The Childhood Asthma Control Test (c-ACT) is a validated tool for determining pediatric asthma control. However, it is not validated in Spanish in Spain. We evaluated the psychometric properties of the Spanish version of the Childhood Asthma Control Test (Sc-ACT) for assessing asthma control in children ages 4–11.
MethodsThis national, multicentre, prospective study was conducted in Spain with asthmatic children and their caregivers. Patients were assessed at 3 visits (baseline, 2 weeks, and 4 months). Clinical variables included: symptoms, exacerbations, FEV1, asthma classification, PAQLQ and PACQLQ questionnaire scores, and asthma control as perceived by physicians, patients and caregivers. The Sc-ACT feasibility, validity, reliability, and sensitivity to change were assessed.
ResultsA total of 394 children were included; mean (SD) time to complete the Sc-ACT was 5.3 (4.4) min. Sc-ACT score was correlated with asthma control as perceived by physician (−0.52), patient (−0.53), and caregiver (−0.51) and with the PAQLQ (0.56) and PACQLQ (0.55) scores. Sc-ACT was found to be significantly related to intensity and frequency of asthma symptoms. Cronbach alpha coefficient (CCα) was 0.81 and intraclass correlation coefficient (ICC) was ≥0.85 for all of the items. The global effect size of Sc-ACT was 0.55. The cutoff point scores of 21 or higher indicated a good asthma control and their MCID was 4 points.
ConclusionThe Spanish version of the c-ACT was found to be a reliable and valid questionnaire for evaluating asthma control in Spanish-speaking children ages 4–11 in Spain.
El cuestionario ACT pediátrico es una herramienta validada para determinar el grado de control del asma. Sin embargo, no está valida en español para España, motivo de evaluar las propiedades psicométricas de la versión en español del cuestionario ACT pediátrico, dirigido a conocer el grado de control del asma en niños de 4 a 11 años de edad.
MétodosEstudio nacional, prospectivo, multicéntrico, desarrollado en España en niños asmáticos y en sus padres. Los pacientes fueron evaluados en 3 visitas (basal, a las 2 semanas y a los 4 meses). Variables clínicas relacionadas: síntomas, exacerbaciones, FEV1, clasificación del asma, escalas de los cuestionarios PAQLQ y PACQLQ y control del asma percibido por el paciente, sus padres y por su médico. Se evaluaron la factibilidad, validez, fiabilidad y sensibilidad del cuestionario ACT.
ResultadosCohorte constituida por 394 niños. La duración media y la DS para completar el cuestionario fue 5,3 (4,4) minutos. La puntuación se correlacionó con el grado de control del asma percibido por su médico (-0,52), por el niño (-0,53) y por sus padres (-0,51), y con las puntuaciones de los cuestionarios PAQLQ (0,56) y PACQLQ (0,55). Se ha observado una estrecha asociación de la puntuación del cuestionario con la intensidad y la frecuencia de los síntomas relacionados con asma. Para todos los items, coeficiente alfa-Cronbach 0,81 y coeficiente de correlación intraclase ≥0,85. El punto de corte de 21 o más indican un muy buen control del asma y su MCID fue de 4 puntos.
ConclusiónLa versión en español del cuestionario ACP pediátrico es fidedigno y válido para evaluar en control del asma en España, en niños de 4 a 11 años de edad.
Achieving and maintaining asthma control is the current goal of therapy. This represents a shift away from using asthma severity assessments to determine the level of asthma control.1 International asthma guidelines consider asthma control in 2 dimensions—impairment and risk. Impairment is defined as the frequency and intensity of symptoms and the functional limitations the patient experiences. Risk is defined as the likelihood of either asthma exacerbations, progressive decline in lung function (for children, reduced lung growth), or risk of adverse effects from medication. Both impairment and risk are valid considerations, which may respond differently to therapy. To evaluate impairment, international guidelines commonly suggest using standardized tools to evaluate the patients’ perception of their asthma control and its effect on daily function. Hence, investigators have developed several assessment tools; commonly used ones include: the Asthma Control Questionnaire (ACQ),2 the Asthma Control Test (ACT),3 ACT Spanish Version,4 the Asthma Therapy Assessment Questionnaire (ATAQ),5 the Asthma Control Score System (ACSS),5 and the Asthma Symptom Utility Index.6 Initially, these assessment tools were developed for use in adults. Use in children requires modifications and currently validated pediatric versions exist for ACQ,7 ATAQ,5 CAN (Control de Asma en Niños),8 and ACT.9 The CAN is a 4-min, self-administered questionnaire evaluating asthma symptoms occurring during the last 4 weeks. This tool is validated in Spanish and has 2 versions: (1) children age 9–14 years, self-administered and (2) children age 2–8, administered by parents/tutors. A pediatric version of ACT, the Childhood Asthma Control Test (c–ACT), was developed to assess asthma control in children between ages 4 and 11.9 The c-ACT is validated and is one of the most commonly used questionnaires worldwide.10 The c-ACT has been validated in various languages in several countries11–15; however; it has not yet been validated in Spanish in Spain.
Over 500 million people worldwide speak Spanish making a validated tool essential.16 Moreover, according to ISAAC (International Study of Asthma and Allergies in Childhood) report, 2 out of 4 countries with the highest prevalence of asthma in children (Costa Rica, 37.6%; Panama, 22.7%) around the world are Latin-American countries where Spanish is the native language. Another Spanish speaking country, Chile, also has a high prevalence of asthma (17.9%).17
We sought to validate the Spanish childhood-ACT (Sc-ACT) in a Spanish speaking population in Spain in order to provide physicians with a tool to more accurately assess childhood asthma control.
