Pharmacological treatment of asthma
Before describing pharmacological treatment of asthma in Paediatrics, we want to make two basic points:
The original guide describes the treatment of both children and adults. In general, evidence is more solid for adults than for children, especially the under-5s. The classification of recommendations takes this into account, with children often given a lower rating than adults, as the recommendation is extrapolated from the adult population. For example, a grade B recommendation indicates that it is based on a systematic review that groups children and adults, in which the results for sub-groups of children are analysed. A grade D recommendation for small children indicates that, as there are no data for this population group, the recommendation is a consensus one, extrapolated from the benefits found for adults and older children.
Relevant new publications. In the original edition of the guide, we mentioned that one of the recommendations could change because of the findings of a study under way. This trial has just been published and we have introduced its finding into this article (reference 13). We also introduce the results of a recent Cochrane review, which provides more data on long-acting Beta-adrenergic bronchodilators (ref. 59).
This edition does not include the chapters on education and organisation or the appendices of the original guide, which can be consulted on the intranet of Osakidetza and at htpp/www.respirar.org or htpp/www.avpap.org
General considerations
The objectives of the pharmacological treatment of asthma are to control symptoms, including nocturnal symptoms and exercise-induced asthma, prevent crises and attain the best possible lung function, with minimum adverse side-effects 1.
In general terms, asthma control is evaluated by the following standards 1:
Minimum daytime and nocturnal symptoms.
Need for minimum relief medication 1.
Absence of crises.
Absence of restrictions on physical activity.
Normal lung function (FEV1 and/or PEF > 80 % of the theoretical value or of the best value).
