Streptococcus pneumoniae (SP) is a human pathogen that involves a high use of antibiotics. The objective of the study was to determine the susceptibility to commonly used antibiotics and their associated risk factors, in order to promote rational use of antibiotics.
Patients and methodsIn a multicentre study was conducted in summer 2009 and winter 2010 on children attending paediatric clinics in the Region of Murcia. A nasopharyngeal sample was collected and an epidemiological questionnaire was completed. The study included 1562 children aged 1 and 4 years old.
ResultsAlmost one-third (31.3%, 489/1562) of children were nasal carriers. A sensitivity study was carried out on 376 isolates, of which 343 were serotyped. Almost two-thirds (61.7%, 964/1562) of children had received at least one dose of PCV7 (heptavalent pneumococcal conjugate vaccine), and 12.8% (44/343) of the isolates belonged to PCV7 serotypes. The prevalence rates of penicillin resistance (meningitis infections criteria CMI>0.06mg/L) were 28.1%; however, this percentage was 54% in PCV7 serotypes. None of the isolates had (MIC>2mg/L), so prevalence rates of susceptibility with non-meningitis infections criteria were 100%. There was a high percentage of erythromycin resistance (45.7%). The factors favouring resistance to penicillin and cefotaxime were the consumption of antibiotics in the previous month and the carrying of vaccine serotypes. On the other hand, the age of 4 years old was a protective factor of resistance. The 14, 35B, 19A, 15A, and 19F serotypes were less susceptible to penicillin.
ConclusionsBoth oral amoxicillin given to outpatients and intravenous penicillin or ampicillin to hospitalized patients are excellent options for the treatment of non-meningeal infections, as seen with pneumonia in these kinds of environments, where there is low incidence of isolates highly resistant to penicillin (CMI≥2mg/L).
Streptococcus pneumoniae (SP) es un patógeno que causa un elevado consumo de antibióticos. Objetivos: conocer la sensibilidad a antibióticos de uso habitual, los factores epidemiológicos asociados y favorecer el uso racional de antibióticos.
Pacientes y métodosEn verano del 2009 y el invierno del 2010 realizamos un estudio multicéntrico en Atención Primaria (AP). Se recogió una muestra nasofaríngea y se cumplimentó una encuesta epidemiológica en 1.562 niños de 1 y 4 años.
ResultadosEl 31,3% (489/1.562) eran portadores nasales (PN). Se realizó un estudio de sensibilidad en 376 aislados, y se serotipificaron 343. El 61,7% (964/1.562) habían recibido al menos una dosis de vacuna antineumocócica conjugada heptavalente (PCV7). El 12,8% (44/343) correspondía a serotipos vacunales (SV). La resistencia a penicilina (criterio meningitis CMI>0,06mg/L) fue del 28%, siendo del 54% para los SV. Para infecciones no meníngeas, el 100% de los aislados eran sensibles a penicilina parenteral (CMI ≤2mg/L). Existe un alto nivel de resistencias para eritromicina (45,8%). Fueron factores favorecedores de resistencia haber tomado antibióticos el mes previo y ser portador de SV tanto para penicilina como para cefotaxima y la edad de 4 años un factor de protección. Los serotipos 14, 35B, 19A, 15A y 19F fueron los menos susceptibles a penicilina.
ConclusionesLa amoxicilina por vía oral para pacientes ambulatorios y la penicilina o ampicilina por vía intravenosa para pacientes ingresados son excelentes opciones para el tratamiento de infecciones neumocócicas no meníngeas, en entornos como el nuestro, con una baja incidencia de aislados con alto nivel de resistencia a penicilina (CMI≥2mg/L).
Streptococcus pneumoniae (SP) is one of the most frequent aetiological agents in community-acquired infections in both children and adults. It causes severe invasive disease, such as sepsis, meningitis and bacteraemic pneumonia, as well as other, less severe yet much more frequent processes such as otitis and sinusitis, so it has a very high impact on public health and leads to extensive use of antibiotics.1 Our aim was to determine the susceptibility of SP to commonly used antibiotics in our geographical region, as well as the relationship of its susceptibility to the analysed epidemiological factors, with the purpose of contributing to a more rational use of antibiotics.
