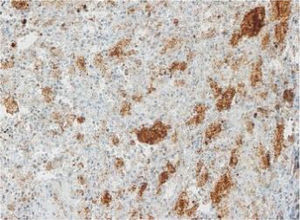
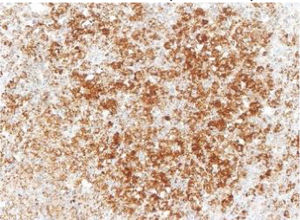
Langerhans cell histiocytosis (LCH) is a disease with heterogeneous manifestations whose immune or neoplastic origin has not been clearly established. Somatic changes in the BRAF proto-oncogene (BRAFV600E variant) are found in more than 50% of LCH lesions. Our aim was to validate the use of immunohistochemistry with the VE1 monoclonal antibody as a simpler and faster approach than molecular techniques for the diagnosis of BRAFV600E-positive LCH.
Between 1976 and 2015, 91 patients underwent evaluation, with a mean age at diagnosis of 4 years (range, 0.08–17). Twenty-five percent had single-system forms of disease (SS-LCH), most frequently involving skin or bone, and 70% multisystemic forms of disease (MS-LCH). The median duration was 4 years, with an overall survival of 94% (95% CI, 91–97). We obtained biobank samples for 13 patients applying the following criteria: (1) Diagnosis of LCH based on the morphological analysis of the haematoxylin-eosin stain and immunohistochemical staining for proteins S100 and CD1a; (2) availability of histological sections in paraffin, and (3) exclusion of bone tissue samples to avoid distortion due to immunohistochemical expression secondary to the decalcification with nitric acid required for the processing of these samples. We did not find significant differences in the clinical characteristics of this subset of patients compared to the total group of patients under consideration, so we considered the sample representative. The presence of BRAFV600E was assessed by quantitative polymerase chain reaction (PCR) (BRAF V600 Mutation Test; Roche®) and with a BRAFV600E immunohistochemistry test (VE1 antibody, VENTANA® Medical Systems). The BRAFV600E immunoreactivity was classified with a semiquantitative scale as negative (−), weak (+), moderate (++) or strong (+++). Samples with strong staining were considered positive.1,2
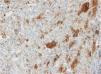
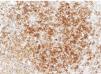
Among the 13 samples under study, BRAFV600E was detected in 6 (46%). The concordance between the diagnosis made by molecular techniques and by immunohistochemistry was 100%, with detection of BRAFV600E by PCR in every sample with strong immunoreactivity (+++) (Table 1).
Clinical characteristics and BRAFV600E results obtained through immunohistochemistry with the VEI antibody (VENTANA® Medical Systems) and real-time PCR (cobas® test, Roche).
| Age at diagnosis | Disease form | Tissue | VE1 IHC | BRAF V600E PCR | Total sample: IHC/PCR | |
|---|---|---|---|---|---|---|
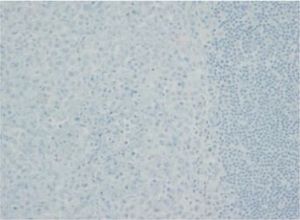
| 1 | 2 years | MS-LCH | Lymph node | Negative | Unmutated | 2 negative (15.4%)/Zero mutated (0%) |
| 2 | 1 month | SS-LCH | Lung | Negative | Unmutated | |
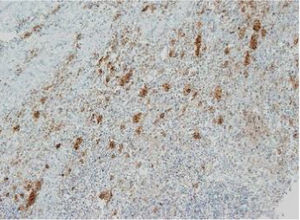
| 3 | 2 months | SS-LCH | Skin | + | Unmutated | 4 weak (30.8%)/Zero mutated (0%) |
| 4 | 1 year | MS-LCH | Skin | + | Unmutated | |
| 5 | 7 months | MS-LCH | Lymph node | + | Unmutated | |
| 6 | 4 years | SS-LCH | Muscle | + | Unmutated | |
| 7 | 8 years | SS-LCH | Soft tissues | ++ | Unmutated | 1 moderate (7.2%)/Zero mutated (0%) |
| 8 | 4 years | SS-LCH | Soft tissues | +++ | Mutated | 6 strong (46.2%)/6 mutated (46.2%) |
| 9 | 13 years | SS-LCH | Orbit | +++ | Mutated | |
| 10 | 1 year | SS-LCH | Skin | +++ | Mutated | |
| 11 | 10 months | MS-LCH | Ear | +++ | Mutated | |
| 12 | 1 month | MS-LCH | Gum | +++ | Mutated | |
| 13 | 1 month | MS-LCH | Skin | +++ | Mutated |
The images correspond to a representative case in the subsets of patients with negative, weak, moderate or strong immunohistochemical staining.
IHQ: immunohistochemistry; MS-LCH, multisystem Langerhans cell histiocytosis; PCR, polymerase chain reaction; SS-LCH, single-system Langerhans cell histiocytosis.
The results suggest that the BRAFV600E variant plays an important role in approximately half the patients with LCH. These genetic changes support the conceptualization of BRAFV600E-positive LCH as a neoplastic disease, given the evidence of clonality in the lesions.3,4 Other authors consider that in the absence of this variant, the basis of LCH could be a process of immune dysregulation causing inflammation.5
The importance of the detection of BRAFV600E in LCH resides in its potential as a therapeutic target, opening up new possibilities for treatment in refractory patients.6 Furthermore, we propose the use of VE1 immunohistochemistry as an alternative to molecular methods for detection of the BRAFV600E variant, as it is both reliable and easy to perform and interpret.
FundingThis study was partly funded by the Fundación CRIS contra el cáncer (https://criscancer.org/es/). The Fundación CRIS contra el cáncer was not involved in the design or implementation of the study or the publication of the manuscript.