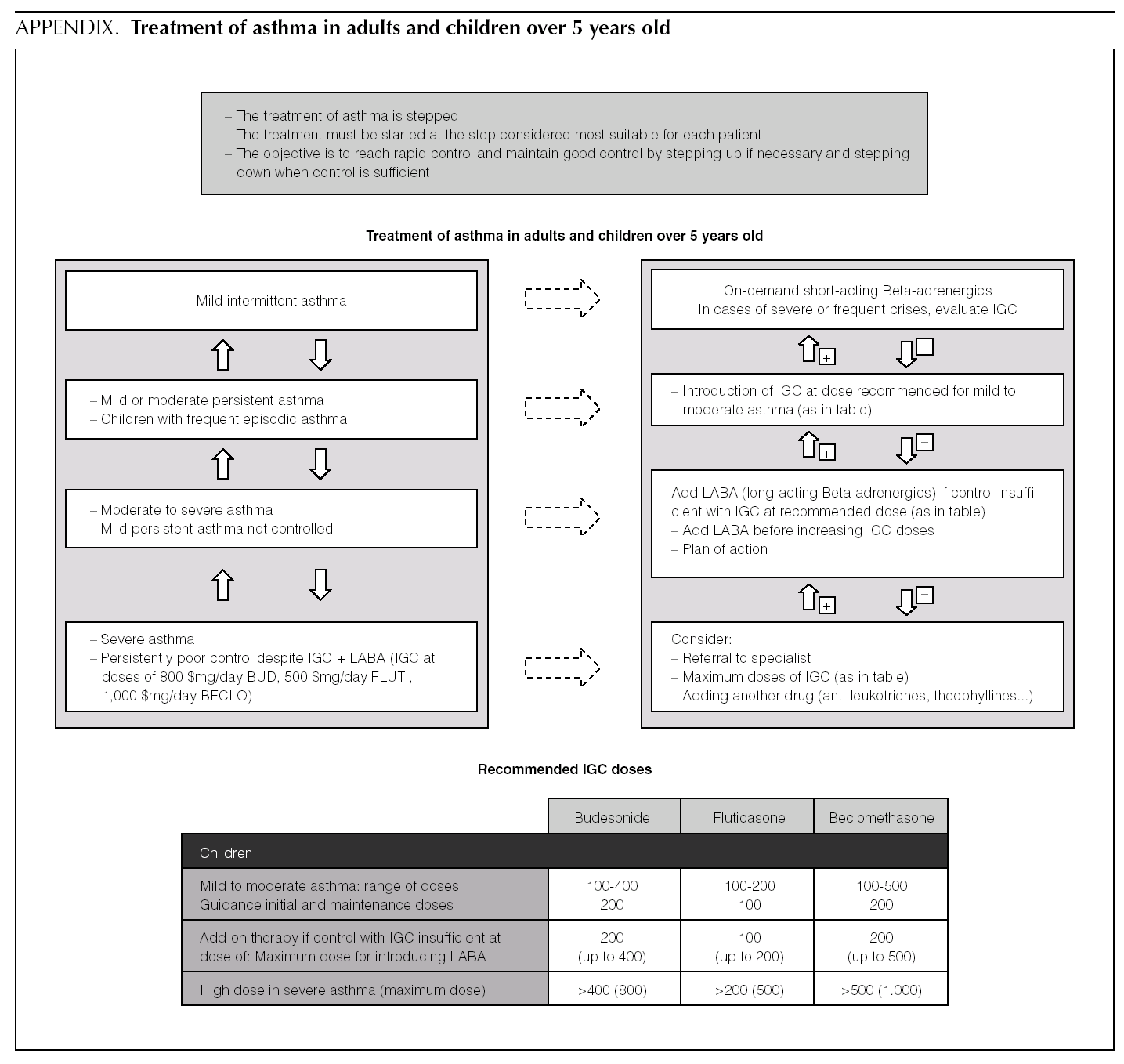
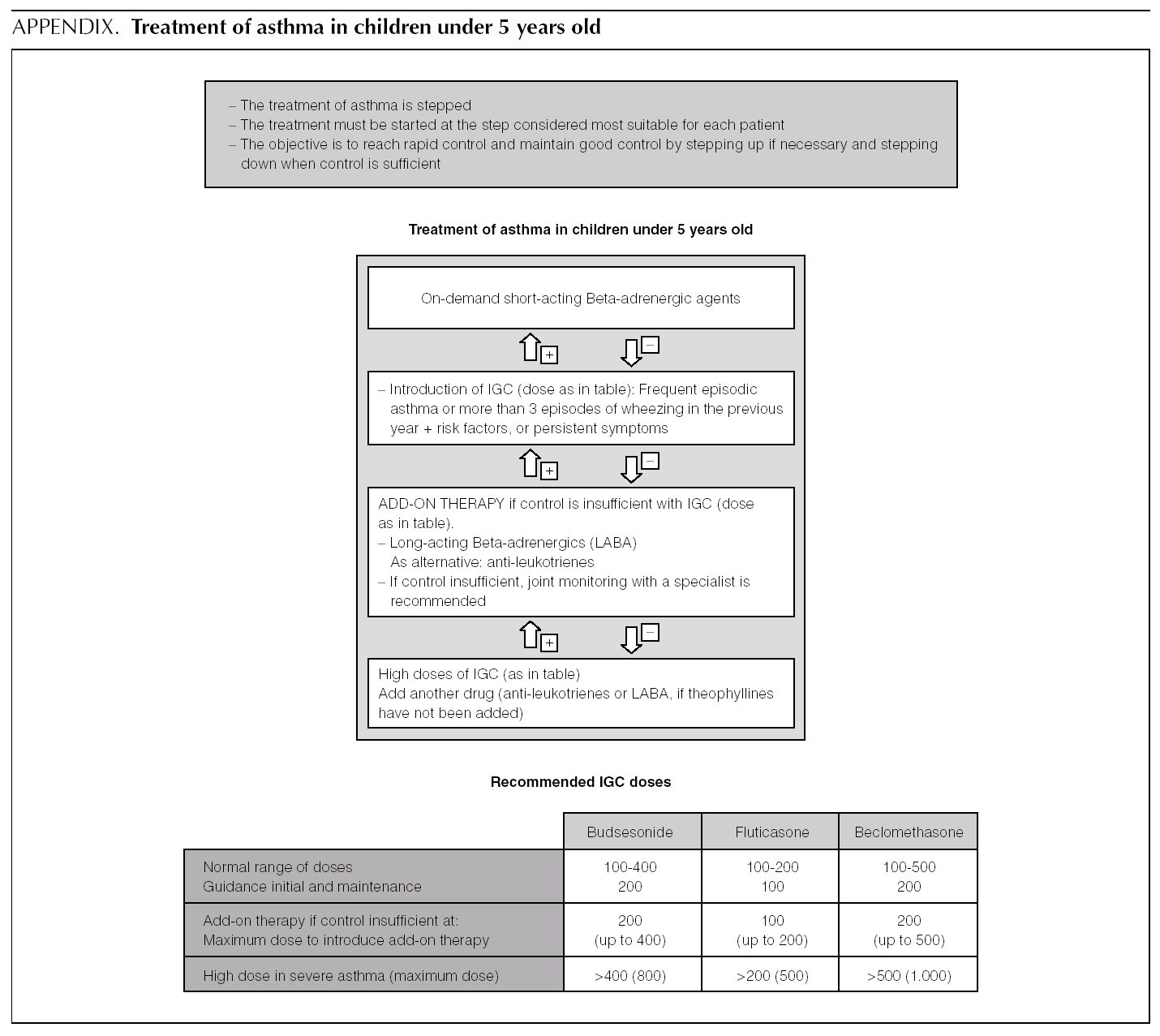
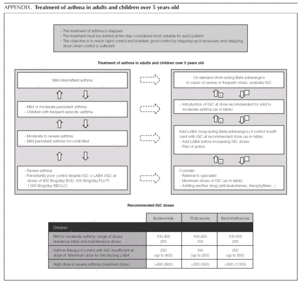
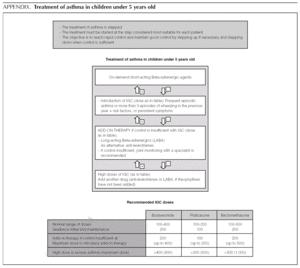
Asthma treatment is stepped. Treatment must be started at the step thought most appropriate for each patient. The objective is to control the condition rapidly and maintain it well controlled, by stepping up the medication if need be and stepping it down when control is sufficient.
Before a change of treatment, compliance with the treatment in place, the inhalation technique and the triggering factors must be evaluated.
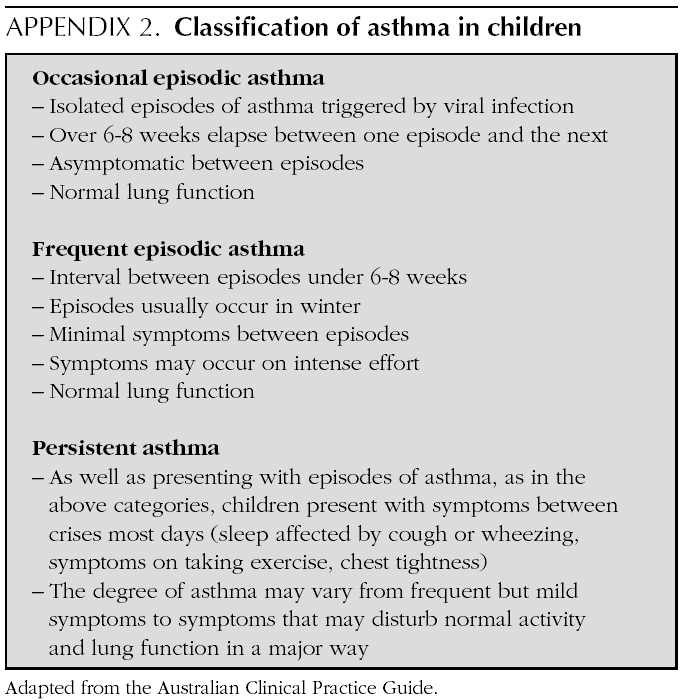
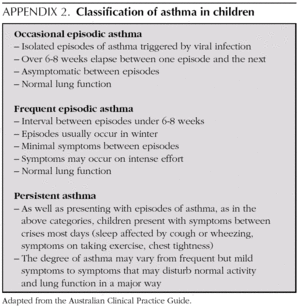
Mild intermittent asthma
QUESTIONS TO BE ANSWERED
How should mild intermittent asthma be treated?
Short-acting Beta-adrenergic agents (Salbutamol, Terbutaline) are the drugs of choice as relief medication, since they act more quickly and/or have fewer adverse effects than other alternatives 1.
Normally mild intermittent asthma can be properly controlled by on-demand beta-adrenergics alone 1-3. However, the introduction of inhaled Glucocorticoids (IGC) should be assessed for patients presenting with serious crises (e.g. requiring hospital admission) or frequent crises (e.g. children with frequent crises who are asymptomatic between crises) 4,5.
Although mild intermittent asthma is characterised by non-frequent symptoms and normal lung function, physiopathological studies indicate that air-way inflammation persists 6.
SUMMARY OF THE EVIDENCE
1++ Short-acting beta-adrenergics are the drugs of choice as relief medication.
4 Normally mild intermittent asthma can be properly controlled by on-demand beta-adrenergics alone 1-3.
Recommendations
D It is recommended that mild intermittent asthma be treated with on-demand short-acting beta-adrenergics.
D Patients with intermittent asthma, but with severe or frequent exacerbations may require chronic treatment with IGC.
(check)Studies are needed to evaluate the long-term impact of IGC background treatment on the prognosis of patients with mild intermittent asthma.