Materials and methodsWe conducted a cross-sectional multicentre study in children 1–4 years of age in the autonomous community of the Region of Murcia (CARM). In 2009, the population of 1-year-old children in the CARM was 18,479, and the population of 4-year-old children was 17,555 (Instituto Nacional de Estadística).2 The participants were children that attended check-ups in the context of the “Well-child programme” and selected by purposive sampling. We included healthy children age 12 months (10–14 months) and 4 years (3.5–4.5 years) that may or may not have been vaccinated with the pneumococcal 7-valent conjugate vaccine (PCV7). We excluded children presenting with fever, chronic diseases, cystic fibrosis, or that were immunosuppressed.
In collaboration with the Asociación de Pediatría Extrahospitalaria y Atención Primaria de la Región de Murcia (Association of Out-of-Hospital Paediatrics and Primary Care of the Region of Murcia [APERMAP]), we obtained the participation of 60 primary care paediatricians from all the health areas of the CARM except one that represents only 10% of the population, and of microbiologists working at the hospitals of each health area where the samples were collected. One paediatrician was appointed coordinator for each participating hospital service area.
Data collectionData was collected over two periods, the summer of 2009 and the winter of 2009–2010. After verbally explaining the study, we obtained informed consent and administered an epidemiological questionnaire that included the following data: health care centre, date of sample collection, age, sex, pneumococcal vaccination status, breastfeeding, school enrolment/child care attendance, antibiotic use in the past month, parental smoking and number of siblings. The preferred approach was to collect this information from the medical records, and it was completed by interviewing the parents whenever necessary. The project was accepted by the Fundación para la Formación e Investigación Sanitarias de la Región de Murcia (Foundation for Public Health Education and Research of the Region of Murcia [FFIS]) and received a grant from the Proyectos Fundación Cajamurcia programme (FFIS/CM09/037).
Microbiological sample managementSamples of nasopharyngeal secretions were collected with a flexible nasal swab (Deltalab, AMIES® medium swab) by the nursing staff in the paediatrics department of each centre. They were sent to the corresponding health area hospital in transport medium and seeded in 5% sheep blood agar plates with optochin disks. They were incubated for 24–48h in a 5% to 10% CO2 atmosphere at a temperature of 37°C. S. pneumoniae was identified by colony morphology, susceptibility to optochin and solubility in bile salts. After being identified, pneumococcal strains were suspended in a skim milk medium (Skim milk Difco®) and stored at −80°C, and then submitted to the referral hospital for susceptibility testing. Later on, they were sent for serotyping to the Laboratorio Nacional de Referencia de Neumococos (National Reference Laboratory for Pneumococcus) of the Instituto Carlos III in Madrid. The penicillin and cefotaxime minimum inhibitory concentrations (MICs) were determined by the Etest method (bioMèrieux) in blood agar plates. For the rest of the antibiotics under study, antimicrobial susceptibility was determined by the disc diffusion method in blood agar plates. After reseeding the strain to be analysed twice in blood agar, an inoculum suspension was prepared and adjusted to the turbidity of a 0.5 McFarland standard. A swab was dipped in the suspension and squeezed against the walls of the tube, and then used to distribute the suspension in three directions on blood agar to obtain a spread plate. After allowing a few minutes for drying, Etest strips for penicillin and cefotaxime were placed on the agar. Disks for the rest of the antibiotics under study (erythromycin, clindamycin, vancomycin, linezolid, rifampicin and levofloxacin) were placed in another spread plate prepared by the same method. Both plates were incubated in a 5–10% CO2 atmosphere at 37°C for 24h. We classified strains into susceptible, intermediate or resistant following the updated 2013 recommendations of the Clinical and Laboratory Standards Institute.3SP ATCC 49619 was the control strain.
