Acute myeloid leukaemia (AML) is the second haematological malignancy in the paediatric population, and one of the leading causes of childhood cancer mortality. Survival is currently around 60%, with no improvement in last decades, suggesting that new therapeutic approaches are needed. The anti-leukaemia effect mediated by the lymphocytes and natural killer (NK) cells of the immune system has been established in haematopoietic stem cell transplantation, and also as adoptive immunotherapy after consolidation chemotherapy schemes.
Patients and methodsA retrospective study was conducted on the clinical characteristics of patients diagnosed and treated for AML in our centre from 1996 to 2014. The mean fluorescence intensities of HLA-I, MICA/B and ULBP1-4, ligands for NK cell receptors, were also analysed in ten new diagnosed leukaemia cases, five myeloid and five lymphoid.
ResultsA total of 67 patients were used in this analysis. With a median follow up of 25 months, the event-free survival was 62% (95% CI: 55–67). Secondary AML, non-M3 phenotype, and the absence of favourable cytogenetic markers had a lower survival. The probability of relapse was 38% (95% CI: 31–45). The expression of HLA-I and ULBP-4 was significantly lower in myeloid than in lymphoid blast cells.
ConclusionsOur clinical results are similar to those described in the literature. Survival did not significantly change in recent decades, and the likelihood of relapse remains high. Myeloid blasts might be more susceptible to the cytotoxicity of NK cells through their lower expression of HLA-I. NK therapy strategies in minimal disease situation could be effective, as reported by other groups.
La leucemia mieloblástica aguda (LMA) constituye la segunda hemopatía maligna en la población pediátrica y una de las principales causas de mortalidad por cáncer infantil. La supervivencia se sitúa alrededor del 60% sin haber mejorado en las últimas décadas, por lo que son necesarios nuevos enfoques terapéuticos. El efecto antileucémico ejercido por los linfocitos y las células natural killer (NK) del sistema inmunológico está bien establecido en el trasplante de células madre hematopoyéticas pero también como estrategia de inmunoterapia adoptiva tras la quimioterapia de consolidación.
Pacientes y métodosDe manera retrospectiva, se analizan las características clínicas de los pacientes diagnosticados de LMA en nuestro centro durante el período 1996–2014. Además en 10 leucemias agudas, 5 linfoides y 5 mieloides, se analizaron la intensidad media de fluorescencia de HLA-I, MICA-B, ULBP1-4, ligandos para los receptores de las células NK.
ResultadosUn total de 67 pacientes formaron parte de este análisis. La supervivencia libre de eventos con una mediana de seguimiento de 25 meses fue del 62% (IC del 95%, 55–67). Las LMA con menor supervivencia fueron las secundarias, las no M3 y las carentes de marcadores citogenéticos favorables. La probabilidad de recaída fue del 38% (IC del 95%, 31–45). La expresión de HLA-I y ULBP-4 fue significativamente menor en los blastos mieloides que en los linfoides.
ConclusionesNuestros resultados clínicos son similares a los descritos en la literatura. No se ha modificado significativamente la supervivencia en las últimas décadas y la probabilidad de recaída sigue siendo elevada. Los blastos mieloides podrían ser más susceptibles a las células NK al expresar menos HLA-I, por lo que estrategias de terapia celular podrían ser eficaces tal y como reportan otros grupos.
Acute myeloid leukaemia (AML) accounts for 20% of childhood leukaemias.1 Approximately 60 cases are diagnosed each year in Spain. Current therapeutic approaches are based on the administration of polychemotherapy, combining high-dose cytarabine with anthracyclines and topoisomerase inhibitors.2 Furthermore, patients that respond poorly to induction therapy, patients considered to be high-risk from the outset (with secondary AML or unfavourable cytogenetic characteristics), or intermediate-risk patients that have a HLA match related donor are candidates for allogeneic haematopoietic stem cell transplantation (HSCT) for AML in first complete remission.3,4