Introduction of preventive therapy with IGC
QUESTIONS TO BE ANSWERED
Inhaled glucocorticoids (IGC): are they the preventive treatment of choice for persistent asthma of whatever level of severity?
Are IGC effective for treating breast-feeding and pre-school children with asthma?
When should IGC treatment start? Are IGC effective in treatment of mild persistent asthma?
What is the effectiveness of various doses of Beclomethasone, Budesonide and Fluticasone? At what dose should the various inhaled corticoids be delivered in asthma maintenance treatment?
Should high doses of IGC be delivered at first, which are then steadily stepped down, or should treatment start with normal doses?
Are IGC delivered once a day as effective in treating mild to moderate asthma as the same dose delivered twice a day?
What are the adverse effects of IGC on bone mineral density, children's growth, ocular toxicity and the suppression of the hypothalamus-hypophysis-suprarenal gland axis?
What role do other preventive treatments have (anti-leukotrienes, chromones, immunotherapy, etc.)?
Efficacy of IGC
IGC improve symptoms and lung function and prevent exacerbations of asthma. Their safety profile is acceptable. They are the most effective preventive treatment for asthma at various degrees of severity 1.
Considerations in breast-feeding and pre-school children
In this age group it is important to distinguish children with wheezing and factors of risk for developing atopic persistent asthma from children with light wheezing only during viral infections and without factors of risk. In this latter group, there is no evidence to favour chronic IGC treatment 7, whereas breast-feeding children with symptoms outside viral episodes or those with factors of risk of developing asthma could benefit from treatment with IGC.
Until a short time ago, the recommendation for use of IGC in the under-5s was based essentially on extrapolation from study data on older children. Recently, a systematic review 8 and various clinical trials 9,10 conducted exclusively in this population group were published. They confirmed the benefits of IGC in improving symptoms and reducing exacerbations. The systematic review 8 includes children from 0 to 6 years old with a medical diagnosis of asthma and excludes trials with intermittent IGC models, so as to avoid the inclusion of children with wheezing due to virus rather than asthma. These studies confirm the efficacy of IGC in small children with a medical diagnosis of asthma. The definition of asthma varied greatly between the studies included in the review (children with episodes of wheezing in the months or weeks prior to the study, persistent wheezing and tendency to atopy, children with a monthly crisis in the preceding three months or symptoms most days...), which shows the effectiveness of IGC across this very heterogeneous age-group.
In children aged 2-5, IGC improved Bronchial Hyper-reactivity (BHR) and lung function in a small short-term trial 10. However, there are no contrasted studies on the long-term impact of IGC treatment on lung function in small children. The evidence comes from studies that include children from the age of 6 and favours early (evolution of the disease less than two years) IGC treatment 11; whereas, if the introduction of IGC treatment is delayed, in the long term lung function does not improve despite IGC treatment 12.
Recently, the PEAK trial 13 studied the efficacy of using Fluticasone versus placebo in 2-3 year-old children with the modified Castro positive prediction index (table 6 in the asthma prognosis chapter), in order to evaluate whether early IGC treatment of young children might modify the course of the disease. The children received 176 mg/ day of Fluticasone (in two doses) or placebo for two years. The results were evaluated in the third year (year of observation with no treatment received). During the treatment period (two years), IGC increased the number of days without symptoms and reduced the frequency of exacerbations and use of relief medication. However, in the third year, no significant differences between the group treated with IGC and that not treated with IGC were found. In the treated group, growth was 1.1 cm less at 24 months, and 0.7 cm at the end of the study (at three years). It was concluded from the study that early IGC treatment of 2-3 year-old children who had over 4 episodes of wheezing and factors of risk for asthma improved symptoms and reduced wheezing episodes while the treatment lasted, but did not modify the asthma prognosis. The risk of wheezing persisted at the end of the treatment, regardless of whether or not IGC had been taken in the preceding two years.
When to initiate IGC treatment. Role of IGC in mild persistent asthma
Till now, the threshold of when to introduce IGC treatment has not been fully clear. In recent years various clinical trials published have tackled this question, especially in mild persistent asthma 11,12,14.
The START study 11 evaluated the efficacy of early treatment (asthma of under two years evolution) with Budesonide (400 μg/day in > 11 years old and 200 μg/day in younger children) versus placebo in 7,165 adults and children (from 6 years of age) with mild persistent asthma. This was defined as those patients with symptoms (wheezing, cough, breathlessness, chest tightness, waking up at night due to symptoms) at least once a week but not daily, and with normal lung function. Early treatment with Budesonide reduced the frequency of severe crises (HR 0.56; CI 95 %: 0.45-0.71, NNT at 3 years old = 43), improved control of symptoms and brought about long-term improvement in lung function. The treatment was tolerated well; in children, the speed of growth was slightly less with Budesonide (0.43 cm/year) in the first two years, but not in the third year 11. Due to the relevance of this study, we have adopted its inclusion criteria as the threshold of when to introduce IGC treatment.
The CAMP study 12 evaluated the efficacy of IGC for 4-6 years in children from 5 to 12 with mild to moderate asthma of several years evolution. This study also found better control of asthma in children treated with IGC (with reduction in the frequency of hospital admissions and visits to Casualty), but unlike the START study no improvement in long-term lung function (post-bronchodilation FEV-1), the parameter proposed as indicator of lung growth, was found. This difference might be because the patients in the CAMP study had asthma of various years evolution. In a stratified analysis of the CAMP study, patients who continued with a loss of lung function showed in the multivariate analysis that the sole predictive factor was peripheral eosinophilia. All these data point to the importance of introducing IGC treatment early.
Some guides 4,5 recommend treating children of any age who present with frequent episodes of wheezing and do not suffer symptoms between crises. This recommendation needs to be confirmed by Randomised Clinical Trials, since at present, for asthma in children without symptoms between crises, it is not possible to establish a clear threshold for when (after how many crises) IGC treatment should be introduced. In 2-3 year-old children with frequent asthma symptoms (over 4 episodes of wheezing in the preceding year and risk factors for asthma), IGC treatment improves the clinical parameters, but does not change the natural course of the disease 13.
SUMMARY OF THE EVIDENCE
1++ IGC are the most effective preventive treatment for asthma of various levels of severity, including mild persistent asthma 11,12,14 in children of all ages 1.
1++ In breast-fed and pre-school children with a medical diagnosis of asthma, IGC improve asthma control, in terms of symptoms, reduction of risk of exacerbations and use of relief medication 8-10. In children aged 2-5, IGC improved BHR and lung function in a short-term trial 10. The definition of asthma varies greatly between studies, showing the efficacy of IGC in this highly heterogeneous age group.