Statistical analysisWe estimated the minimum sample size at 318 children for each of the four groups, assuming that 30% of the children were carriers, with an accuracy of 5% for a confidence level of 95%. We compared the means using Student's t test. We analysed the association between qualitative variables with Pearson's chi square test. We performed a multivariate logistic regression analysis to identify factors associated with resistance that included all variables that reached or approximated statistical significance in the univariate analysis. We performed the same statistical analysis in children 1 year of age stratifying by child care attendance. All results were considered significant for α<0.05.
ResultsThe study included 1562 children; 729 were 1 year of age (387 in the summer, 342 in the winter) and 833 were 4 years old (445 in the summer, 388 in the winter). Out of all the included children, 61.7% (964/1562) had received at least one dose of the PCV7 vaccine and 58% (905/1562) had received two or more doses. The total percentage of nasal carriers was 24.6% (205/832) in the summer and 38.9% (284/730) in the winter (P<.001), and the mean value for the entire cohort under study was 31.3% (489/1.562).
We lost 122 of the 489 isolates due to various circumstances (freezer malfunction, contamination, lysis during sample transport, etc.) so the antibiogram was performed in 367. Another 24 isolates were lost in the transfer to the Laboratorio Nacional de Referencia de Neumococos, so 343 were serotyped. Thus, we will refer to two figures in our susceptibility analysis, 343 when discussing the serotyped isolates, and 367 when referring to isolates tested for antimicrobial susceptibility.
We recovered 44 isolates (12.8%) covered by the PCV7 vaccine that corresponded to 4 serotypes: 16 isolates each of serotypes 14 and 23F, 10 isolates of serotype 19F and 6 of serotype B2. Another 83 isolates (24.2%) corresponded to the 6 additional serotypes covered by the PCV13 vaccine, with predominance of serotypes 6A and 19A, with 36 and 31 isolates, respectively. Two hundred and sixteen isolates (63%) were not covered by either vaccine.
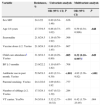
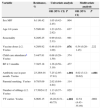
Table 1 shows the rate of penicillin resistance by route of administration and the disease being treated. Of the 367 isolates, those that met the criteria for nonmeningeal infection treated with oral penicillin V included 103 resistant isolates (28%), 101 with intermediate resistance (MIC, 0.12–1mg/L) and two with high-level resistance (MIC, 2mg/L). All the isolates that met the criteria for nonmeningeal infection treated with parenteral penicillin were susceptible. The main serotypes associated with resistance can be seen in Table 2, with the highest percentages being found for serotypes 14 (87.5%), 35B (71.4%), 19A (54.8%), 15A (44%) and 19F (37.5%). The percentage of resistance in the serotypes covered by the PCV7 was 54%, while it was 18.4% for the remaining serotypes (P<.001). The multivariate analysis (Table 3) showed that being a vaccine serotype (VT) carrier (P=.004) and having used antibiotics within the past month (P<.001) were risk factors for penicillin resistance for the entire sample, while attending a child care centre was a risk factor for one-year-old children (P=.049) and age 4 years was a protective factor compared to age 1 year (P=.048).
Susceptibility of S. pneumoniae to penicillin, cefotaxime and erythromycin. Breakpoints applied: CLSI 2013.
| Susceptible/Number of isolates | Intermediate/Number of isolates | Resistant/Number of isolates | % Nonsusceptible | |
|---|---|---|---|---|
| Pneumococcus 367 | ||||
| Parenteral penicillin, meningeal infection (MIC) | ≤0.06264 | ≥0.12103 | 28 | |
| Parenteral penicillin, nonmeningeal infection (MIC) | ≤2367 | 40 | ≥80 | 0 |
| Oral penicillin (penicillin v) (MIC) | ≤0.06264 | 0.12–1101 | ≥22 | 28 |
| Cefotaxime, meningeal infection (MIC) | ≤0.5345 | 120 | ≥22 | 6 |
| Cefotaxime, nonmeningeal infection (MIC) | ≤1365 | 22 | ≥40 | 0.5 |
| Erythromycin (MIC) | <0.25199 | 0 | >1168 | 45.7 |
MIC mg/L: minimum inhibitory concentration.