The survival of patients with AML has improved considerably in the past 40 years, mostly due to advances in supportive care. However, survival has plateaued at 60% in recent decades. Our knowledge of the genetic heterogeneity of AML and its importance in prognosis is increasing by the day.5,6 There are cytogenetic changes that carry a good prognosis, such as t(8;21)(q22;q22), inv(16)(p13.1q22) or t(16;16)(p13.1;q22), t(15;17)(q22;q12), the presence of which corresponds to an 80% survival with chemotherapy alone, while unfavourable changes such as 5q, t(6;9)(p23;q34), monosomy 7 and complex karyotypes are refractory to chemotherapy and have survival rates of no more than 40% even with HSCT.6–8
Thus far, the potential of these genetic changes as therapeutic targets has not had a significant impact on survival.9,10 Recently, and complementing advances on genetic and epigenetic phenomena, an immunobiological approach to AML is being developed.11,12 Such an approach is supported by different observations: (a) the crucial role of HSCT3,13; (b) the success of cellular adoptive immunotherapy with post-transplant donor lymphocyte infusions against minimal residual disease and/or mixed chimerism14,15; and (c) the favourable clinical and preclinical experience with antibodies such as gemtuzumab ozogamicin (anti-CD33) and bi-specific T-cell engagers (CD33/CD3, AMG 330).16,17
In this regard, the Perugia group led by Dr Velardi demonstrated the antileukaemic effect of haploidentical HSCT in adult patients with AML through the donor-derived natural killer (NK) cells.18,19 Later on, the St Jude group confirmed these findings in childhood AML and pioneered an NK cell infusion regimen for consolidation therapy in patients with low-to-intermediate risk AML.20 We know that the antileukaemic activity of NK cells is regulated by the recognition by inhibitory and activating receptors of their ligands in blast cells.21 These ligands correspond to human leucocyte antigen class I (HLA-I) for inhibitory receptors, and major histocompatibility complex (MHC) class I-related chain A/B (MICA/B) and UL16-binding proteins 1-4 (ULBP 1-4) for activating receptors.22
This study offers a retrospective review of our experience in the management of childhood AML and describes the expression of NK cell receptor ligands in 10 cases of acute leukaemia (5 myeloid and 5 lymphoid), comparing this expression in both types of acute leukaemia with the purpose of contributing additional data in support of incorporating NK cell adoptive immunotherapy to conventional chemotherapy protocols.
Patients and methodsPatientsWe collected data retrospectively on the epidemiology, morphology (French-American-British [FAB] classification)23 or immunological classification as proposed by the EGIL European group,24 cytogenetic changes, treatment and outcomes of cases of AML diagnosed between 1996 and 2014 in the Paediatric Haemato-Oncology Unit of the Hospital Universitario La Paz in Madrid, Spain (Table 1). Patients were treated consecutively with the successive versions of the protocol developed by the Sociedad Española de Hemato-Oncología Pediátrica (Spanish Society of Paediatric Haemato-Oncology; SHOP-1996, SHOP-2001, SHOP-2007). We excluded patients with AML and Down syndrome from the analysis. We obtained the informed consent of the legal guardians of the patients prior to biological assessment, and the study was approved by the Ethics Committee of the Hospital La Paz.
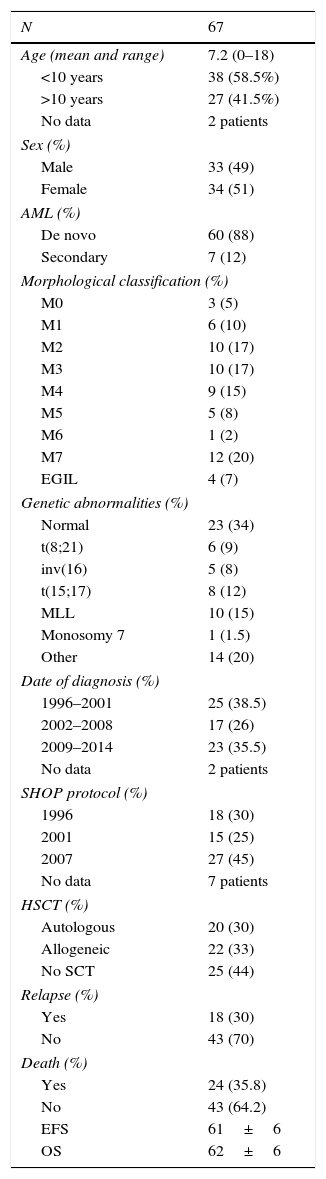
Characteristics of paediatric patients given a diagnosis of AML between 1996 and 2014 in our unit.
