Patients with invasive pneumococcal disease (IPD) may require admission into paediatric intensive care units (PICU). The aim of this study is to analyse the epidemiological, clinical, and microbiological characteristics associated with IPD that may require admission to the PICU.
Material and methodsA prospective study was conducted on cases of IPD diagnosed in three Paediatric Hospitals in Barcelona between January 2012 and June 2016. An analysis was made of the associations between the admission to PICU and the epidemiological, clinical, and microbiological variables.
ResultsA total of 263 cases with IPD were included, of which 19% (n=50) required admission to PICU. Patients with septic shock (7; 100%), meningitis (16; 84.2%), and those with complicated pneumonia (23; 15.2%) were admitted to the PICU. The most frequent complications were pulmonary (35.2%) and neurological (39.5%). The ratio between admission and non-admission to PICU was 4.7 times higher in subjects with an underlying disease. The serotypes associated with PICU admission were 19A (23% of the total of this serotype), serotype 14 (20%), serotype 3 (17%), and serotype 1 (12.5%).
ConclusionsIPD required PICU admission in cases of septic shock and meningitis, and less so with complicated pneumonia. The percentage of admissions is greater in children with an underlying disease. Admission into the PICU involves a longer stay, complications during the acute phase, as well as sequelae, particularly neurological ones. The serotypes of the patients that were admitted to PICU were predominantly vaccine serotypes.
La enfermedad neumocócica invasora (ENI) puede requerir ingreso en la unidad de cuidados intensivos pediátricos (UCIP). El objetivo de este trabajo es analizar las características epidemiológicas, clínicas y microbiológicas asociadas a la ENI que predisponen el ingreso en la UCIP.
Material y métodosEstudio prospectivo de casos diagnosticados con ENI en tres hospitales pediátricos de Barcelona entre enero de 2012 y junio de 2016. Se analizaron las asociaciones entre el ingreso en la UCIP y las variables epidemiológicas, clínicas y microbiológicas.
ResultadosSe incluyeron 263 casos con ENI. El 19% (n=50) requirió ingreso en la UCIP. El 100% (7) de los pacientes con shock séptico, 84,2% (16) con meningitis y 15,2% (23) con neumonía complicada ingresaron en la UCIP. Las complicaciones más frecuentes fueron pulmonares (35,2%) y neurológicas (39,5%). La razón entre ingreso y no ingreso en la UCIP fue 4,17 veces mayor en los sujetos con enfermedad de base. Los serotipos asociados al ingreso en la UCIP fueron el 19A (23% del total de este serotipo), el 14 (20%), el 3 (17%) y el serotipo 1 (12,5%).
ConclusionesLa ENI requiere ingreso en la UCIP en caso de shock séptico y meningitis, no así, de entrada, la neumonía complicada. El porcentaje de ingresos es mayor en los niños con enfermedad de base. El ingreso en la UCIP conlleva una estancia más prolongada, así como complicaciones durante la fase aguda y secuelas, sobre todo, neurológicas. Los serotipos de los pacientes que ingresaron en la UCIP fueron, predominantemente, serotipos vacunales.
Invasive pneumococcal disease (IPD) continues to cause significant morbidity and mortality in the paediatric population of Spain, even after the introduction in our country of the pneumococcal conjugate vaccine.1 The incidence of IPD in children under 5 years in Spain ranges between 16.8 (2012) and 12.3 (2015) per 100000 inhabitants.1,2
In some cases, it requires admission to the paediatric intensive care unit (PICU) due to the severity of the symptoms. Some forms of IPD, such as sepsis or meningitis, require intensive care from onset, with management based on the continuous monitoring and intensive treatment that these forms require once they are diagnosed. Other forms, such as complicated pneumonia, do not always require admission to the PICU to be cured and could be managed initially in a hospital without paediatric intensive care services.3
The aim of our study was to analyse the epidemiological, microbiological and clinical characteristics of cases of IPD potentially requiring admission to the PICU.