Most frequent serotypes and resistance patterns in descending order of percentage of nonsusceptibility to penicillin.
| Serotype | Number of strains | PENI-NS and (%) | ERYTHRO-R and (%) |
|---|---|---|---|
| 14 | 16 | 14 (87.5) | 12 (75) |
| 35B | 14 | 10 (71.4) | 1 (7.1) |
| 19A | 31 | 17 (54.8) | 20 (64.5) |
| 15A | 25 | 11 (44) | 21 (84) |
| 19F | 10 | 6 (37.5) | 10 (62.5) |
| 6A | 36 | 9 (25) | 15 (41.6) |
| 23F | 16 | 3 (18.7) | 7 (43.7) |
| 11A | 21 | 0 | 17 (80.9) |
| 6C | 16 | 0 | 7 (43.7) |
| 15B | 16 | 0 | 5 (31.2) |
| 23A | 14 | 0 | 9 (64.3) |
ERYTHRO-R: erythromycin-resistant; PENI-NS: penicillin-nonsusceptible.
Univariate and multivariate analysis of resistance to penicillin applying a MIC>0.06mg/L breakpoint.
| Variable | Resistance, % | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Sex M/F | 24.1/22 | 0.89 (0.54–1.46) | .636 | ||
| Age 1/4 years | 27.7/19.5 | 0.86 (0.73–1.02) | .073 | 0.59 (0.34–0.996) | .048 |
| Seasonality | 21.8/24.5 | 1.16 (0.70–1.92) | .566 | ||
| Vaccine doses≥2. Yes/no | 21.9/24.3 | 0.88 (0.53–1.45) | .607 | ||
| Child care attendancea Yes/no | 31.9/18.3 | 0.48 (0.29–0.80) | .005 | 0.32 (0.10–0.997)a | .049 |
| BF≥3 months | 23.9/22.2 | 1.10 (0.67–1.82) | .708 | ||
| Antibiotic use in past month. Yes/no | 52.6/19.3 | 4.63 (2.31–9.30) | <.001 | 4.82 (2.36–9.82) | <.001 |
| Parental smoking. Yes/no | 24.1/22.6 | 1.09 (0.61–1.92 | .777 | ||
| Number of siblings≥2. Yes/no | 17.5/24.1 | 0.67 (0.32–1.39) | .284 | ||
| VT carrier. Yes/No | 54.5/18.4 | 5.32 (2.75–10.32) | <.001 | 6.42 (1.79–23.05) | .004 |
BF, breastfeeding; F, female; M, male; VT, vaccine serotype.
Bold values are statistically significant.
As for susceptibility for cefotaxime, the MIC of 22 (6%) of the 367 isolates was greater than 0.5mg/L, and of these 22, 63.6% corresponded to VTs (10 were serotype 14). We found two isolates with high-level resistance to cefotaxime with the breakpoints for meningeal infection (MIC=2mg/L) that corresponded to serotypes 14 and 19. We did not detect any isolates with a MIC above 2mg/L. The risk factors for bacterial resistance identified by the multivariate analysis (Table 4) were VT carriage (P<.001) and antibiotic use in the past month (P<.001).
Univariate and multivariate analyses of resistance to cefotaxime applying a MIC>0.5mg/L breakpoint.
| Variable | Resistance, % | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Sex M/F | 6.11/6.42 | 1.05 (0.42–2.45) | .904 | ||
| Age 1/4 years | 5.59/6.80 | 1.23 (0.52–2.92) | .637 | ||
| Seasonality | 6.28/6.25 | 0.99 (0.42–2.33) | .991 | ||
| Vaccine doses≥2. Yes/no | 4.29/8.92 | 0.46 (0.19–1.09) | .076 | 0.54 (0.20–1.45) | .222 |
| Child care attendancea Yes/no | 5.44/7.81 | 0.68 (0.29–1.59) | .374 | ||
| BF≥3 months | 7.38/5.18 | 1.36 (0.58–3.19) | .477 | ||
| Antibiotic use in past month. Yes/no | 23.26/4.01 | 7.25 (2.95–17.81) | <.001 | 8.02 (3.12–20.62) | <.001 |
| Parental smoking. Yes/no | 9.78/5.09 | 2.02 (0.84–4.84) | .114 | ||
| Number of siblings≥2. Yes/no | 17.50/24.12 | 1.13 (0.37–3.45) | .829 | ||
| VT carrier. Yes/no | 6.90/6.15 | 16.28 (6.51–40.75) | <.001 | 12.54 (4.43–35.49) | .0001 |
BF, breastfeeding; F, female; M, male; VT, vaccine serotype.