1++ In 2-3 year-old children with over 4 episodes of wheezing in the preceding year and risk factors for asthma, IGC treatment improves symptoms and reduces wheezing episodes while treatment lasts, but does not change the natural course of the disease at 3 years 13.
1++Early IGC treatment in mild persistent asthma in adults and children from 6 years of age reduces severe crises, improves symptom control and improves long-term lung function 11.
1++ In children between 5 and 12 with asthma of several years evolution, IGC improve short-term, but not long-term lung function 12.
RECOMMENDATIONS
A IGC are the preventive drugs of choice.
A IGC are the treatment of choice for breast-fed and pre-school children with a medical diagnosis of asthma*.
A All adults and children from the age of 6 with mild persistent asthma** must receive IGC.
A Early introduction (at under 2 years evolution) of IGC treatment for mild persistent asthma**, in both adults and children over 6, is recommended.
B In young children with frequent wheezing episodes (over 4 in the preceding year) and factors of risk for asthma***, IGC treatment can be recommended to improve symptoms, although there is no evidence that it modifies the prognosis of the disease.
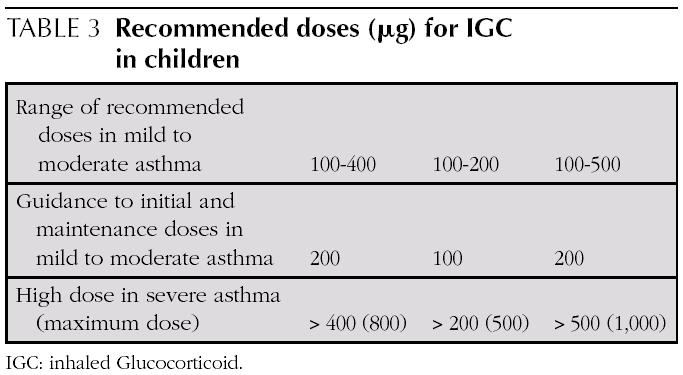
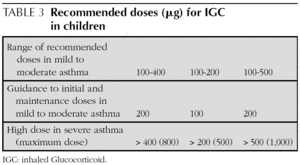
Initial and maintenance doses of IGC. Equivalence (Table 1)
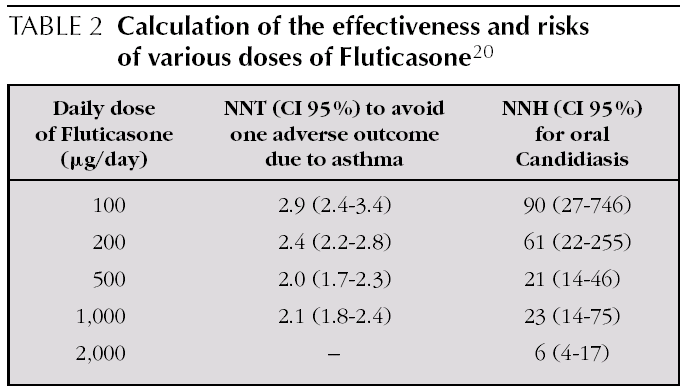
Questions relating to IGC doses have been tackled in several systematic reviews that evaluate the dose-response curve for Beclomethasone, Budesonide and Fluticasone and comparisons between them 23. There is high concordance in recommending the minimum effective dose of IGC.
IGC have a relatively flat dose-response curve for efficacy. However, side effects such as dysphony or oropharyngeal Candidiasis are more common at higher doses.
For Budesonide, 80 % of the benefit is achieved at doses of 400 μg/day, and 90 % at doses of 300-600 μg/day 22.
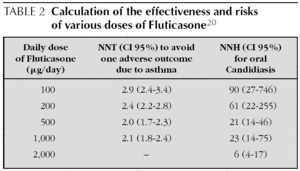
With Fluticasone, moving from 200 μg/day to 500 μg/ day brings scarcely any additional benefit and yet is linked to a higher rate of Candidiasis 20,21. Powell, starting from Cochrane review data, calculated the NNT to avoid one adverse asthma outcome and the NNH to cause oral Candidiasis for various ranges of Fluticasone doses in mild to moderate asthma (see table 2) 20.
Regarding the efficacy and comparative safety of the various IGC available on the market, it is now well established that both the efficacy and adverse effects of IGC are an effect of the entire class and, therefore, are common to all the IGC available on the market. The equivalence between the various IGC is clear, with Fluticasone requiring half the dose of Budesonide or Beclomethasone due to its greater strength 23. Fluticasone, compared with Budesonide or Beclomethasone at doses of 1:2, produces light improvements in lung function, but is linked to greater local adverse effects (Pharyngitis) 23. These data support the use of Fluticasone at half the dose of Beclomethasone and Budesonide.
Most long-term clinical trials are conducted with Budesonide or Fluticasone and, to a lesser degree, with Beclomethasone.
There are other IGC not sold in our country, such as Mometasone, which, like Fluticasone, have an effect similar to Beclomethasone and Budesonide at half the dose, but their relative safety has not been established 1. Ciclesonide is another new IGC still not sold and whose efficacy and safety are not well established 24.
On initial dosage, a recent Cochrane review 16 concluded that, in mild to moderate asthma, similar control of symptoms and of lung function is achieved by starting with low to moderate doses as by a step-down strategy (starting with high doses, then gradually reducing them). Moderate initial doses (≥ 400 to < 800 μg) were lightly more effective than low doses (< 400 μg/day) in improving PEF (difference of means, 11.14 L/min; CI 95 %, 1.34-20.93) and nocturnal symptoms. No benefits were found at higher doses (except a trend to improve BHR). The results were consistent for the various inhalation appliances and IGC, in both children and adults.
Another factor to bear in mind is the possibility of using IGC as a single daily dose. This aspect was studied in several trials that compared the model of one daily dose with two in both children and adults 25-31. In addition, the START study used the model of Budesonide once a day in mild intermittent asthma 11. In general, the data supporting the use of the single daily dose are more consistent with Budesonide than with Fluticasone (there are not sufficient data for Beclomethasone to enable any recommendation to be made). The data are also more consistent for mild asthma than for moderate asthma.
SUMMARY OF THE EVIDENCE
1++ Most patients with mild to moderate asthma can be adequately treated with low-moderate doses of IGC 17-22. The data do not apply to severe asthma; and some patients may benefit from high doses.
Fluticasone, when compared with Budesonide or Beclomethasone at 1:2 doses, improves lung function slightly, but is linked to more local adverse effects (Pharyngitis) 23. These data support the use of Fluticasone at half the dose of Beclomethasone and Budesonide.
