High-flow nasal cannula (HFNC) is a safe and effective treatment in bronchiolitis in paediatric wards. The optimal flow on starting HFNC is still unknown. The main aim of this study was to determine if there were differences in clinical outcome of patients according the initial flow.
MethodsA prospective, observational and analytical study was conducted between 2014 and 2016 on infants admitted with bronchiolitis and who required HFNC. Two cohorts were established according to the initial flow: cohort 1: flow 15L/min (HFNC-15), and cohort 2: flow 10L/min (HFNC-10). Treatment failure was defined as the presentation of apnoea or the absence of clinical improvement in the first 12–24h. Multivariate probabilistic models were built to identify predictive variables of treatment failure.
ResultsA total of 57 patients were included. The median age was 4 months (IQR 2–13), and 54% received treatment with HFNC-10 and 46% with HFNC-15. In HFNC-15 cohort, respiratory rate (RR) decreased in the first hour, and in the HFNC-10 cohort in the first 6h (P=.03). In HFNC-10 cohort, treatment failure rate was 71%, compared to 15% of HFNC-15 (P<.01). Admission to PICU was required in 35% of the HFNC-10 group vs 18% in HFNC-15 (P=.11). No adverse effects were found.
ConclusionsThe use of HFNC 15L/min in bronchiolitis treatment in paediatric wards is safe and effective, achieves a faster improvement of respiratory rate and has a lower treatment failure rate.
La oxigenoterapia de alto flujo (OAF) es un tratamiento seguro y eficaz de la bronquiolitis en las plantas de hospitalización. Se desconoce cuál es el flujo óptimo para iniciar esta terapia. Nuestro objetivo es analizar si hay diferencias en la evolución de los pacientes según el flujo inicial empleado.
MétodosDurante el periodo 2014-2016 se realizó un estudio clínico observacional y analítico de cohortes prospectivas en lactantes ingresados por bronquiolitis que precisaron OAF. Se establecieron dos cohortes en función del flujo inicial: cohorte 1: flujo 15l/min (OAF-15); cohorte 2: flujo 10l/min (OAF-10). El fracaso terapéutico se definió como la presentación de pausas de apnea o a la ausencia de mejoría clínica en las siguientes 12-24h. Se construyeron modelos probabilísticos multivariantes para identificar variables predictivas de fracaso terapéutico.
ResultadosSe incluyeron 57 pacientes. Mediana de edad, 4 meses (RIQ 2-13). Recibieron tratamiento con OAF-10 el 54% y con OAF-15 el 46%. En la cohorte OAF-15 la frecuencia respiratoria empezó a disminuir en la primera hora y en la cohorte OAF-10 a partir de las 6 primeras horas (p=0,03). En la cohorte OAF-10 ocurrió fracaso terapéutico en el 71%, frente al 15% de la OAF-15 (p<0,01). Precisaron ingreso en la UCIP el 35%, en la cohorte OAF-10 vs el 18% en la OAF-15 (p=0,11). No se encontraron efectos adversos en ninguna de las cohortes.
ConclusionesLa OAF a 15l/min en el tratamiento de la bronquiolitis es segura y eficaz, consigue una mejoría precoz de la frecuencia respiratoria y tiene un menor porcentaje de fracaso terapéutico.
Bronchiolitis is the leading cause of hospital admission in infants aged less than 1 year. Approximately 10–15% of hospitalised patients eventually require admission to paediatric intensive care units (PICUs). Supportive care is an essential part in the management of patients with bronchiolitis, including maintenance of appropriate nutrition and hydration and an adequate oxygen saturation.1 In this context, high-flow oxygen therapy delivered through nasal cannula (HFNC) was a modality initially used in the PICU setting that, with the development of inpatient paediatrics as a specialty, has started to be used in paediatric wards with positive outcomes. At present, HFNC is considered a safe and effective therapy for the management of bronchiolitis in inpatient wards.2
High-flow oxygen therapy acts by delivering an increased volume of air and oxygen to the airways using a higher flow of humidified and heated gas, which increases the inspiratory flow and delivers oxygen more efficiently to the alveoli, thus decreasing the work of breathing and improving gas exchange.3–5 Although there are standardised protocols for its administration, some aspects continue to be debated, such as the optimal initial flow of oxygen or the best timing for initiating HFNC.