Bold values are statistically significant.
The rate of erythromycin resistance was 45.8% (168/367) (Table 1). When it came to specific serogroups (Table 2), the rate was higher than 75% in 15A, 11A and 14, and higher than 60% in 19A, 19F and 23A. The percentage of resistant isolates of VTs was 68.5%, while it was 40.8% for isolates of nonvaccine serotypes (NVTs) (P<.001). Of the 264 penicillin-susceptible pneumococci, we considered 99 (37.5%) resistant to erythromycin, with a predominance of serotypes 15A, 11A, 19A, 23A, 6A and 6C. We found statistically significant differences when we compared erythromycin resistance in penicillin-susceptible versus nonsusceptible isolates (37.5% and 67%, respectively), which was higher in nonsusceptible isolates (P=.003).
Resistance to clindamycin was detected in 110 strains (29.9%), with predominance of serotypes 19F (10 isolates) and 33F (7 isolates), all the isolates of which were resistant; and serotype 15A, with resistance found in 72% of the isolates (18/25).
As for combined antibiotic resistance, 69 strains (18.8%) were resistant to penicillin and erythromycin, and 45 (12.3%) were multidrug-resistant (resistance to 3 or more antibiotics). The predominant serotypes in both cases were 19A, 15A and 14.
We did not find resistance to any of the other antibiotics under study (linezolid, levofloxacin, rifampicin and vancomycin) in any of the isolates.
DiscussionWe conducted this study eight years after the PCV7 was introduced in Spain, and in consideration of the impending marketing of the new 10- and 13-valent conjugate vaccines. We wanted to determine the susceptibility to commonly used antibiotics of the circulating pneumococcal strains in the population of healthy children, as well as the influence of the epidemiological factors under study, as respiratory infections caused by SP (otitis, sinusitis, pneumonia) result in a high use of antibiotics, especially at the primary care level.
The inappropriate use of antibiotics is an important public health problem, as it has been associated with an increase in bacterial resistances.1 Spain is one of the countries with the highest use of antibiotics and the highest rates of resistance in Europe.1,4
The mechanism of penicillin resistance is based on alterations in penicillin-binding proteins (PBPs).5 These alterations lead to various degrees of resistance, from very low levels in susceptible strains, to intermediate- or high-level resistance.5 Two mechanisms of macrolide resistance have been reported. The MLSB phenotype involves the enzymatic modification of the ribosomal target (changes in the ermB gene that encodes the production of methylases that methylate the 23S subunit of rRNA, preventing macrolide binding to the target). This mechanism results in cross-resistance to all macrolides (14-, 15- and 16-membered), lincosamides and streptogramins. This is the most prevalent phenotype in Spain and it may have a constitutive or an inducible expression. The M phenotype consists in an active antibiotic efflux system (mef(E) gene). This mechanism confers resistance to 14- and 15-membered macrolides, lincosamides and streptogramins.5
The patterns of resistance vary widely between countries, and they evolve through time.1 Spain, along with France, has had the highest rates of resistance in Europe,4 and there has been a decline in penicillin resistance in recent years. The factors associated with these changes are a decrease in the use of antibiotics, the introduction of the new vaccines, and other factors that are less understood, such as the expansion of existing clones.1,6–9
When it came to penicillin in meningeal infections, our study found that 28% of the strains were nonsusceptible (MIC>0.06mg/L), figures that were similar to those in some studies conducted in Spain8 and lower than the figures reported in other studies in Spain and abroad.6,9–11 Consistent with the reports of other authors, when we applied the criteria for meningeal infection and parenteral penicillin (resistance, MIC>2mg/L), 100% of pneumococci were susceptible.10,12,13 The rates of resistance were higher in VT strains (54% in our study) than in NVS strains (18%), consistent with the existing literature.1,7,9 Thus, there was a significant decrease in penicillin resistance compared to studies conducted in Spain before the introduction of conjugate vaccines (64%14 and 68%15) or in the early years of PCV7 use, and it was associated with this vaccine and with the decrease in antibiotic use.9,16