1++Initial dose. In mild to moderate asthma, similar control of symptoms and lung function is attained by starting with low to moderate doses as when starting with high doses and stepping them down gradually. Moderate initial doses are lightly more efficacious than low doses in improving PEF and nocturnal symptoms. No benefits were found with higher doses 16.
1+ In adults and children over 4-5 years old with stable mild asthma requiring IGC, the delivery of IGC in a single daily dose has a similar efficacy to when the same dose is divided in two 25-31. The studies are more consistent for Budesonide.
In patients with moderate asthma, delivery with Fluticasone Accuhaler® in a single daily dose is slightly less effective than when taken in two doses, though it is clinically similar in mild asthma. There are not sufficient data to allow recommendation of Beclomethasone in a single daily delivery.
RECOMMENDATIONS
A Most of the patients with mild to moderate asthma can be adequately treated with low to moderate doses of IGC.
A 1:1 equivalence between Budesonide and Beclomethasone is assumed; and 1:2 of Fluticasone with these.
AInitial dose. In children who require IGC, the recommendation is against starting with high doses and stepping them down gradually. It is recommended that IGC treatment starts at the dose appropriate for the severity of the asthma (normally low or moderate doses).
AFrequency of dose. Children over 4-5 with stable mild asthma can be treated with IGC in one daily dose. Data are more solid for Budesonide.
√ There is more uncertainty for patients with moderate asthma.
On moving from two doses a day to just one, the response must be watched to ensure that the patient is still properly controlled.
Safety of IGC: systemic adverse effects
SUMMARY OF THE EVIDENCE
Growth in children:
1+ Evidence shows consistently that there is a reduction in the speed of growth of approximately 1 cm or less during the first year of treatment of children with mild to moderate asthma. However, this effect is small and does not seem to last over time 11,12,32-35.
2+ Evidence on the repercussion of IGC on adult height is consistent: they appear to have little repercussion on adult height 32,33,35.
Osteoporosis and bone mineral density (BMD):
1+ The use of IGC at low to moderate doses has no effects on BMD in children 3,11,34,36.
2+ Observational studies have found no association between consumption of IGC by children and adolescents and increased risk of fractures 37.
1+ In adults with asthma, IGC doses of up to 1,000 μg/ day for 2-3 years are not associated with effects on BMD or vertebral fractures. Higher doses were linked to biochemical markers of increased bone thinning 38,39.
Ocular toxicity:
2+ In children and young adults, low to moderate doses have no significant effects on the incidence of subcapsular cataracts or glaucoma, but it should be borne in mind that in this population the base risk is practically nil 11,12,33.
Suppression of the hypothalamus-hypophysis-suprarenal gland axis:
4 In general, children treated with low-medium doses of IGC may experience clinically insignificant effects on this axis 33,34.
However, on rare occasion, cases of adrenal crises associated with the use of high doses of Fluticasone (> 500 μg/ day, up to 1,500 μg/day) have been described 40.
RECOMMENDATIONS
A Low or moderate doses must be used for children who require IGC. At the doses recommended (low or moderate doses) IGC are safe, and the risk benefit balance is favourable. Use of high doses confers very little extra benefit and exposes patients to unnecessary risk of local and systemic adverse effects.
√ Doctors and nurses providing health education for asthma patients should tell them of the good safety profile of IGC, as well as the importance of taking them at proper doses and without interrupting treatment.
Other preventive single-therapy treatments (chromones, anti-leukotrienes, immunotherapy)
Chromones. A Cochrane 41 review gathered studies (mostly crossover ones) with cromoglycate of a total of 1,074 children. No significant differences between cromoglycate and placebo were found for the days without symptoms, nor for other variables. In addition, the review suggests a possible publication bias. For Nedochromil, the CAMP trial 12 showed they were less effective than IGC.
Anti-leukotrienes. Evidence on the comparative efficacy of anti-leukotrienes and IGC is taken from a systematic Cochrane review 42. This concluded that, at doses of 400 μg of Beclomethasone or equivalent (200 μg Fluticasone), IGC are clearly superior to 10 mg/day Montelukast or 20 mg/12h Zafirlukast in preventing exacerbations, as well as in improving lung function and control of symptoms and reducing need for relief medication. The superiority of IGC is clear at 4-6 weeks and is maintained for over 37 weeks. In children, with a single open clinical trial lasting 24 weeks included in the systematic review, caution is needed when extrapolating results. This study 43 was conducted on children from 6 to 11 with mild asthma: no differences were found between Montelukast and Beclomethasone, but the trial did not meet the previously defined equivalence criteria.
Immunotherapy. A Cochrane review 44 with 3,506 patients evaluated the efficacy of specific immunotherapy (domestic mites, pollen, animal skin, mould, multiple allergens) versus placebo for asthma. A significant reduction in symptoms and the use of medication, and improvement in specific and non-specific BHR were found. No effect on lung function was seen. Only one study comparing immunotherapy and IGC was found, with data insufficient to establish conclusions. In retrospective and prospective studies, systemic adverse reactions with immunotherapy were described, at a frequency of one per 1,250-2,206 injections. Most reactions were mild. Deaths due to immunotherapy were very rare (with estimates running from one per million to one per two million injections). Optimum doses and duration of immunotherapy are not clearly established. Further studies are needed to determine which patient sub-groups could benefit most from the treatment.
Alternative therapies
In our area, Homeopathy is probably the alternative therapy that is most commonly used in asthma, especially for children. This is why health professionals should bear this kind of treatment in mind. They need to know what evidence underlies alternative therapies in order to provide full information.
Homeopathy. A Cochrane review 45 analysed the data from 6 trials of variable quality that had a total of 556 patients. They used various homeopathic treatments, which prevented the combination of their findings. No trial reported significant differences on validated scales of symptoms and results on lung function were contradictory.
Acupuncture. A Cochrane review 46 analysed the data from 11 studies with 324 patients. The information in the trials was deficient and their quality was inadequate. There was variation in the kind of acupuncture and in the results measured. No significant or clinically relevant differences were found between acupuncture and simulated acupuncture. However, in one review of cases 47, isolated cases of pneumothorax and other severe adverse effects were found.
Manual therapy (including chiropractice and massages). There is a Cochrane review 48 with 5 clinical trials, most of which were defective. No significant differences were found between chiropractic spinal manipulation and a simulated manoeuvre. It was not possible to find conclusions on other manual therapies, such as physiotherapy.
SUMMARY OF THE EVIDENCE
1+ Chromones have little effect when they are used as a treatment to prevent asthma in both children and adults 12,41.
1+ Compared with placebo, anti-leukotrienes are modestly effective in improving symptoms and lung function 1.
1++ In adults with mild to moderate asthma, IGC are clearly superior to anti-leukotrienes in preventing exacerbations, improving lung function and controlling symptoms. Anti-leukotrienes are well tolerated in the long term, but the abandonment of treatment because of their poor control of asthma is much more common than for patients taking IGC 42.