| N | 67 |
|---|---|
| Age (mean and range) | 7.2 (0–18) |
| <10 years | 38 (58.5%) |
| >10 years | 27 (41.5%) |
| No data | 2 patients |
| Sex (%) | |
| Male | 33 (49) |
| Female | 34 (51) |
| AML (%) | |
| De novo | 60 (88) |
| Secondary | 7 (12) |
| Morphological classification (%) | |
| M0 | 3 (5) |
| M1 | 6 (10) |
| M2 | 10 (17) |
| M3 | 10 (17) |
| M4 | 9 (15) |
| M5 | 5 (8) |
| M6 | 1 (2) |
| M7 | 12 (20) |
| EGIL | 4 (7) |
| Genetic abnormalities (%) | |
| Normal | 23 (34) |
| t(8;21) | 6 (9) |
| inv(16) | 5 (8) |
| t(15;17) | 8 (12) |
| MLL | 10 (15) |
| Monosomy 7 | 1 (1.5) |
| Other | 14 (20) |
| Date of diagnosis (%) | |
| 1996–2001 | 25 (38.5) |
| 2002–2008 | 17 (26) |
| 2009–2014 | 23 (35.5) |
| No data | 2 patients |
| SHOP protocol (%) | |
| 1996 | 18 (30) |
| 2001 | 15 (25) |
| 2007 | 27 (45) |
| No data | 7 patients |
| HSCT (%) | |
| Autologous | 20 (30) |
| Allogeneic | 22 (33) |
| No SCT | 25 (44) |
| Relapse (%) | |
| Yes | 18 (30) |
| No | 43 (70) |
| Death (%) | |
| Yes | 24 (35.8) |
| No | 43 (64.2) |
| EFS | 61±6 |
| OS | 62±6 |
We analysed ten bone marrow aspirate samples collected at the time of diagnosis and corresponding to five patients with AML and five with acute lymphoblastic leukaemia by means of multiparametric flow cytometry with a BD FACSCanto II system. We determined the mean fluorescence intensity (MFI) of HLA-I, the ligands of the KIR inhibitory receptors in NK cells, the ligands for NK group inhibitory receptors, and MICA-B, ULBP-1, ULBP-2, ULBP-3 and ULBP-4, ligands of the NKG2D activating receptors, in leukaemic cells previously selected by size and internal complexity. We used the following antibodies: HLA-I-PE (clone: G46-2.6), manufactured by Becton, Dickinson and Company; and MICA-PE (clone: 159227), MICB-PE (clone: 236511), ULBP-1-PE (clone: 170818), ULBP-2/5/6-APC (clone: 165903), ULBP3-PE (clone: 166510) and ULBP4-PE (clone: 709116) manufactured by R&D Systems. The expression level was defined as the ratio of the mean fluorescence intensity of the tested antibodies and the measured mean autofluorescence intensity of cells.
Statistical analysisWe have expressed the data as mean and standard deviation unless otherwise noted. The statistical calculations were performed with the Software Package for the Social Sciences (SPSS) version 12.0 for Windows. The event-free survival (EFS), with event defined as relapse or death, was the primary outcome variable of the study and analysed by means of the Kaplan–Meier method and the log-rank test with a 95% confidence interval. We also calculated the probability of relapse and of death unrelated to the disease (toxic death) by the same method. We used the nonparametric Mann–Whitney U test for the statistical comparison of nonparametric variables. Statistical significance was defined as P<.05.
ResultsPatient characteristicsBetween 1996 and 2014, a total of 67 patients (33 male and 34 female) with a mean age of 7.2 years (range, 0–18) received a diagnosis of AML and were treated in our unit in adherence with the SHOP-1996 (30%), SHOP-2001 (25%) or SHOP-2007 (45%) protocols. The epidemiological characteristics of the patients with AML managed with the different versions of the SHOP protocol were homogeneous.
In 60 patients (88%) the diagnosis was de novo AML, while 7 patients (12%) received a diagnosis of secondary AML or AML related to a myelodysplastic syndrome. Table 1 summarises the morphology and cytogenetic changes in these cases. Cytogenetic changes were found in 66% of the patients, while 34% had normal cytogenetics. Approximately one third of the patients (29%) had cytogenetic changes associated with a favourable prognosis, such as t(8;21), inv(16) and t(15;17); 20% had cytogenetic changes of unknown prognosis; and 16.5% of patients had unfavourable cytogenetic changes, MLL gene rearrangement and monosomy 7.
Twenty-five patients (44%) were treated with chemotherapy alone. Twenty (30%) underwent autologous HSCT (the last one on April 30, 2012) and twenty-two patients (33%) underwent allogeneic HSCT (7 from an HLA-identical related donor, 7 from an HLA-identical unrelated donor, 4 from umbilical cord blood, and 4 from a haploidentical donor).