There is considerable variability in the flow rate applied at initiation of HFNC: in most studies, it has been determined based on body weight, but other authors report its determination based on clinical severity or the judgment of the clinician.6 Some studies suggest that a flow rate of 2L/kg/min can achieve a pharyngeal pressure of 4cmH2O or greater, but the maximum flow that can be used in the management of infants with HFNC has not been established.5 After 7 years of clinical experience using this treatment in the paediatric ward, we consider that many infants and toddlers could benefit from the use of higher flow rates from the beginning of treatment.
The aim of our study was to assess the safety of HFNC for management of bronchiolitis in the paediatric ward and to analyse whether different initial flow rates (10 vs 15L/min) were associated with differences in the effectiveness of HFNC in terms of clinical severity and the probability of treatment failure.
Patients and methodsDuring the 2014–2016 period, we performed a prospective observational and analytical clinical cohort study in children aged less than 24 months hospitalised due to bronchiolitis who required HFNC. The setting of the study was a tertiary hospital with a general paediatrics ward with 38 beds that manages 1750 admissions a year. We defined bronchiolitis as a first or second acute episode of wheezing preceded by manifestations of viral respiratory illness in children aged less than 2 years.7 Bronchiolitis was the leading cause of admission in this age group during the period under study.
We used consecutive sampling to include patients aged 1–24 months admitted with bronchiolitis to the paediatric ward that required HFNC and for whose participation we obtained a signed written consent from the parents. We excluded newborns, children admitted directly to the PICU, children in whom HFNC was prescribed in the emergency department of our hospital and were admitted with the aforementioned inclusion criteria, children in whom HFNC was initiated at a different hospital, and children in whom HFNC was contraindicated due to radiographic evidence of pneumothorax, facial malformations or a tracheostomy tube.
Patients admitted to the paediatric ward were evaluated by general hospital paediatricians, and if HFNC was indicated, were included in the HFNC protocol. We assessed the severity of illness by means of the Wood-Downes-Ferres (WDF) score8 (Table 1). The criteria for initiation of HFNC in the paediatric ward were: worsening of breathing difficulty as established by an increase in the WDF score to 8 points or higher, a decrease in oxygen saturation (SatO2) to less than 92% despite use of conventional oxygen therapy, or the presence of 2 or more apnoeic pauses per hour.
Wood-Downes clinical score modified by Ferres.
| Points | Wheezing | Retractions | RR (bpm) | HR (bpm) | Breath sounds | Cyanosis |
|---|---|---|---|---|---|---|
| 0 | None | None | <30 | <120 | Normal and symmetrical | No |
| 1 | End expiration | Subcostal | 31–45 | >120 | Regular and symmetrical | Yes |
| 2 | Entire expiratory phase | 1+intercostal | 46–60 | Markedly reduced | ||
| 3 | Inspiration and expiration | 2+nasal flaring | Silent thorax |
Bronchiolitis severity: mild, 1–3 points; moderate, 4–7 points; severe, 8–14 points.
HR, heart rate; RR, respiratory rate.
In our usual clinical practice, some patients start HFNC with a flow rate of 10L/min and others with a flow rate of 15L/min, depending on the prescribing physician, so patients were assigned to one of 2 initial flow rate groups based on the judgment of the physician that was in charge at the ward at the time of prescription. In both groups, the fraction of inspired oxygen (FiO2) was titrated to achieve a SatO2 of 92% or greater with humidified air at a temperature of 35–37°C. Thus, 2 groups were defined for analysis based on the initial flow rate: the HFNC-15 group, with an initial flow of 15L/min, and the HFNC-10 group, with an initial flow of 10L/min. High-flow oxygen therapy was delivered with the Fisher & Paykel® MR850® humidification system and Fisher & Paykel Optiflow Junior nasal cannulas in infant size (OPT-316).