As was seen in other studies, VT carriage,9,10 younger age,11 child care attendance and antibiotic use in the previous month1,10 were associated with resistance. Serotype 14 among the PCV7 VTs, 19A among the 13-valent VTs, and serotypes 15A and 35B had the highest rates of penicillin resistance, similar to what has been observed in other studies.1,10
Ninety-four percent and 99.4 percent were susceptible to cefotaxime in meningeal and nonmeningeal infections, respectively, consistent with other reports.10
When it comes to macrolides, resistance to erythromycin has been associated with penicillin resistance.1 We also observed this association in our study. We found a very high percentage of resistance to erythromycin of 45.8%, similar to that of a study conducted before the introduction of the PCV714 and higher than the rates reported in other Spanish studies before and after the introduction of this vaccine. This resistance is strongly associated with a widespread use of macrolides.1,7,9,14 We also found an association between macrolide resistance and VTs, similar to what has been observed by other authors.9 The literature describes an initial decrease in resistance following the introduction of the PCV7 due to a reduction in the prevalence of VTs, and a subsequent increase in resistance due to the emergence of NVTs such as 19A with high rates of resistance.1,11,13
We found resistance to more than one antibiotic in 69 strains (18.8%), which did not diverge significantly from previous reports.8
We found a percentage of clindamycin resistance of 29.9%. In this study we did not determine the macrolide resistance phenotype, but the seemingly better performance of clindamycin compared to macrolides could be due to the presence of strains with inducible MLSB phenotypes or M phenotypes.
The rates of multi-drug resistance (to 3 or more antibiotics) in Europe, while widely variable, are of approximately 15%.1 In our study we found multi-drug resistance in 45 isolates (12.3%), a proportion close to the European mean.
In nonmeningeal infections, the change in the affinity of PBPs that confers a degree of resistance to some isolates can be overcome by higher concentrations of penicillin.5,16,17 Therefore, in regions like ours, with a low prevalence of pneumococcal high-level penicillin resistance (MIC>2mg/L), amoxicillin may be used as the first-line agent in appropriately vaccinated children with mild to moderate disease with a suspected pneumococcal aetiology, such as pneumonia.18 Parenteral penicillin or ampicillin can be used in patients that require admission to the hospital.16,18 This strategy may also be used in the outpatient management of acute otitis media, as doses of 80–90mg/kg/day yield middle ear fluid amoxicillin levels that meet pharmacokinetic and pharmacodynamics parameters, with times with concentrations above the MIC that allow the treatment of infections by all intermediately-resistant pneumococci (MIC, 0.12–1.0mg/L) and many highly-resistant pneumococci (MIC>2mg/L).19 Similarly, in cases of acute sinusitis, high doses of amoxicillin can achieve adequate concentrations in nasal secretions to overcome resistance attributable to alterations in PBPs.20
Third-generation cephalosporins are an excellent option for treating nonmeningeal infections in patients with incomplete vaccination, in areas with a high prevalence of penicillin-resistance pneumococcus (MIC>2mg/L) and in severe forms of disease, such as empyema.18
Whenever a pneumococcal aetiology is suspected in meningeal infections, the patient should be treated with cefotaxime and vancomycin due to their synergistic effect until the causative agent is identified and its susceptibility determined, as 6% of the isolates had intermediate resistance to cefotaxime and two isolates had a MIC of 2mg/L, and both of these circumstances were associated with treatment failure when the patient was treated with cefotaxime or ceftriaxone as monotherapy.21
The use of macrolides in respiratory tract infections should be restricted given the high rate of resistance to these agents, and it should only be considered in cases of suspected atypical pneumonia 18 or of uncomplicated disease in patients with type 1 beta-lactam hypersensitivity.22
The main strengths of this study are the time when it was conducted, just prior to the introduction of new vaccines to the market, and the number of samples collected, despite the excessive number of samples lost. However, as it usually occurs in this type of study, there was no randomisation since participation was voluntary, so the sample may not be fully representative. We did not collect data on the dosage and duration of previous antibiotic therapy and we did not assess for multiple colonisation, which may have biased the final results.