MethodsStudy designWe conducted a prospective study in children aged 2 to 59 months given a diagnosis of IPD between January 1, 2012 and June 30, 2016 managed in 3 children's hospitals in Barcelona, Spain (Hospital Sant Joan de Déu, Hospital Materno-Infantil Vall d’Hebron and Hospital HM Nens) with an estimated cumulative catchment population under 5 years of 116279 inhabitants (30.4% of the total population aged <5 years in Catalonia).2 The first 2 hospitals have a PICU and are the 2 referral PICUs in Catalonia, although there are other PICUs in the region.
We defined IPD as clinical manifestations of infection in addition to isolation of Streptococcus pneumoniae or detection of DNA corresponding to the LytA gene and an additional capsule gene by reverse transcription polymerase chain reaction (RT-PCR) in a sample collected from a normally sterile site.
Demographic, clinical and epidemiological variablesFor each case of IPD, we collected data on the following demographic, clinical and epidemiological variables: age, sex, date of birth, date of onset, date of hospital admission, form of IPD (meningitis, septic shock, complicated or uncomplicated pneumonia, occult bacteraemia and other, less frequent forms), complications, admission to PICU and length of stay, presence of medical risk factors (sickle cell anaemia, congenital or acquired asplenia, infection by human immunodeficiency virus, cochlear implant, severe congenital immunodeficiency requiring treatment, chronic heart disease requiring treatment or surgically corrected, chronic respiratory disease including asthma treated with high-dose steroids (methylprednisolone at a dose >1mg/kg/day for longer than 1 month), cerebrospinal fluid fistula, chronic renal failure including nephrotic syndrome requiring dialysis, immunosuppressive therapy or radiotherapy in the past 6 months, solid organ or haematopoietic stem cell transplantation, and insulin-dependent diabetes mellitus), date of discharge and outcome at discharge (discharge without sequelae, discharge with sequelae, death). We considered that pneumonia was complicated in case of lung consolidation with more than one of the following: pleural effusion greater than 10mm, loculated pleural effusion, parapneumonic empyema, necrotising pneumonia or pulmonary abscess. We assessed for the presence of sequelae at 6 months from diagnosis.
Identification, serotyping and classification of S. pneumoniaeStrains isolated from culture were serotyped by means of the Quellung reaction or a dot blot assay in the Pneumococcus Reference Laboratory of the Centro Nacional de Microbiología (National Centre of Microbiology) in Majadahonda (Madrid, Spain). In patients in which the pneumococcal strain could not be isolated from culture, serotyping was performed by means of RT-PCR, which allows detection of 43 different serotypes/serogroups with the multilocus sequence typing (MLST) method, which is recognised at the international level.
Since PCR does not differentiate between serotypes 6A and 6C, 7F and 7A, 9V, 9A and 9N and 19F, 19B and 19C, we only considered serotypes 6A, 7F, 9V and 19F as vaccine serotypes once their identification was confirmed by the Pneumococcus Reference Laboratory in Majadahonda.2
Genotyping from samples was performed at the Molecular Biology Laboratory of the Hospital Sant Joan de Déu with the MLST method. We identified allelic profiles and sequence types with the software available at http://pubmlst.org/spneumoniae/. We analysed sequence types and assigned specific clonal complexes using the eBURST algorithm.
Criteria for admission to the PICUThe children admitted to the PICU met all or some of the following criteria: IPD with severe haemodynamic instability that did not improve with administration of 1 or 2 boluses of fluids; diagnosis of meningitis; development of acute respiratory distress syndrome defined as a ratio of arterial oxygen partial pressure (PaO2) to fractional inspired oxygen (FiO2) of 200 to 250; need for invasive or non-invasive mechanical ventilation; signs of acute renal failure (2-fold or greater increase in serum creatinine relative to baseline over a 12–24h interval); disseminated intravascular coagulation; Glasgow coma scale score of less than 8.3
Vaccination against pneumococcusWe obtained the vaccination status of each patient with IPD from the official vaccination history card, the records of the public or private primary care centre that managed the child routinely or the patient health records. We defined correct vaccination as having received every dose of pneumococcal 13-valent conjugate vaccine (PCV13) scheduled up to the patient's age as established by the summary of product characteristics.4
Statistical analysisWe assessed the association between categorical variables with the χ2 test or the Fisher exact test, and the association between continuous variables with the Student t test. To estimate the strength of the association between the variables under study and admission to the PICU, we calculated odds ratios (ORs) with the corresponding 95% confidence intervals (CIs). We defined statistical significance as a P-value of .05. All tests were two-tailed.