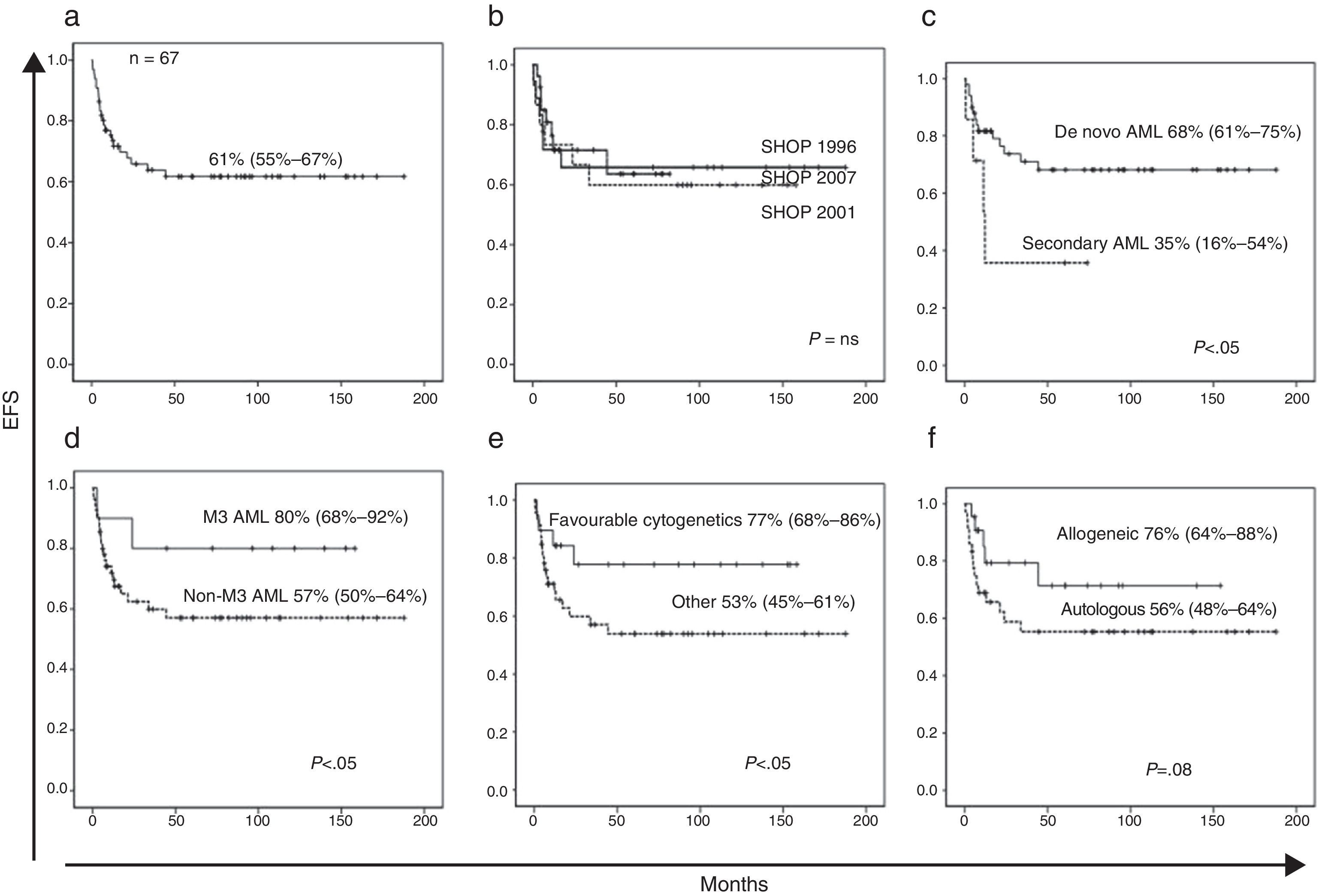
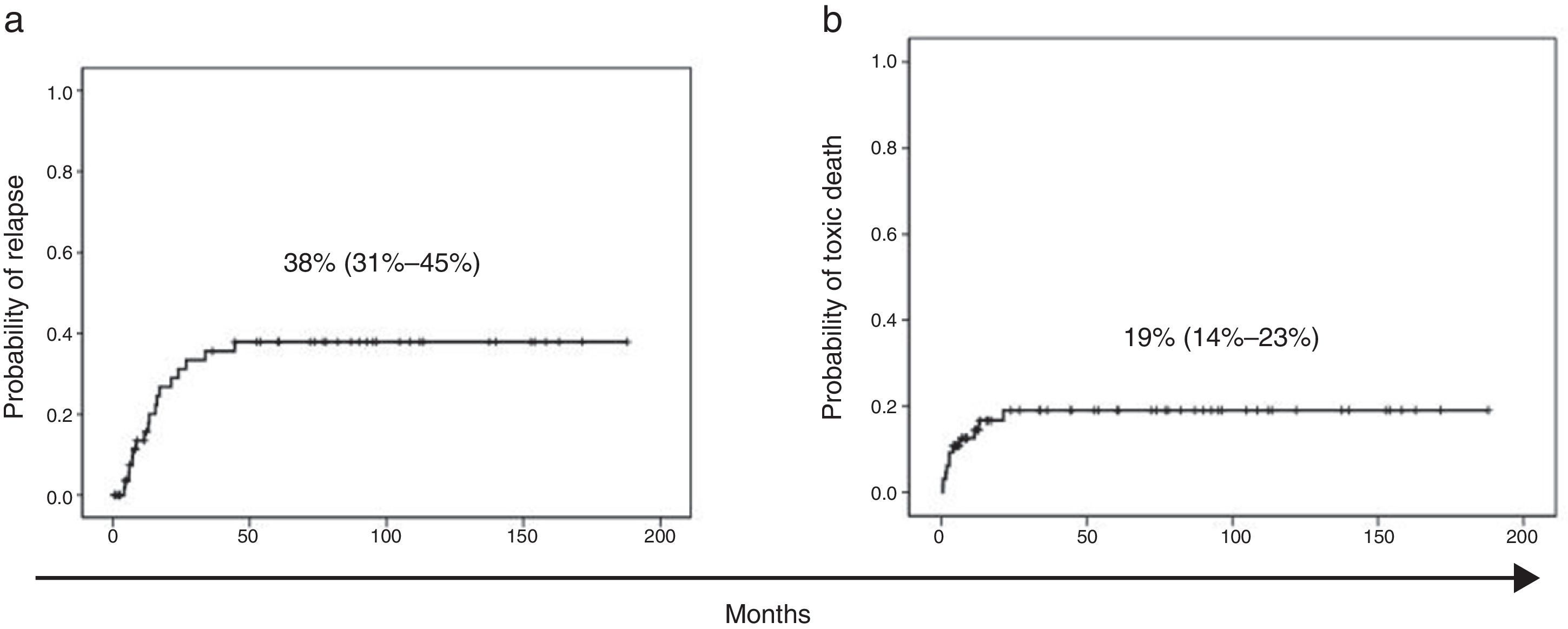
At the time of this writing, 24 of the patients have died, 13 of them as a result of the disease and 11 due to treatment-related complications (infection in 9 patients and haemorrhage in 2). With a median followup of 26 months, the EFS of the patients with AML included in our series was 61% (95% CI, 55–67%) (Fig. 1a) and the overall survival was 62% (95% CI, 56–68%). The probability of relapse was 38% (95% CI, 31–45%) and the probability of death unrelated to AML was 19% (95% CI, 14–23%) (Fig. 2a and b).
(a) Event-free survival (death and relapse). (b) Event-free survival by SHOP protocol version. (c) Event-free survival in de novo vs secondary AML. (d) Event-free survival in M3 AML vs non-M3 AML. (e) Event-free survival in AML cases with favourable translocations t(8;21), inv(16), t(15;17) compared to other cases. (f) Event-free survival in patients that underwent allogeneic HSCT vs patients that did not.
The univariate analysis of EFS did not reveal any differences between sex, age groups or versions of the SHOP protocol (Fig. 1b). We found significant differences in EFS between de novo AML and secondary AML (68% [95% CI, 61–75%] vs 35% [95% CI, 16–54%]), M3 AML and non-M3 AML (80% [95% CI, 68–92%] vs 