In children, including the under-5s 49, Montelukast is safe in the short term and causes a slightly greater clinical improvement than placebo, but there are not sufficient studies comparing it with other therapies 42.
1+ Immunotherapy is more effective than placebo in improving symptoms and reducing the need for medication, but its greater efficacy than IGC is not established. In addition, the possibility of grave systemic side-effects must be borne in mind 44.
1+ There is not sufficient evidence to evaluate reliably the role of homeopathy in asthma 5.
RECOMMENDATIONS
A IGC continue to be the preventive treatment of choice, as they are more effective than chromones or anti-leukotrienes.
A As anti-leukotrienes are less effective than IGC, their use in maintenance monotherapy for children and adults is not recommended.
A The use of chromones for asthma is not recommended, as their efficacy is very limited.
A Immunotherapy is more effective than placebo in improving symptoms and reducing the need for medication. As its efficacy versus IGC is not established and it may cause adverse side-effects, its use as monotherapy is not recommended.
A The use of alternative therapies, such as homeopathy, acupuncture or manual therapies, is not recommended.
Therapy added to IGC
QUESTIONS TO BE ANSWERED
When should a second drug be added to preventive treatment with IGC?
Are long-acting beta adrenergic agents (LABA) the drugs of choice as an add-on therapy?
In mild persistent asthma not controlled by IGC, is it preferable to add a LABA than to increase the IGC dose?
Are there differences between delivering IGC and LABA in a single inhalation appliance and delivering them separately?
What is the role of theophyllines, anti-leukotrienes and other treatments as add-on therapy?
What is the role of LABA delivered on demand versus delivery at set doses?
General considerations. Long-acting Beta-adrenergic agents (LABA) as therapy added to IGC
Before adding a new drug to preventive therapy with IGC, compliance with treatment and inhalation techniques need to be checked, triggering factors evaluated, and continuity of care examined 1.
Of the possible therapeutic options, LABA (Salmeterol and Formoterol) continue to be the drugs of choice as add-on therapy 1-3,50,51. The exact dose of IGC at which the introduction of add-on therapy with LABA is recommended before increasing the IGC dose is not at all clear. In general, the various guides recommend introducing a LABA when control is not achieved by doses of 200-400 μg/day of IGC in children 1,2,51.
In terms of safety of LABA, tolerance to them is generally good. In the systematic review by Walters 52, greater risk of adverse reactions was found for them than for placebo (OR 1.35, CI 95 % 1.03-1.77). Head-aches were more common with LABA and no differences were found in trembling or palpitations. A non-significant tendency was found in children to more exacerbations with LABA than with placebo, which is why we examine LABA in children in detail. Finally, it must be noted that the SMART clinical trial (population > 12 years old) was halted because, at an intermediate analysis 53, a small but significant increase in asthma-related deaths was found when taking Salmeterol, particularly in Afro-American patients and in patients not taking IGC. Therefore, in countries such as the United States, the instructions accompanying LABA include a warning to this effect.
LABA in children
In the studies conducted solely on children 33,54-57 and in the sub-group analysis conducted in a systematic review 52, LABA demonstrably improved symptoms and lung function. Data on exacerbations are less definite and require further study. Recently, a clinical trial on the association of Formoterol and Budesonide was published. This study, which included children from 4 years of age as well as adults, showed a consistent reduction in the frequency of exacerbations at all ages 58, but did not provide data on the sub-group of children. In a Cochrane review that included poorly controlled patients with moderate asthma 59, published after the composition of this guide, but which we mention here due to its importance, the addition of LABA to IGC (mean dose 400 μg/ day) versus doubling the IGC dose (800-1,000 μg/day) improved lung function and reduced symptoms and relief medication. However, it found no statistically significant differences in frequencies of crises. No heterogeneity between trials was found, despite the inclusion of participants of various ages. Meta-regression suggested that the benefit of LABA on exacerbations could fall with the duration of the treatment and with higher doses of IGC in the combination. The review included few studies of children. However, it needs to be borne in mind that, in our environment, according to the instructions, Formoterol is not recommended for children under 6 years old and Salmeterol is not recommended for children under 4.
LABA in mild persistent asthma
One of the points that has changed from previous guides is the role of LABA in mild persistent asthma. Thus, the OPTIMA trial 14 evaluated the efficacy of Budesonide at low doses versus Budesonide plus Formeterol in mild persistent asthma in patients over 12, whether treated previously with IGC or not. In the group of patients who were not receiving IGC previously, Budesonide at doses of 200 μg/day plus Formoterol was equally efficacious as Budesonide alone in limiting the number of severe exacerbations. However, both treatments were better than placebo: RR of severe exacerbations of Budesonide vs. placebo 0.40 (CI 95 %: 0.27-0-59). In the group of patients who were receiving IGC previously, 4 treatments were compared: 200 μg/day Budesonide, 200 μg/day Budesonide + Formoterol, 400 μg/day Budesonide, and 400 μg/day Budesonide + Formoterol. In this group of patients, adding Formoterol to 200 or 400 μg of Budesonide reduced by half the risk of severe exacerbations per patient/year (RR 0.57; CI 95 %, 0.46-0.72). The 400 μg/ day dose was lightly more efficacious than the 200 μg one in improving symptoms and lung function, but showed no differences in exacerbations. Compared with 400 μg/day Budesonide, the association of 200 μg/day Budesonide with Formoterol was more efficacious in reducing the rate of exacerbations (RR 0.71, CI 95 %, 0.52-0.96). The results of this study are useful for clarifying the strategy to follow for mild persistent asthma, as they show us that many patients with mild asthma can benefit from IGC at low doses and, if the control continues to be insufficient, the most favourable option is to add a LABA.
Combinations of IGC and LABA in a single appliance
There are no differences in efficacy between delivery the combination of an IGC and a LABA in the same appliance versus use of two different appliances. For the association of Fluticasone and Salmeterol, 4 clinical trials (lasting 12-28 weeks) with a total of 1,375 patients (children and adults) have been published. Findings were similar in terms of lung function, symptoms and relief medication. Nor were there any differences in adverse effects 60-63. The meta-analysis of the 4 trials was associated with a statistically significant improvement in favour of the association, although the benefit observed (5.4 L/min) was clinically irrelevant 64.
There is no direct evidence to suggest that combinations improve compliance with treatment. Associations may be useful in patients with stable asthma, provided that the combined inhaler matches the separate requirements of each medicine. As possible benefits, they may help the patient not to stop taking the inhaled corticoid and they are not more expensive. Possible disadvantages are that they may favour the maintenance of the patient on more medication than he/she strictly needs and that it may be more difficult to adjust the dose.