ConclusionsWith the ultimate end of improving the use of antibiotics, our data support the use of high-dose oral amoxicillin in most mild to moderate respiratory tract bacterial infections, such as otitis, sinusitis and community-acquired pneumonia, while in patients that require parenteral antibiotherapy, penicillin and ampicillin would be the first-line therapy for uncomplicated nonmeningeal pneumococcus infections. We found a high rate of macrolide resistance, so the use of these agents should be restricted to very specific situations. We observed the highest rates of resistance in serotypes 14, 19A and 15A. Being a VT carrier, antibiotic use within the past month, age 1 year compared to 4 years, and child care attendance were risk factors for penicillin resistance.
FundingThis project was accepted by the Fundación para la Formación e Investigación Sanitarias de la Región de Murcia (FFIS) and received a grant from the Proyectos Fundación Cajamurcia program (FFIS/CM09/037).
Conflicts of interestThe authors have no conflicts of interest to declare.
Hospital Universitario (University Hospital [HU]) Virgen de Arrixaca: Ángela Casquet Barceló, Guillermo Nieves González, José M. Calderón Sánchez, Luisa Camps Martínez, Nadia Sayed Sancho, M. Ángeles Chumilla Valderas, Joaquina Villalobos Pérez, M. Matilde Cuenca Gómez, Fuensanta Costa Guirao, Begoña Pelegrin López, Rosario Hurtado del Cerro, José Saura Sánchez Parra, Francisco España, Inmaculada Martínez Artero.
HU Morales Meseguer: Antonio Iofrío de Arce, Luisa M. García Sandoval, Jesús Meca Garrido, Enrique Gutiérrez, Beatriz Garnica Martínez, Rosa M. Pérez Tomás, Esperanza Moreno Gomáriz, M. Dolores Hernández-Gil, M. José Vicente Fernández.
HU Reina Sofía: Gonzalo Sanz Mateo, Fuensanta Alemán Lorca, Alí Ghandour Houmani, Enrique López Conesa, Silvia Martínez García, Cristina Cañavate González, Mercedes Gutiérrez Pérez, and Antonio Lao García.
HU Los Arcos-Mar Menor: Rosa M. Sánchez Andrada, Isabel Cascales Barceló, María Vera Lorente, Maria Teresa José Hernangómez Cuesta, Monserrat Martínez López, Josefina Martínez Garre and Jose Maria Ojeda Escuriet.
HU Rafael Méndez: Sebastián Lorente García, Francisco Pérez Navarro, Carmen Nelia Vicente de Jiménez, Manuel García Moreno, Teresa Domínguez Rodríguez, Juan Francisco Soriano Ibarra and Francisco Jaldo Alba.
HU Santa Lucía: M. Pilar Espejo García, M. Paz Ortuño del Moral, Lorena Conesa Hernández, M. Dolores Rosique Conesa, Clara Ferrández García, Gloria Heredero García, Javier Peñalver Manrrubia, M. Teresa Fábrega Valverde, M. Eugenia Serna de Miquel, Eva Rodríguez Martínez, Ana González Pacanowska, Teresa Vidal Vidal.
HU Yecla-Altiplano: Juan José Vigueras Abellán, M. Eugenia Fuentes Sebastián, Josefina Romero Ortiz, Lucía González-Moro Azorín, José M. Ibáñez García and Víctor Sánchez Quiñones.
Please cite this article as: Alfayate-Miguélez S, Ruiz Gómez J, Sanchez-Solis de Querol M, Guerrero Gómez C, Cámara Simón M, Ortiz Romero MM, et al. Sensibilidad de Streptococcus pneumoniae en niñosportadores sanos en Murcia (España). An Pediatr (Barc). 2015;83:183–190.