We defined treatment failure as:
- -
Presence of apnoeic pauses after initiation of HFNC, or
- -
Increase in RR, HR or WDF score or decrease in SatO2 in the first 12–24h, or
- -
Plateau in RR, HR, SatO2 or WDF score values (or improvement by less than 15% the baseline value).
We collected clinical and epidemiological data, including age, weight, gestational age at birth, personal medical history and results of the rapid respiratory syncytial virus (RSV) test. We designed a data collection form for the documentation during treatment with HFNC of the following variables: body temperature, respiratory rate (RR), heart rate (HR) and SatO2. These parameters were recorded at the time HFNC was prescribed, at 1, 3, 6, 12 and 24h of treatment, and thereafter once a day until treatment was discontinued. The physicians conducting the study administered the WDF scale on a daily basis. We collected data on all associated treatments, admission to the PICU, complications and tolerability of HFNC.
We performed the statistical analyses with the statistics software SPSS version 21.0 (IBM SPSS Statistics; Armonk, NY, USA) and GraphPad Prism version 7 (La Jolla, CA, USA). We have expressed quantitative variables as median and interquartile range, and qualitative variables as percentages. In the comparative analysis, we used the Fisher test to compare qualitative variables, and for quantitative variables we used the Mann–Whitney U and the Kruskal–Wallis tests for unpaired samples and the Friedman test for paired samples. To analyse the time elapsed to treatment failure, we used Kaplan–Meier survival curves and the Mantel–Cox test.
To analyse the factors that could have an influence on treatment failure and admission to the PICU, we fit 2 logistic regression models where we included all variables that were statistically significant in previous analysis or that are clinically relevant, including age, underlying disease, weight, WDF score and initial flow (HFNC-10 vs HFNC-15).
We present the results of the logistic regression analysis in terms of odds ratios (OR) and 95% confidence intervals (CIs). To select and compare regression models, we used the Akaike informative criterion (AIC) and the Bayesian information criterion (BIC). We chose the model with the lowest AIC and BIC values, which are indicative of a better fit. We ruled out the possibility of colinearity in the models. Statistical significance was defined as a P-value of less than .05. The study was approved by the Clinical Research Ethics Committee of the Hospital Gregorio Marañón (File 57/18).
ResultsGeneral resultsDuring the period under study, the paediatric ward admitted 481 patients with bronchiolitis, of whom 57 met the inclusion criteria. The median age of the sample was 4 months, and 50% had bronchiolitis caused by RSV. Table 2 summarises the baseline characteristics of the patients in the sample.
Baseline characteristics and clinical outcomes in patients, overall and by initial HFNC flow rate group.
| All (n=57) | HFNC-10 (n=31) | HFNC-15 (n=26) | P | |
|---|---|---|---|---|
| Age (months) | 4 (2–13) | 4 (2–10) | 8 (2.2–14) | .41 |
| Severity score | 9 (8.2–9) | 9 (9–9) | 9 (8–9) | .87 |
| Sex (% male) | 56 | 58 | 54 | .79 |
| Underlying disease (%) | 21 | 19 | 22 | .75 |
| Preterm birth (%) | 14 | 16 | 12 | .72 |
| Weight (kg) | 7.2 (5.3–9.3) | 6.5 (5.0–9.4) | 7.4 (5.4–9.4) | .75 |
| Pathological X-ray findings (%) | 60 | 88 | 63 | .07 |
| Days of HFNC | 4 (3–6) | 4 (3–6) | 5 (3–6) | .47 |
| Mean LOS | 8 (5–9) | 8 (5–11) | 8 (5–9) | .90 |
| Admission to PICU | 21% | 35% | 18% | .11 |
| Treatment failure | 46% | 71% | 15.3% | <.01 |
HFNC, high-flow oxygen therapy with nasal cannula; LOS, length of stay; PICU, paediatric intensive care unit.
Values expressed as median and interquartile range or percentages.