Ethical considerationsThe study was approved by the ethics committees of the 3 participating hospitals. The guardians of the patient signed an informed consent document stating that they understood the study, accepted to participate and had the right to withdraw at any time.
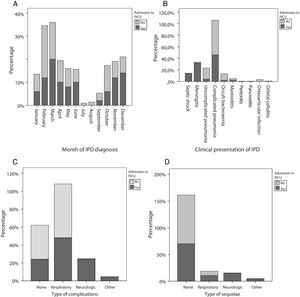
ResultsThe study included 263 patients with IPD (155 male and 108 female), with a mean age of 45 months (standard deviation [SD], 37.1), out of who 50 (19%) required admission to the PICU at some point in the course of disease (Group 1). The remaining 213 patients did not require admission to the PICU (Group 2). The incidence of IPD peaked in February (n=54; 20.5%) and March (n=44; 16.7%), while admission to the PICU peaked in March (n=10; 20%) and December (n=7; 14%) (Fig. 1A).
The length of stay was significantly longer in patients that required admission to the PICU (19.8±12.5 days) compared to those that did not (9.6±5.8 days) (P<.001). We found that 46.7% of patients with underlying disease required admission to the PICU compared to 17.3 of patients that were previously healthy. The ratio of the number of patients admitted to the PICU to the number not admitted was 4.17 times greater in patients with underlying disease compared to previously healthy patients, and this association was statistically significant (OR, 4.17; 95% CI, 1.44–12.12).
The forms of IPD found in the total sample were (n; %): complicated pneumonia (152; 57.4%), uncomplicated pneumonia (43; 16.3%), occult bacteraemia (25; 9.5%), meningitis (19; 7.2%), septic shock (7; 2.7%), mastoiditis (7; 2.7%), osteoarticular infection (7; 2.7%), orbital cellulitis (2; 0.8%), peritonitis (1) and pancreatitis (1). In group 1, the patients admitted to the PICU included 100% of patients with septic shock (OR, 5.95; 95% CI, 4.53–7.82), 84.2% of patients with meningitis (OR, 32.94; 95% CI, 9.11–119.10) and 15.2% of patients with complicated pneumonia (OR, 0.57; 95% CI, 0.30–1.50), although in the latter case the association with PICU admission was not statistically significant. Group 2, composed of the patients that did not require PICU admission, included 95% of the patients with uncomplicated (OR, 0.18; 95% CI, 0.04–0.75) (Table 1 and Fig. 1B).
Characteristics of patients with invasive pneumococcal disease, compared based on the need for admission in the paediatric intensive care unit.