57% [95% CI, 50–64%]) or presence and absence of favourable cytogenetic changes (77% [95% CI, 68–86%] vs 53% [95% CI, 45–61%]) (Fig. 1c–e). The probability of relapse in patients with favourable cytogenetic changes was not significantly lower (29% vs 42%, P=.07). We did not find statistically significant differences in the probability of relapse or of toxic death between the different versions of the SHOP treatment protocol (Table 2).
Probability of relapse and toxic death for different versions of the SHOP protocol in our case series.
| % | SHOP-1996 | SHOP-2001 | SHOP-2007 | P |
|---|---|---|---|---|
| Relapse | 22% (95% CI, 11–33) | 32% (95% CI, 19–45) | 45% (95% CI, 33–57) | .6 (ns) |
| Toxic death | 33% (95% CI, 23–43) | 14% (95% CI, 6–22) | 8% (95% CI, 6–14) | .2 (ns) |
CI, confidence interval; ns, not significant.
The number of allogeneic HSCTs has increased significantly with the SHOP-2007 protocol compared to previous versions (SHOP-2007, 53%; SHOP-2001, 25%; SHOP-1996, 26%). Conversely, the number of autologous HSCTs has decreased with the current protocol (SHOP-2007, 15%; SHOP-2001, 58%; SHOP-1996, 46%). When we analysed the impact of HSCT on EFS, we observed a positive trend that was not statistically significant (76% [95% CI, 64–88%] vs 56% [95% CI, 48–64%]; P=.08) (Fig. 1f).