Continuous variables (age, severity score, mean LOS, days of HFNC and weight) compared with Mann–Whitney U test. Categorical variables (sex, presence of underlying disease, pathological X-ray findings, preterm birth, admission to PICU and treatment failure) compared with the Fisher exact test.
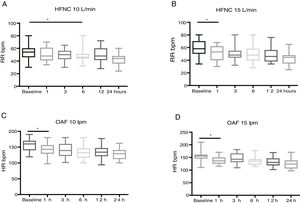
High-flow oxygen therapy in patients with bronchiolitis achieved significant decreases in HR, RR and the WDF score in the first hours of treatment (Fig. 1).
Twelve patients (21%) required admission to the PICU, and the types of respiratory support they received in this unit were continued HFNC in 25%,3 non-invasive ventilation in 67%8 (CPAP/BiPAP), and invasive mechanical ventilation in 8%1 (Fig. 2).
Assessment of safetyAll patients tolerated HFNC well. None required sedation. We did not observe any complications associated with the use of HFNC, such as pneumothorax, epistaxis or pressure ulcers in the nose.
Group comparisonWe conducted a comparative analysis of the patients grouped according to the initial flow rate used for HFNC; 54% of patients (n=31) started treatment with 10L/min (HFNC-10 group) and 46% (n=26) with 15L/min (HFNC-15 group). Both groups were comparable in terms of age, sex, underlying disease, weight, WDF score, additional treatments used and the prevalence of a viral aetiology (Table 2). Table 2 summarises the comparison of the outcomes in both cohorts.
In the HFNC-15 group, the RR started to decrease significantly starting at 1h of treatment (P=.01), compared to 6h in the HFNC-10 group (P=.03) (Table 3).
Changes in respiratory rate and heart rate in the hours following initiation of high-flow oxygen therapy by flow rate used.
| Time (h) | RR (HFNC-10 group) | P | RR (HFNC-15 group) | P | HR (HFNC-10 group) | P | HR (HFNC-15 group) | P |
|---|---|---|---|---|---|---|---|---|
| 0 | 51 (46–58) | 58 (50–70) | 160 (140–170) | 155 (145–161) | ||||
| 1 | 49 (44–62) | .93 | 53 (40–62) | .01 | 143 (129–156) | <.01 | 136 (125–149) | <.01 |
| 3 | 50 (43–56) | .37 | 48 (44–54) | .04 | 139 (122–160) | <.01 | 142 (131–162) | .04 |
| 6 | 43 (40–50) | .03 | 48 (39–58) | .04 | 132 (115–146) | <.01 | 135 (121–143) | .01 |
| 12 | 40 (38–52) | .15 | 46 (38–56) | <.01 | 134 (121–147) | <.01 | 130 (118–146) | <.01 |
| 24 | 41 (34–46) | .03 | 44 (34–48) | <.01 | 129 (112–140) | <.01 | 123 (106–137) | <.01 |
HFNC, high-flow oxygen therapy with nasal cannula; HR, heart rate; RR, respiratory rate.
Values expressed as median and interquartile range.
We analysed compared RR and HR values by means of the Mann–Whitney U test.
In the HFNC-10 group, overall decrease in RR in the first 3h was of 2% (from 51 to 50bpm) and in the first 24h of 19% (from 51 to 41bpm), while in the HFNC-15 group the overall decrease was of 18% in the first 3h (from 58 to 48bpm) and 24% in the first 24h (from 58 to 44bpm). Therefore, the reduction in RR was significantly quicker and greater in the HFNC-15 group compared to the HFNC-10 group (Fig. 3).
When it came to the HR, we observed a significant decrease in both groups from the first hour of HFNC (P<.01) (Table 2 and Fig. 3), and the same was the case of the WDF score, which improved significantly in both groups in the first 24h of treatment (P<.01).