| PICU (n=50) n (%) | No PICU (n=213) n (%) | P (Student t) | Raw OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|---|
| Age (months) | 36.9±36.1 (26.7–47.2) | 47.6±37.4 (42.6–52.7) | .067 | – | – |
| Sex | |||||
| Male | 28 (18.1%) | 127 (81.9%) | .220.639 | 0.86 (0.46–1.6) | 0.37 (0.12–1.19) |
| Female | 22 (20.4%) | 86 (79.6%) | |||
| Hospital length of stay in days | 9.6±5.8 (8.8–10.4) | 19.8±12.5 (16.2–23.4) | <.001 | – | – |
| Underlying disease | |||||
| Yes | 7 (46.7%) | 8 (53.3%) | .011 | 4.17 (1.44–12.12) | 4.97 (0.61–40.64) |
| No | 43 (17.3%) | 205 (82.7%) | |||
| Clinical presentation | |||||
| Septic shock | |||||
| Yes | 7 (100%) | 0 (0%) | <.001 | 5.95 (4.53–7.82) | |
| No | 43 (16.8%) | 213 (83.2%) | |||
| Meningitis | |||||
| Yes | 16 (84.2%) | 3 (15.8%) | <.001 | 32.94 (9.11–119.10) | 15.27 (2.39–97.67) |
| No | 34 (13.9%) | 210 (86.1%) | |||
| Complicated pneumonia | |||||
| Yes | 23 (15.2%) | 128 (84.8%) | .07 | 0.57 (0.304–1.50) | |
| No | 27 (24.1%) | 85 (75.9%) | |||
| Uncomplicated pneumonia | |||||
| Yes | 2 (4.7%) | 41 (95.3%) | .009 | 0.18 (0.04–0.75) | 0.21 (0.03–1.41) |
| No | 48 (21.8%) | 172 (78.2%) | |||
| Bacteraemia | |||||
| Yes | 1 (4.0%) | 24 (96.0%) | .081 | 0.16 (0.02–1.21) | 0.11 (0.01–1.31) |
| No | 49 (20.6%) | 189 (79.4%) | |||
| Other presenting symptoms | |||||
| Yes | 1 (5.6%) | 17 (94.4%) | .232 | 0.24 (0.03–1.81) | |
| No | 49 (20%) | 196 (80%) | |||
| Acute complications | |||||
| None | 12 (12.9%) | 81 (87.1%) | Ref. | ||
| Neurologic | 12 (85.7%) | 2 (14.3%) | <.001 | 33.3 (7.17–154.8) | 7.53 (0.83–68.23) |
| Respiratory | 24 (15.8%) | 128 (84.2%) | .536 | 0.613 (0.33–1.14) | |
| Other | 2 (50%) | 2 (50%) | .18 | 4.4 (0.6–31.9) | |
| Sequelae | |||||
| None | 33 (14.4%) | 194 (85.5%) | |||
| Neurologic | 7 (87.5%) | 1 (12.5%) | <.001 | 37.1 (4.44–309.9) | |
| Respiratory | 5 (22.7%) | 17 (77.3%) | .54 | 1.4 (0.48–3.93) | |
| Other | 2 (66.7%) | 1 (33%) | .028 | 9.4 (0.84–106.2) | |
| PCV13 vaccination | |||||
| Complete | 5 (11.1%) | 40 (88.9%) | (.14) | 0.48 (0.18–1.29) | |
| Incomplete or unvaccinated | 45 (20.6%) | 172 (79.4%) | |||
CI, confidence interval; OR, odds ratio; PICU, paediatric intensive care unit; PCV13, pneumococcal conjugate 13-valent vaccine.
* We applied the continuity correction to the χ2 test when at least 1 expected frequency <5.
Quantitative data expressed as mean±standard deviation (95% CI).
In both groups 1 and 2, 64.4% of patients with IPD experienced some form of complication; 57.8% were respiratory complications and 5.3% neurologic complications. Of the patients with neurologic complications during the acute phase, 85.7% required admission to the PICU (OR, 33.3; 95% CI, 7.17–154.8), as did 15.8% of the patients with respiratory complications (OR, 0.613; 95% CI, 0.33–1.14) (Fig. 1C).
Of the 260 patients with IPD for who follow-up data were available, 85.5% were free of sequelae at 6 months. Two patients died and 1 did not attend the follow-up visit. Out of the patients with immediate sequelae, 22 (8.5%) had respiratory sequelae and 8 (3.1%) neurologic sequelae (including 2 patients with hearing loss); 1 patient had high blood pressure, 1 required a radical mastoidectomy and 1 had cavernous sinus thrombosis. In group 1, 14.3% of patients developed neurologic sequelae (OR, 37.1; 95% CI, 4.4–309-9), followed in frequency by respiratory sequelae in 10.2% (OR, 1.4; 95% CI, 0.48–3.93) (Fig. 1D).
Of all patients with IPD, 84.8% (223/263) had not received antibiotherapy at the outpatient level before admission, and 20.6% of these subset of patients (46/223) belonged to group 1 (OR, 0.43; 95% CI, 0.145–1.26; P=.115).