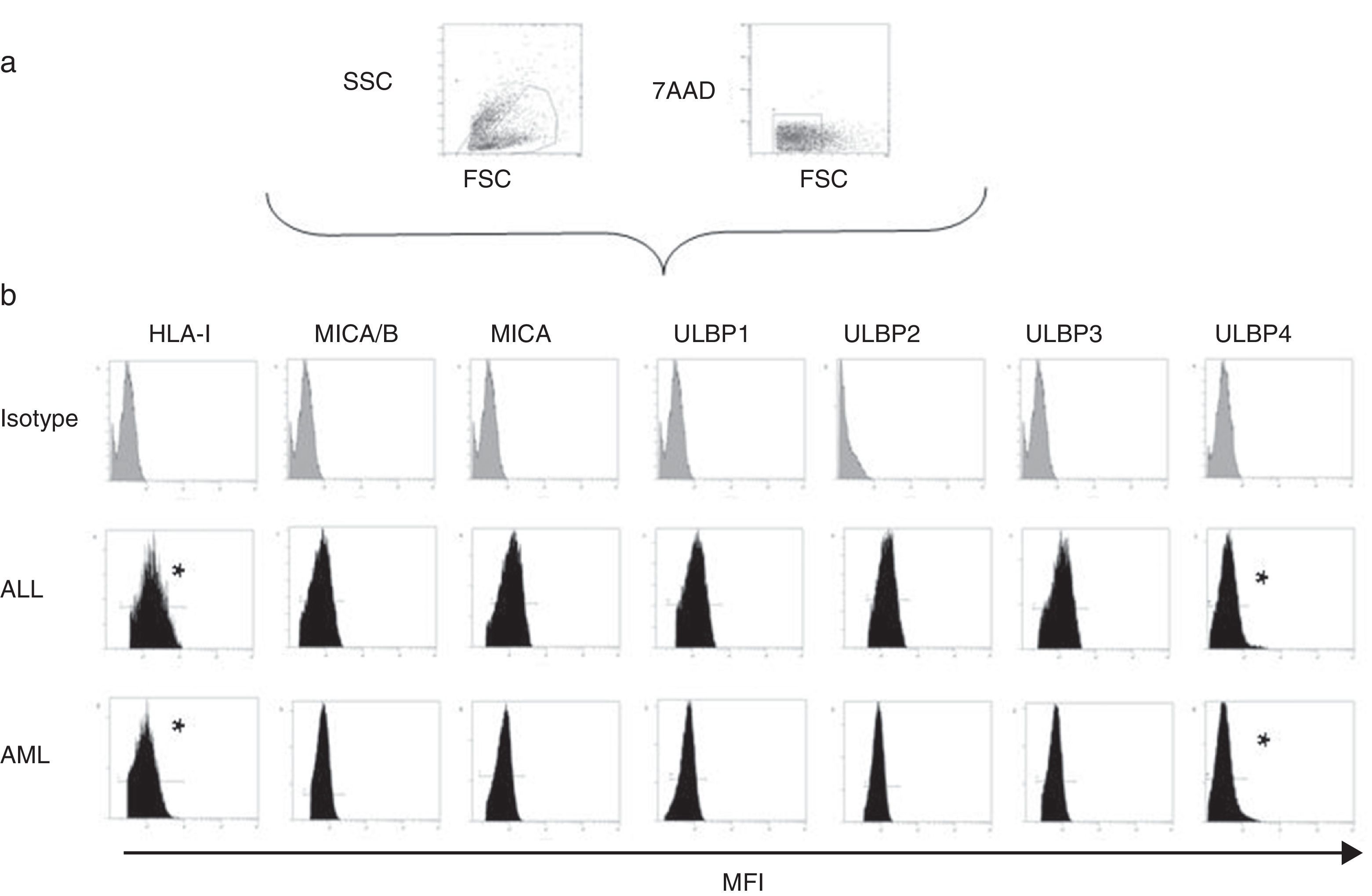
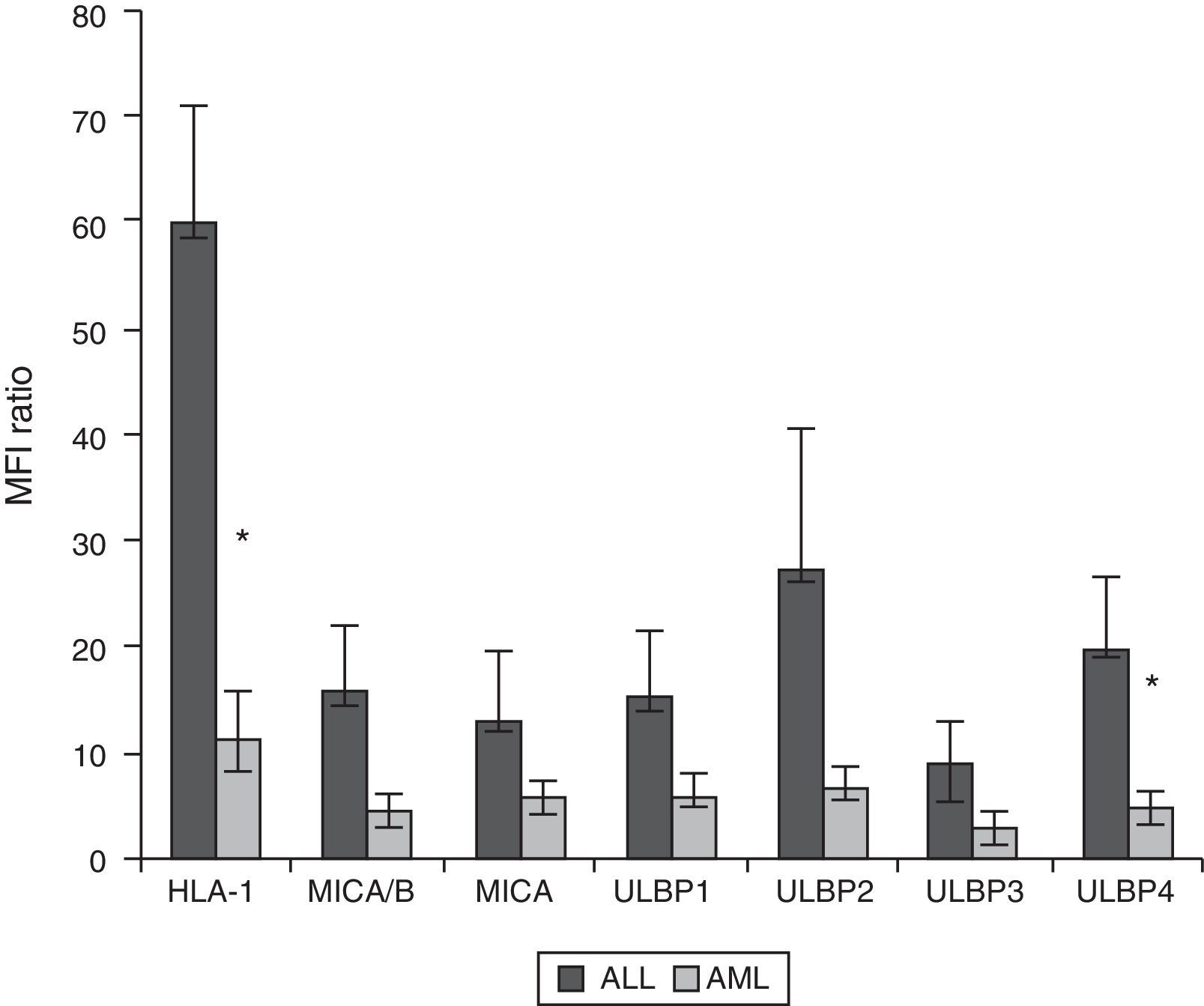
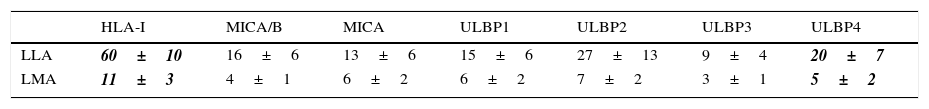
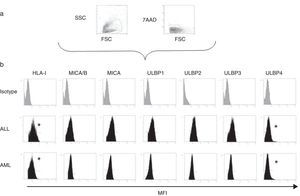
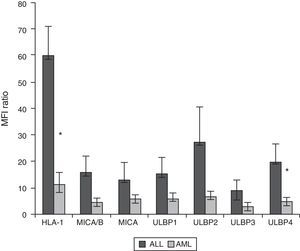
Expression of HLA class I and NKG2D ligands in leukemic blasts of paediatric patientsWe used multiparametric flow cytometry to measure the expression of HLA-I ligands, MICA/B and ULBP-1, ULPB-2, ULPB-3 and ULBP-4 in 10 samples of leukemic blasts analysed based on size and complexity (Table 3). We observed that the mean fluorescence intensities of HLA-I and ULBP-4 were significantly lower in myeloid blasts than in lymphoid blasts (HLA-I, 11±3 vs 60±10 [P<.05]; ULBP-4, 5±2 vs 20±7 [P<.05]). The differences in the MFIs of MICA/B, ULBP-1, ULBP-2 and ULBP-3 were not statistically significant (Figs. 3 and 4).
Ratio of the mean fluorescence intensity (MFI) of myeloid and lymphoid blasts. The experiment involved the analysis of 10 cases of acute leukaemia, 5 myeloid and 5 lymphoid.
| HLA-I | MICA/B | MICA | ULBP1 | ULBP2 | ULBP3 | ULBP4 | |
|---|---|---|---|---|---|---|---|
| LLA | 60±10 | 16±6 | 13±6 | 15±6 | 27±13 | 9±4 | 20±7 |
| LMA | 11±3 | 4±1 | 6±2 | 6±2 | 7±2 | 3±1 | 5±2 |
Values are expressed as mean±standard deviation. The values shown in bold and italics were statistically significant.
(a) Scheme for the selection of leukaemic blasts based on size, cell complexity, CD45 expression and viability. (b) Mean fluorescence intensity of ligands of inhibitory and activating receptors in myeloid and lymphoid blasts. We show the data for a single patient per group as an example. *Statistically significant difference.
