrome.28
Technological advances in high-throughput sequencing in recent years have allowed the identification of new causative genes, especially in patients with non-classic phenotypes. Among the salient ones are ANKRD11, which encodes a protein involved in transcriptional regulation,24 or MAU2 and BDR4, which encode proteins that interact with NIPBL29,31 (Fig. 3).
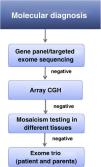
This substantial clinical and genetic heterogeneity, especially in individuals with non-classic/mild or atypical CdLS, can pose a barrier to molecular diagnosis. Therefore, the use of next-generation sequencing techniques allowing simultaneous evaluation of a large number of genes is recommended for the initial approach to genetic diagnosis (Fig. 4). In recent years, the general approach has been to use of panels that include genes known to cause CdLS and other genes associated with neurodevelopmental disorders, or clinical exome sequencing to simultaneously evaluate up to 7000 genes associated with disease. On the other hand, given the improvement of molecular technologies, the reduction in the costs of sequencing and the automation of data analysis, it is possible that in upcoming years whole-exome or even whole-genome sequencing will replace these techniques.
If all these techniques yield negative results, performance of array comparative genomic hybridization (CGH) is recommended to assess for chromosomal anomalies or copy number variants.
The presence of somatic mosaicism in some individuals (10%–13%) may pose an additional difficulty and require analysis in specialised laboratories of other tissues, such as oral mucosa specimens or fibroblasts, and the use of highly sensitive molecular diagnostic techniques when results in blood samples turn out negative.32,33
If this still fails to yield a diagnosis, patient-parent trio whole exome sequencing is recommended. The parental specimens are then used to filter the variants identified in the index case (Fig. 4).
However, despite the significant progress made to date, there is still a considerable number of individuals in which genetic diagnosis is not achieved, which suggests the existence of causative genes and mechanisms of mutation that have yet to be identified.
Genotype-phenotype associationAlthough most individuals with classic CdLS have a pathogenic variant in gene NIPBL associated with the characteristic facial morphology and, in some cases, reduction deformities in the extremities, there are also patients with non-classic phenotypes (Fig. 5). The pathogenic variants of NIPBL are uniformly distributed through the coding region, but missense variants are more frequent in the HEAT repeat domain and are associated with a milder presentation.32,34
Individual with pathogenic variants of the SMC1A or SMC3 genes exhibit fewer structural anomalies and less severe growth retardation compared to individuals with pathogenic variants in NIPBL; however, they have significant intellectual disability that is moderate to severe.35 Patients with pathogenic variants in the SMC1A present with slightly flatter and wider eyebrows and a long and wide nasal ridge, in some cases, they develop refractory epilepsy with an early onset.36 Patients with pathogenic variants in the SMC3 gene often present with subtle or no synophrys, and 56% have heart defects.37
Individuals with a heterozygous pathogenic variant of RAD21 usually have no major structural anomalies, and tend to present milder cognitive impairment and GER, with the latter limited to early childhood.38 They usually exhibit growth delay, minor skeletal deformities and facial features that overlap with those of CdLS.
Male patients with hemizygous pathogenic variants in the HDAC8 gene have the characteristic facial features of CdLS accompanied by delayed anterior fontanelle closure, hooding of the eyelids, orbital hypertelorism, pigmentary mosaicism, dental anomalies and, most saliently, a broad/bulbous nasal tip and a friendly personality. Growth delay is not as significant, with a lower frequency of postnatal growth retardation and microcephaly. Some of these patients may also exhibit severe reduction deformities in the extremities. In female patients, the presentation tends to be milder, but the severity is strongly associated with the X chromosome inactivation pattern.39
Genetic counsellingMost CdLS cases are sporadic, especially in cases involving genes with autosomal dominant inheritance: NIPBL, SMC3 and RAD21. In the case of pathogenic variants in X-linked genes (SMC1A and HDAC8), male patients tend to have more severe presentations compared to female patients. In these families, there are cases of parents with mild disease and the risk of transmission depends on the sex of the affected parent.3
More than 99% of patients with classic CdLS have a de novo heterozygous pathogenic variant in the NIPBL gene. This means that the risk of recurrence in future siblings is very low (1–2%) and chiefly due to the possibility of germline mosaicism in one of the parents.40 In families in which a pathogenic variant has been identified in any of the genes associated with CdLS, prenatal genetic screening could be performed to detect or rule out the presence of the variant in the foetus.
Treatment and follow-upAt present, there is no curative treatment for CdLS, but there are symptomatic and palliative treatments that the paediatrician must be aware of. The main medical complication in classic CdLS (associated with the NIPBL gene) is GER, which is present in most patients and must be treated early and aggressively conforming to the validated treatment protocols in the paediatric literature. In some instances, when the patient does not respond well to the standard of care, alternative measures need to be used, such as nasogastric or gastrostomy tube feeding in the first months or years of life. Some patients ultimately require surgical treatment (Nissen fundoplication). When it comes to the digestive system, it is important to keep in mind the possible presence of intestinal malrotation that could become complicated, manifesting with constant or recurrent acute abdominal pain (due to obstruction or volvulus), bringing the patient to the emergency department (usually within 2 years from birth) where, if the cause is not identified early, the outcome could be death.3
Hearing should be tested from birth (otoacoustic emission test); in the case of negative or uncertain results, auditory evoked potential testing should be performed so that, should sensorineural hearing loss be detected, hearing aid devices (audiphone or cochlear implant) can be placed as soon as possible, preferably before the age when speech starts to develop. The evaluation should also include an echocardiogram and an abdominal ultrasound examination to assess for the presence of structural anomalies in the heart and the organs in the abdominal cavity (kidneys).
As regards psychomotor development, most patients with CdLS require intervention to stimulate language development (speech-language therapy) and motor development (physical and/or occupational therapy). Some patients may need pharmacotherapy to manage hyperactivity and/or attention deficit.
In November 2023, the XII CdLS World Conference was held in Zaragoza by the Reference Centre for CdLS in collaboration with the Asociación Española del Síndrome de Cornelia de Lange (Spanish Association of CdLS) and the CdLS World Federation. The congress gathered the main experts worldwide, who shared the latest advances in the knowledge of the syndrome. In the clinical area, we ought to highlight the novel technique of sensory neuromodulation to treat refractory epilepsy in patients with CdLS and pathogenic variants of the SMC1A gene, which has proven effective in 3 out of 4 treated patients. The importance of contemplating the possibility of intestinal malrotation in any child with CdLS presenting to the emergency department with acute abdominal pain was also addressed. In respect of anaesthetic procedures in children with CdLS, presenters insisted that experience has demonstrated that they are well tolerated by patients. In the basic research section, evidence was presented confirming that the absence of cohesin gives rise to changes in DNA transcription, in addition to novel 3D models of cohesin that could help identify potential therapeutic targets in CdLS. Animal models are also being developed to start clinical trials of Ataluren for treatment of epilepsy in patients with CdLS-SMC1A. Results from single-cell sequencing studies were presented that evinced the presence of transcriptional dysregulation in the early stages of embryonal development in a mouse model. The first findings on the subject of cellular senescence in CdLS, obtained with pluripotent stem cells derived from patients with CdLS, were also presented.
FundingStrategic Health Action programme (Acción Estratégica en Salud [AES], Instituto de Salud Carlos III, Ministry of Science, Innovation and Universities of Spain): Project PI23/01370; Government of Aragón (Diputación General): Research Group B32-20R.
Conflicts of interestThe authors have no conflicts of interest to declare.