Theophylline, anti-leukotrienes and other treatments as therapy added to IGC
Theophylline. As an add-on therapy, this improves lung function and symptoms, but adverse effects are common 1. A Cochrane review 65 that collected the trials on children and adults to compare the efficacy of Theophylline versus LABA concluded that LABA are similar or somewhat superior to Theophylline in FEV1 and in the number of nights without symptoms, and that with LABA adverse effects are fewer (RR 0.44, CI 95 %, 0.30-0.63), (NNT 9CI 95 %, 8-50). Fewer Central Nervous System or gastrointestinal events were described.
Oral Beta-adrenergic agents. As add-on therapy, they improve lung function and symptoms, but adverse effects are common 1.
Anti-leukotrienes. The effectiveness of anti-leukotrienes as a therapy added to IGC has been the subject of study with various comparisons: versus placebo, versus doubling the IGC dose and versus LABA. The comparison that has most practical relevance is the LABA one. There is a marked lack of clinical trials in children of anti-leukotrienes as an add-on therapy.
Anti-leukotrienes vs placebo in patients who are symptomatic despite IGC treatment. Adding Montelukast whilst maintaining the same IGC dose improves the symptoms and control of the asthma 66-68.
Anti-leukotrienes vs doubling the IGC dose. Montelukast added to 800 μg/day of Budesonide had a similar effect to 1,600 μg/day of Budesonide in a trial conducted in adults 67,68. Nevertheless, the IGC dose is on the "flat" part of the dose-response curve.
Anti-leukotrienes vs LABA. A recent Cochrane review compared the two treatments in adults not properly controlled by low doses of IGC 69. Adding a LABA is more effective than adding an anti-leukotriene regarding the risk of exacerbations that need oral corticoids: RR 0.83 (CI 95 %, 0.71-0.97), NNT at 48 weeks 38 (CI 95 %, 23-247), and was also better in improving lung function, symptoms and relief medication. More studies of children are needed to compare the efficacy of anti-leukotrienes versus LABA as a therapy added to IGC.
We found no trial that evaluated the effectiveness of anti-leukotrienes as a therapy added to IGC plus LABA.
Areas of recent research
Association of Budesonide-Formoterol: set model versus flexible model. Recently several trials on the use of the Budesonide-Formoterol combination at set dose versus flexible dose have been published 70-75. These show that the flexible model enables medication to be stepped down, as against the set model. In addition, two studies showed a benefit in the reduction of exacerbations 71-73. These studies were all of adults, had methodological failings and were of limited use (they require an action plan and considerable practice in order to modify the dose in line with the symptoms and the PEF monitoring in some studies 73). All of this makes it hard to formulate concrete recommendations for children. In addition, those who received the set model may be over-treated, which could partly explain why the need for medication was lower in the flexible model group.
Association of Budesonide-Formoterol as treatment of maintenance and relief. A recent double-blind clinical trial 58 compared the effectiveness of the Budesonide/Formoterol 80/4.5 association as maintenance and relief treatment, versus the same association as maintenance treatment + a short-acting Beta-adrenergic agent as relief and versus 320 μg Budesonide as maintenance + a short-acting Beta-adrenergic as relief for one year in 2,760 asthma patients, both children and adults (4-80 years old), with mild to moderate asthma. To measure the main result, the trial used severe exacerbations. The group with Budesonide/Formoterol as maintenance and relief had lower risk of severe exacerbations than the group with this association + short-acting Beta-adrenergic as relief (HR 0.50; CI 95 %, 0.40-0-64) or than the group with Budesonide + short-acting Beta-adrenergic as relief. If exacerbations because of the drop in PEF are excluded and only those needing medical intervention are included, the result is still favourable to Budesonide-Formoterol as maintenance and relief (NNT = 10). The speed of growth of children treated with higher doses of Budesonide was lower than in the other two combinations. No over-use of the Budesonide-Formoterol association as relief was seen. Judging by the results of this study and the size of the differences, it appears that early treatment rather than the dose of IGC or Formoterol is what conditions a good response to treatment.
Complete control and good control of asthma by the Fluticasone-Salmeterol association: GOAL study. The results of the year-long double-blind GOAL clinical trial were recently published 76. The study looked at 3,421 patients over 12 years old whose asthma was not controlled and who were taking three levels of treatment: without IGC, doses ≤ 500 μg of Beclomethasone or equivalent and doses between 500 and 1,000 μg Beclomethasone or equivalent. The objective of the study was to find what percentage of patients reached "complete control" or "good control" with the Fluticasone/Salmeterol association versus Fluticasone alone. The first stage of the study, lasting 12 weeks, is to gradually step up the dose until reaching "complete control" or the maximum dose. At the second stage of the study, for the rest of the year, the patients remained at these doses, without any gradual stepping down. The most novel aspect of this study is probably the result variable, consisting of various clinical and functional criteria taken together. At each of the three levels, the association of Fluticasone and Salmeterol was superior to Fluticasone alone in terms of the percentage of patients who attained complete control and good control. This study made clear that asthma could be controlled in a high number of patients. 41 % of the patients taking the association achieved complete control after one year of treatment, along with 28 % with Fluticasone alone. Good control was achieved in 71 % of patients treated with the association and 59 % with Fluticasone alone. Nevertheless, it should be underlined that, by the end of the study, 68 % in the association group and 76 % in the Fluticasone alone group were at the highest permitted dose. The frequency of nasal Pharyngitis was high in both groups (13-14 %) and oral Candidiasis ran at 2-3 %. Reductions were seen in Cortisol levels. However, the conditions of the trial were not those of normal practice, since compliance was near 90 %. This conditions the real effectiveness of this model in clinical practice in our environment (its applicability).
SUMMARY OF THE EVIDENCE
1+ In children from 4-5 years old on, adding a LABA improves lung function and symptoms 33,52,54-57. Further studies to evaluate the effect of LABA on exacerbations in children are needed.
1++ In adults and children over two with mild persistent asthma and poorly controlled with low doses of IGC, adding a LABA to low doses of IGC (200-400 μg/day of Budesonide) reduces exacerbations and the days with symptoms and improves lung function and other variables. The benefit is greater than that attained by doubling IGC doses. In patients not treated previously with IGC, adding low doses of Budesonide reduces exacerbations by half; these patients obtain no additional benefits with LABA 14.
1+ There are no differences in efficacy between using the combination of an IGC and a LABA in an appliance versus using two appliances 60-64.
1+ In adults not sufficiently controlled by IGC, LABA are superior to anti-leukotrienes in reducing exacerbations and in improving symptoms and lung function 69.
1+ The combination of Formoterol and Budesonide used as relief and maintenance medication is effective in preventing severe crises 58.