The results of our retrospective series are similar to those reported in studies of paediatric patients with AML both in Spain and abroad. The latest report of the Spanish Registry of Childhood Tumours (RETI) for the 1980–2013 period described an overall 5-year survival of 54% for years 1995–1999, 59% for 2000–2004 and 62% for 2005–2007. The European group NOPHO recently reported an EFS of 55% in their NOPHO-AML2004 protocol25; and the group at the St Jude's Hospital in Memphis, United States, a 5-year EFS of 63% in 230 patients during the 2000–2008 period in their AML-02 protocol.26 Based on our data, the event-free survival in childhood AML has not changed substantially with the past three versions of the SHOP protocol and plateaued more than two decades ago, remaining in suboptimal values. Furthermore, consistent with the current knowledge, the survival for secondary ALM (treatment-related or arising from a myelodysplastic syndrome) is much lower than survival for de novo ALM.27 On the other hand, the heterogeneity of AML is demonstrated by the EFS of promyelocytic AML (FAB M3), which far exceeds that of the rest of AMLs (80% vs 57%, P<.05), underscoring the positive impact of all-trans retinoic acid on survival and the need to find molecular targets in other types of AML.28
In our series, cytogenetic changes also had an important impact on prognosis. Thus, the presence in a third of our patients of changes considered to be favourable, such as t(8;21), inv(16) or (15;17), was associated with a higher survival that was probably due to a lower probability of relapse, although this result was not statistically significant due to the small sample size. The use of allogeneic HSCT as a treatment strategy has been increasing in recent protocols, along with a decrease in autologous HSCT. Advances in cytogenetic testing methods that allow the identification of high-risk changes may have played a role in this development. We found that allogeneic HSCT was associated with an improving trend in EFS, consistent with the findings of other case series.3,29 A greater sample size may have allowed us to find significant differences in our patients.
The causes of death in the patients in our series were relapse and treatment-related causes (infection and haemorrhage). The rate of toxic death has tended to decrease with successive protocols, while relapse has continued to be an important event with no change in frequency.30 The small sample size of our study did not allow us to identify prognostic factors associated with the probability of relapse. We believe that a 38% relapse rate is still too high. The probability of relapse is nearly 30% in the subset of patients with favourable cytogenetics. Thus, we believe that all patients could benefit from new therapeutic approaches.
In addition to this retrospective study, this article presents the preliminary results on the expression of ligands of inhibitory and activating receptors by malignant myeloid and lymphoid blasts, which we analysed with the aim of explaining the increased susceptibility of AML to NK cell cytotoxicity described by research groups in other countries.31 Consistent with the existing evidence, the mean fluorescence intensities of HLA-I, ligands of the KIR inhibitory receptors, were significantly lower in myeloid than in lymphoid blasts, suggesting a greater sensitivity to NK cell cytotoxicity mediated by their inhibitory receptors. When it came to the expression of MICA/B and ULBP ligands, we only found significant differences in the expression of ULBP-4, which suggests that activating pathways play a lesser role than inhibitory pathways, consistent with the findings of other authors.32 Along the same lines, it has been described that decreased expression of ligands of activating receptors in patients with AML constitutes an immune escape mechanism.33 Recent research has explored haploidentical NK cell transplantation for consolidation treatment of paediatric patients with low-to-intermediate-risk AML in whom HSCT is not indicated.20,34 The results have been quite promising, with a 0% toxicity and a 100% relapse-free survival after a median followup of more than 3 years.34 Biotechnology currently allows us to isolate NK cells from healthy donors by aphaeresis. Furthermore, two new cell products are currently being studied in Spain: NK cells stimulated with IL-15 obtained from haploidentical donors by aphaeresis (NK-IL-15), and activated and expanded NK cells (AENKs) from the peripheral blood of patients (autologous AENKs) or from haploidentical donors (haploidentical AENKs). To date, there is no evidence of significant toxicity in patients with advanced disease (>2 reports, based on the NCI-CTCAE v 3.0),35 so this cell-based therapeutic approach could be incorporated in a national prospective multicentric study, especially in patients in remission that do not meet the criteria for allogeneic HSCT.
In conclusion, the rate of HSCT in AML is still not optimal, and relapse constitutes a significant problem. Research needs to be conducted on diagnostic, therapeutic and monitoring procedures with the goal of improving on current survival. In this regard, NK cell transplantation could be an approach worth developing, considering the importance of immunological factors in AML.
Conflict of interestsThe authors have no conflict of interests to declare
This study was partially funded by the Fondo de Investigación Sanitaria (FIS, project PI12/01622), and the CRIS Cancer Foundation (http://www.criscancer.org/es/index.php).
Please cite this article as: González B, Bueno D, Rubio PM, San Román S, Plaza D, Sastre A, et al. Aspectos inmunológicos de la leucemia mieloblástica aguda. An Pediatr (Barc). 2016;84:195–202.