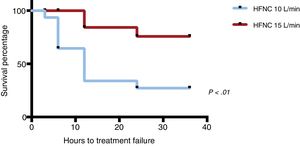
When it came to treatment failure, the proportion was of 71% in the HFNC-10 group compared to 15% in the HFNC-15 group (P<.01) (Fig. 2). Overall, treatment failure in most patients happened within 12–24h from initiation. In the HFNC-10 group it occurred earlier, 6–12h after initiating HFNC. The Kaplan–Meier survival analysis showed that patients in the HFNC-10 group that experienced treatment failure did so before patients in the HFNC-15 group (P<.01) (Fig. 3). The 1 patient that required intubation belonged to the HFNC-10 group.
In the HFNC-10 group (n=31), HFNC was effective in 9 patients (29%), who did not require increases in flow rate at any point and completed the treatment in the paediatric ward, but it failed in 22 patients (71%). Of the patients in whom treatment failed in the HFNC-10 group, 14 (64%) required an increase in flow rate to 15L/min due to lack of improvement in the first few hours of treatment, but continued to be managed in the paediatric ward, while 8 (36%) required admission to the PICU (Fig. 2).
In the HFNC-10 group, treatment failure involved the development of apnoeic pauses in 3 patients (13.5%), increases in RR or HR or decreases in SatO2 in 73% (16/22 patients) or an insufficient decrease in RR or HR or insufficient increase in SatO2 in 13.5% (3 patients). The 8 patients that required admission to the PICU were the 3 that experienced apnoeic pauses and 5 of the patients that exhibited worsening of severity parameters despite delivery of HFNC (Fig. 2).
In the HFNC-15 group (n=26), treatment was effective in 85% of the patients, who completed the treatment in the paediatric ward, while only 4 patients (15%) required transfer to PICU due to the fact that the maximum flow allowed at the ward level was 15L/min (Fig. 2). In the HFNC-15 group, treatment failure in all cases was due to worsening of the RR, HR or SatO2 within 24h from initiation, and in no case did patients experience apnoeic pauses during their care in the ward.
Multivariate analysisIn the first logistic regression model, treatment failure was the dependent variable, and we found that younger age (odds ratio [OR]=0.84 (95% CI: 0.75–0.95), P<.01) and membership in the HFNC-10 group at treatment initiation (OR=26.80 (95% CI: 4.83–150), P<.01) were the 2 independent variables that predicted treatment failure (Table 4).
Multivariate analysis.
| Predictor | Treatment failure*, OR (95% CI) | P | PICU**, OR (95% CI) | P |
|---|---|---|---|---|
| Age | 0.84 (0.75–0.95) | <.01 | 0.82 (0.68–0.99) | .04 |
| Severity score | 3.20 (0.84–13.7) | .09 | 2.08 (0.72–6.00) | .17 |
| Underlying disease | 0.32 (0.03–2.77) | .30 | 0.46 (0.05–3.65) | .46 |
| HFNC initial flow (10/15L/min) | 26.8 (4.83–150) | <.01 | 2.22 (0.46–10.1) | .31 |
| Weight (kg) | 0.86 (0.60–1.23) | .42 | 0.92 (0.61–1.36) | .67 |
AIC, Akaike informative criterion; BIC, Bayesian informative criterion; CI, confidence interval; HFNC, high-flow oxygen therapy with nasal cannula; OR, odds ratio; PICU, paediatric intensive care unit.
We analysed age, weight and severity score as continuous variables and the presence or absence of underlying disease and the initial flow rate as dichotomous variables. The treatment failure and PICU admission dependent variables were dichotomous (logistic regression). Significant results presented in boldface. The asterisks (* and **) indicate the goodness of fit of each model.
In the second model, the dependent variable was admission to the PICU. In this model, we found that age was the sole independent variable that accounted for the need for PICU admission in our sample (OR=0.82; 95% CI, 0.68–0.99; P=.04) (Table 4).
Table 4 presents the values of the Nagelkerke R2, the AIC and the BIC for these models.
DiscussionThe main objective of this study was to identify the optimal initial flow rate for HFNC for management of bronchiolitis in the paediatric ward, and to assess the safety of this oxygen therapy modality in this setting. First of all, we confirmed that the use of HFNC for bronchiolitis in the paediatric ward is an effective measure that improves severity parameters in the first hours of treatment and is also safe, as we observed no relevant side effects; our findings also suggest that an initial flow rate of 15L/min is associated with a more rapid improvement of the RR and a lower proportion of treatment failure compared to an initial flow rate of 10L/min (Table 4).