We found that 50.4% (132/263) of patients, without differences between groups, had received at least 1 dose of pneumococcal conjugate vaccine. Only 28.1% were correctly vaccinated for their age (8.7% with PCV7; 2.3% with PVC10 and17.1% with PCV13). We did not find an association between the need for admission to the PICU (group 1) and complete PCV13 vaccination status (OR, 0.48; 95% CI, 0.18–1.3) (P=.135).
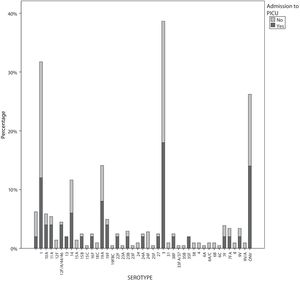
The serotypes identified most frequently in both groups were serotype 3 (53 [20.2%], of who 17% required PICU admission), serotype 1 (48 [18.3%], of who 12.5% required PICU admission), other nonvaccine serotypes (33 [12.5%], of who 21% required PICU admission), serotype 19A (17; 6.5%, of who 23% required PICU admission) and serotype 14 (15 [5.7%], of who 20% required PICU admission). The serotypes identified most frequently in patients of group 1 were (n, %): serotype 3 (9; 18%), other nonvaccine serotypes (7; 14%), serotype 1 (6; 12%) and serotype 19A (4; 8%). The most frequent nonvaccine serotypes in group 1 were serotypes 10A (2; 4%) and 11 (2; 4%) (Fig. 2). There was only 1 case of vaccine failure in this group, corresponding to a patient with infection by serotype 3. When it came to genotyping, the only salient finding in group 1 was detection of sequence type 306, corresponding to serotype 1, in 4/6 cases; the rest of sequence types and clonal complexes varied and were not associated significantly with PICU admission.
DiscussionAdmission to the PICU is a possible development in the context of IPD, so the exploration of the epidemiological, microbiological and clinical factors may be associated with an increased likelihood of admission to the PICU is a relevant pursuit. In our study, we identified factors associated with an increased probability of admission to the PICU, such as age less than 1 year at the time of diagnosis, the presence of underlying disease that increases the risk of IPD, specific presentations of IPD such as septic shock or meningitis and the months of the year corresponding to seasonal viral disease outbreaks.
The two seasonal peaks in the incidence of IPD and the peak in the proportion of patients with IPD requiring PICU admission coincided with the annual outbreaks of bronchiolitis due to respiratory syncytial virus (RSV) and influenza, as described in previous studies.5–9 However, while the incidence of IPD peaked in February and March (and the influenza outbreak occurred between week 50 of 2013 and week 12 of 201410), the months corresponding to the highest proportion of IPD cases requiring PICU admission were November and December (overlapping with the RSV season that happens between week 40 of one year and week 4 of the following year10). This could be explained by different factors, such as the lower age of patients with bronchiolitis, the virulence of the virus involved in the RSV or flu season, and the previous health of the patient.6,7
Outpatient management with antibiotherapy previous to hospital admission does not have a protective effect against admission to the PICU, as we found in our series and in reviewing the work of other authors.11
The total length of stay was greater in patients admitted to the PICU, which was consistent with the findings of previous studies.12 When it came to the forms of IPD that required admission to the PICU, we found that the only feature present in 100% of the patients admitted to the PICU was haemodynamic instability. Although a diagnosis of meningitis was in itself a criterion for admission to the PICU, it was not always made at the outset and patients with a discharge diagnosis of meningitis that were not admitted to the PICU had favourable outcomes. This was because 39.5% of the patients with meningitis admitted to the PICU had complications during the acute phase, as was observed in other studies, such as the one published by Casado et al.11 Most children with IPD admitted to the PICU recovered and were free of sequelae. The most frequent sequelae were neurologic, followed by respiratory sequelae. Only 15.2% of patients with complicated pneumonia with pleural effusion or empyema required admission to the PICU, which demonstrates that these patients may be managed in a hospital without an intensive care unit under close monitoring.11,13
Although the most prevalent serotypes (especially 19A) are covered by the PCV13, 11.8% of the patients with complete PCV13 vaccination were admitted to the PICU compared to 18.5% of patients without complete vaccination (P=.23), a difference that was not statistically significant. Thus, in our study, complete vaccination with PCV13 did not exhibit a protective effect as concerned admission to the PICU. The only case of vaccine failure in a patient that required intensive care involved serotype 3, against which the PCV13 proved to be less effective compared to other serotypes, as found in previous studies.14–16 Asner et al.16 reported vaccine failure in 26% of patients that required admission to the PICU, with many of these cases involving serotype 3. We ought to mention 2 serotypes that are not included in the PCV13, 10A and 11A, which, while infrequent among our patients, are associated with a high proportion of PICU admission. In a prospective study conducted in Switzerland that included 117 patients with IPD, patients with meningitis, especially those with infection by non-vaccine serotypes, were most likely to require admission to the PICU.16
When it came to the serotypes involved in IPD, in our study serotype 3 was the one corresponding to the highest proportion of affected patients requiring admission to the PICU, which may be attributable to the characteristics of this serotype, which has capsule proteins resulting in greater virulence,17,18 and the lower efficacy of the PCV13 against this serotype, described in the previous literature.14,17,18 This was also the serotype most frequently associated with development of IPD and with vaccine failure in our study.