RECOMMENDATIONS
B The added therapy of choice is LABA.
B In children poorly controlled by low/moderate doses of IGC, addition of a LABA is recommended before increasing the IGC dose.
√ The use of anti-leukotrienes in children under 4 not properly controlled by IGC can be countenanced, since LABA have no tested indication in this age-group. However, no studies of anti-leukotrienes as an add-on therapy for small children exist.
√ Studies are required to establish whether anti-leukotrienes provide additional benefit as therapy added to IGC plus LABA.
Insufficient control despite treatment with moderate doses of IGC + LABA
QUESTION TO BE ANSWERED
What is the best treatment option for patients not controlled by moderate doses of IGC and LABA?
In a small number of patients, asthma is not properly controlled by moderate-high doses of IGC (over 400 μg/ day in children) plus a LABA. In these patients, once their compliance with treatment, inhalation technique, the triggering factors and the continuity of care have been reviewed, another drug must be added or high IGC doses be used. However, there are hardly any studies that evaluate this situation, which means that the recommendations for this group of patients are based on consensus 1.
As there are no trials comparing the different options, the choice of one or another must be based on the adverse effects, the preferences of the patient or the cost; and the treatment's effectiveness must be evaluated in each individual case. The duration of the "therapeutic test" will depend on the result to be evaluated. For example, the prevention of nocturnal waking requires a relatively short period of time (days to weeks), whereas to evaluate exacerbations or consumption of oral corticoids more time will be needed (weeks to months). If there is no response to treatment, this must be interrupted and another tried.
RECOMMENDATIONS
√ If control is insufficient, despite taking IGC + LABA (IGC dose of 400 μg/day), consider one of the following interventions:
Increase the dose of IGC to 800 μg/day.
Add theophyllines
Add anti-leukotrienes
√ Consider referral to specialist care.
How to step down treatment
Clinical practice guides concur that asthma treatment is step-by-step and that steps up or down should be taken as a function of the control of the illness. As we have already seen, there is a lot of literature on the effectiveness of various drugs in stepping up and also trials that evaluate the savings on corticoids by the introduction of other drugs (LABA, anti-leukotrienes). However, very few clinical trials evaluate how to come down a step.
Recently a relevant clinical trial on the effects of reducing the IGC dose in patients with stable moderate-severe asthma for a year versus maintaining the initial dose was published. This study concluded that the IGC dose can be reduced by half in 49 % of patients with moderate-severe asthma 77. The patients were included in the study if they were well controlled (good control defined by the level of symptoms and relief medication) in the last month, if there were no visits to the doctor or hospital admissions since the previous control, and if PEF > 80 % in 8 of the 14 days prior to the study. The controls set were quarterly.
Another possible strategy in mild-moderate asthma could be the reduction of the IGC dose from 2 deliveries to one a day: a 12-week trial 78 concluded that the quality of life was not changed by going from a model of 200 μg Budesonide twice a day to 200 μg once a day, but did not evaluate the effects on other results (exacerbations, etc.).
In a small trial lasting 2 years with 37 patients with mild asthma, it was made clear that the dose could be reduced, although treatment interruption was associated with worsening of the asthma 79. Other studies 12,80,81 also described deterioration in asthma and BHR on interruption of treatment. All this indicates that in patients with asthma in clinical remission or with intermittent symptoms inflammation of the air-ways persists.
SUMMARY OF THE EVIDENCE
1++ In patients with well controlled asthma of different degrees of severity, the IGC dose can be reduced by half without compromising the control of the disease. This objective is achieved in 49 % of patients.
2+ Cessation of IGC treatment is linked to worse control of asthma in quite a lot of patients.
RECOMMENDATIONS
A In patients with stable asthma treated with moderate-high doses of IGC, it is recommended that the dose should be stepped down to the minimum effective dose.
C Cessation of IGC treatment is linked to worse control of asthma in quite a lot of patients.
√ Asthma treatment is step-by-step. Just as it is stepped up when control is insufficient, stepping down is equally important in order to use the minimum effective medication to maintain adequate control.
√ To reduce treatment gradually, a period of stability of at least three months is recommended.
√ During the reduction, control of the disease must be evaluated periodically (quarterly, for example).
Choice of inhalation appliance
A recent systematic review regarding the efficacy of various kinds of inhalation appliances to deliver IGC and short-acting Beta-adrenergic agents for asthma and COPD in adults and children found that there were no differences in clinical efficacy between the various kinds of inhalation appliances, provided that the delivery technique was correct 82. A subsequent systematic review on children from 5 to 15 corroborated these findings 83.
In the case of 0-5 year-olds, there is no scientific evidence to conclude that one kind of appliance is more efficacious than another in managing chronic asthma.
The choice of inhalation appliance in long-term asthma treatment must depend on the evidence available and the patient's age, bearing in mind patient's preference and cost.
SUMMARY OF THE EVIDENCE
1++ In children of 5-12 with stable asthma, pMDI with spacer chamber is as efficacious as any other method of inhalation 83.
4 In 0-5s there is no evidence to show that one kind of appliance is more effective than another in managing chronic asthma.
RECOMMENDATIONS
D In children of 5-12 with stable asthma, pMDI with chamber is as efficacious as any other method of inhalation. The choice of inhalation appliance must be based on the preference and skill in use of the patient.
D In 0-5s, pMDI with spacer chamber is the method of choice, with a mask for infants under 3.
√ Health professionals must teach patients how to use inhalers correctly. In addition, inhalation technique must be periodically appraised.
*In children < 5, the definition of asthma varies greatly between the different studies. Despite this, IGC treatment was shown to be efficacious in this highly heterogeneous age group: it includes children on the basis that they present with frequent symptoms, or have frequent wheezing episodes or by the number of their crises. In some studies the tendency to atopy is taken into account as an inclusion criterion. Trials with intermittent models of IGC are excluded.
**The definition of mild persistent asthma in the START study 11 is: wheezing, cough, breathlessness, tightness, nocturnal waking due to symptoms at least once a week but not every day, and normal lung function.
***Children with positive modified Castro index: over 4 wheezing episodes in the preceding year (episodes of > 24 hours duration, at least one of which was confirmed by a doctor) and one major criterion (history of asthma in a parent, atopic dermatitis diagnosed by a doctor, allergic sensitisation to at least one air allergen) or two minor criteria (wheezing unrelated to colds, eosinophils in blood > 4 %, allergic sensitisation to proteins in milk, eggs or nuts).
© Basque Health Service/Osakidetza.
Correspondence: Dra. M. Callén Blecua.
Bera-bera, 78, 2º. 20009 San Sebastián. Spain.
E-mail: mcallen@apge.osakidetza.net