A growing number of articles confirm the benefits of HFNC in the paediatric ward setting for management of different respiratory illnesses, such as bronchiolitis, asthma or laryngitis, and respiratory failure from other causes.2,9–13 However, some aspects are still under debate, such as the optimal initial flow rate or the best time to initiate treatment, which is evinced by the wide variability found in the HFNC protocols.8
Due to the lack of consensus on the calculation of the initial flow rate, some authors calculate it based on clinical criteria such as age, weight or a clinical severity score. Some studies have used flows between 2 and 9L/min that were subsequently adjusted based on the work of breathing and SatO2. In many studies, the initial flow was calculated based on body weight, usually for delivery of 2L/kg/min, which appears to achieve adequate alveolar recruitment and reduce work of breathing,8,14–21 to a maximum of 8–12L/min, and some studies have applied initial flows of 1–3L/kg/min but to a maximum of 8L/min, and found no adverse events.17 Most of these studies, some of which were conducted in paediatric wards, used a maximum flow rate of 10L/min, and found this treatment to be safe.8,15,22–24 We did not find any studies in the literature reporting the use of 15L/min in the paediatric ward, although some authors have suggested that flow rates higher than those usually applied (10L/min) could be useful.24
In our study, in patients treated from the beginning with 15L/min, the RR started to decrease significantly from the first hour of treatment, whereas in the group of children initially treated with 10L/min this decrease happened from hour 6 of treatment, which delayed improvement in the patient. Furthermore, the decrease in RR was greater in the HFNC-15 group compared to the HFNC-10 group. We observed significant improvements in HR and the WDF score, with no difference between groups. We ought to underscore that we did not observe significant side effects in either group, and thus our findings suggest that initiating HFNC with a flow rate of 15L/min in children aged less than 2 years with bronchiolitis (with the exception of newborns, and, until data from clinical trials become available, with caution until the patient's weight reaches 5–7kg) would lead to quicker improvement and a higher probability of success.
In some studies conducted in the PICU setting, up to 25% of patients treated with HFNC experienced treatment failure and required a higher level of respiratory care.16,25 The age and weight of the patient are factors currently debated in the literature, as some authors have found an association with treatment failure and others have not.26 Different authors have analysed the association with treatment failure of other clinical factors, such as the SatO2, pCO2 and severity score, with inconclusive results.26–28 In our study, neither the WDF score nor the body weight of patients were associated with treatment failure, but age was.
In our study, treatment failure occurred in the first 12–14h, and in the HFNC-10 group it failed within the first 12h in as many as 50% of the patients. Other authors have reported treatment failure in the first 6–12h,15,26,29 which suggests that optimising treatment in the first few hours can have a direct impact on the effectiveness of this approach.
There were a series of limitations in our study. It was a pilot study conducted in a single centre with a relatively small sample. It was not a randomised clinical trial, and patients were assigned to groups based on the usual practices of the clinician in charge of their case.
Another limitation was that we did not monitor the partial pressure of carbon dioxide. Nevertheless, we obtained relevant data that suggest that it would be worth it to conduct a multicentric, randomised, blind clinical trial to confirm the findings of this study.
In conclusion, the use of HFNC for management of bronchiolitis in the paediatric ward setting was safe and effective, and our findings suggest that initiating treatment with a high-flow rate of 15L/min achieves a quicker decrease in clinical severity parameters and is associated with a lower frequency of treatment failure.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the nursing staff and the patients and their families.
Please cite this article as: González Martínez F, González Sánchez MI, Pérez-Moreno J, Toledo del Castillo B, Rodríguez Fernández R. ¿Cuál es el flujo inicial idóneo en la oxigenoterapia de alto flujo para el tratamiento de la bronquiolitis en las plantas de hospitalización? An Pediatr (Barc). 2019;91:112–119.