These results evince the need of ongoing surveillance of pneumococcal serotypes involved in IPD and their genotypes to have updated information on the aetiological agents of IPD in the local population at all times.19
There are limitations to our study. First of all, the sample only included 30.4% of the population under 5 years in Catalonia, as we only studied IPD in 3 of the hospitals in the region. Secondly, only 2 of the 3 participating hospitals had a PICU, and while these 2 units are the referral PICUs in Catalonia, there are other hospitals that manage patients with IPD. Third of all, there may have been biases associated with the documentation of clinical data in the health records, and some pneumococcal strains were not analysed, meaning the resulting information was not available for 100% of the cases. Lastly, data collection in the last year included in the study period ended in June, which means that the study did not include data corresponding to the bronchiolitis season of that year.
ConclusionsIn our series, the presence of septic shock and/or neurologic involvement in the context of IPD was associated with admission to the PICU, and the proportion of patients admitted to the PICU was higher in the group of patients with underlying disease. The frequency of PICU admission increased during the annual bronchiolitis season. Admission to the PICU was associated with a longer length of stay in hospital, a higher prevalence of complications in the acute phase and a higher prevalence of sequelae, mainly neurologic. The most frequent serotypes involved in the group of patients admitted to the PICU were vaccine serotypes (19A, 14, 3 and 1). Admission to the PICU in the context of IPD is necessary in patients that develop septic shock or neurologic complications. In case of complicated pneumonia, it is possible to manage the patient in the paediatric ward under close monitoring.
FundingNational R&D& programme, Instituto de Salud Carlos III-Vice Directorate General of Evaluation and Promotion of Research in Health Care. Grant no. P111/02081, PI 11/2345; European Regional Development Fund (ERDF) and Agencia de Gestión de Ayudas Universitarias y de Investigación (AGAUR). Grant no. 2017 SGR 1342.
Declaration of Competing InterestNo conflicts of interest.
Juan José García García, Angela Domínguez, Fernando Moraga Llop, Alvaro Díaz Conradi, Mariona Fernández de Sevilla, Sebastià González Peris, Pilar Ciruela, Magda Campins, Carmen Muñoz-Almagro, Cristina Esteva, Conchita Izquierdo, Sonia Uriona, Johanna Martínez Osorio, Anna Solé Ribalta, Gemma Codina, Nuria Soldevila y Lluís Salleras Sanmartí.
Please cite this article as: Díaz-Conradi A, García-García JJ, González Peris S, Fernández de Sevilla M, Moraga Llop F, Ventura PS, et al. Características de los pacientes con enfermedad neumocócica invasora que requieren ingreso en la unidad de cuidados intensivos. An Pediatr (Barc). 2021;94:19–27.
Appendix A lists the members of the Group for the Study of Invasive Pneumococcal Disease in Catalonia (Barcino Group).
Previous presentation: This study was presented as an oral communication at the Annual Congress of the Asociación Española de Pediatría, June 6–8, 2019, Burgos, Spain.