Materials and methodsThis prospective, multicenter, assessment-tool validation study was performed in Spain with asthmatic children ages 4–11. Approximately 40 pediatricians enrolled 10 children each: 5 children between the ages of 4 and 8 and 5 children between the ages of 9 and 11. Each group enrolled 3 children with mild, persistent asthma; 1 child with moderate, persistent asthma; and 1 child with severe, persistent asthma. All children had GINA (Global Initiative for Asthma)-classified asthma, with persistent asthma in the last 6 months.1,18 Patients and parents/tutors were required to understand the questionnaire and be available to return at 2 and 16 weeks after admission and keep the same tutor during the entire study. Exclusion criteria included: other chronic respiratory illness (e.g., cystic fibrosis, bronchopulmonary dysplasia, primary ciliary dyskinesia); cardiorespiratory malformations; acute pneumonia or lower respiratory illness; toxic abuse; mental illness or incapacity (patient or parent/tutor); participation in other clinical trials at the same time of the study or planning to do so in the next 4 months; or inability to read, write, or understand Spanish.
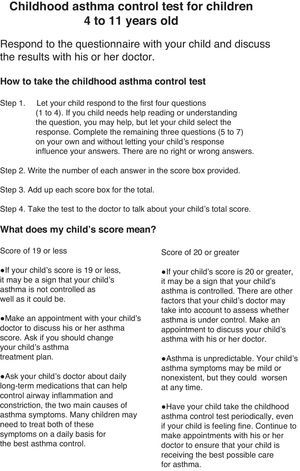
The c-ACT is a 2-part, 7-item questionnaire using a Likert scale that addresses asthma control within the previous 4 weeks. Part 1 is a self-assessment using 4 questions about the child's perception of asthma control, limitation of activities, coughing, and awakening at night. Part 2 is completed by the parent/tutor describing daytime complaints, daytime wheezing, and awakenings at night. The sum of all scores yields the c-ACT score ranging from 0 (poorest asthma control) to 27 (optimal asthma control). A cutoff point of ≤19 indicates uncontrolled asthma. The Spanish translation and linguistic validation of the c-ACT were a forward–backward translation, a method developed by Mapi Research Institute.
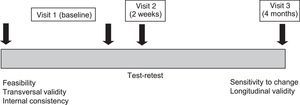
Visit 1 captured demographic information, asthma characteristics, exacerbations, and medication use. Forced spirometry was performed according to ATS/ERS (American Thoracic Society/European Thoracic Society) recommendations.19 Percentage of normality values used the equations proposed by Zapletal.20Table 1 presents the assessment schedule and Fig. 1 presents a timeline of data collection.
Assessment schedule.
| Variablesa | Visit 1 (Baseline) | Visit 2 (2 weeks) | Visit 3 (16 weeks) |
|---|---|---|---|
| Criteria for entrance into the study | √ | ||
| Demographic questionnaire of the patients and their tutors | √ | ||
| Date of asthma diagnosis | √ | ||
| Classification of asthma severity | √ | ||
| Lung function (FEV1) | √ | √ | √ |
| Asthma symptoms | √ | √ | √ |
| Asthma exacerbations | √ | √ | √ |
| Asthma treatment | √ | √ | √ |
| Co-morbidities | √ | √ | |
| Sc-ACT questionnaire | √ | √ | √ |
| PAQLQ and PACQLQ questionnaires | √ | √ | |
| Asthma control perception according to patient (9–11 years old) | √ | ||
| Asthma control perception according to tutor | √ | ||
| Changes in asthma control perception according to patient (9–11 years old) | √ | √ | |
| Changes in asthma control perception according to tutor | √ | √ | |
| Asthma control perception according to pediatrician | √ | ||
| Changes in asthma control perception according to pediatrician | √ | √ | |
| Reasons for withdrawal from the study | √ | ||
Fig. 2 presents the Sc-ACT questionnaire, which was completed on all visits. At Visits 1 and 3 the validated Spanish version of the Pediatric Asthma Quality of life Questionnaire (PAQLQ)21–23 and the Spanish version of the Pediatric Asthma Caregiver's Quality of life Questionnaire (PACQLQ)21,22 were administered.
The study was approved by the hospital's Ethics and Research Committee at Hospital del Niño Jesus (Madrid, Spain); written informed consent and permission were obtained from parents/tutors.
Socio-demographic and clinical characteristics were evaluated by using a mean±standard deviation (SD) or median with interquartile range (IQR) for continuous variables and n (%) for categorical variables. The validation of Sc-ACT was performed by evaluation of psychometric properties using: feasibility, validity, reliability, and sensitivity to changes.
Feasibility was evaluated by using descriptive analysis of the proportion of children who did not answer each question, the proportion of children who did not answer any question, and the mean time required to complete the questionnaire. Answer omission was evaluated from the proportion of patients who answered all questions.
Validity was evaluated transversally by analyzing the relationship between the questionnaire score with the clinical variables (FEV1, asthma symptoms, and exacerbations); with the scores from the PAQLQ and PACQLQ tools; and with asthma control perception by the pediatricians, by the children (9–11 years of age), and by their tutors using either a Pearson correlation or an ANOVA, when appropriate. To evaluate longitudinal validity, we analyzed the relationship between the changes observed in the questionnaire score for the clinical variables during the follow-up period, and the changes in asthma control observed by the pediatrician. These changes were analyzed either with an ANOVA (qualitative variables) or a Pearson correlation (quantitative variables).
Reliability was evaluated by using the internal consistency of the variables within the same score using the Cronbach α coefficient (CCα); we expected a CCα≥0.7. The reliability of test–retest was evaluated by using the questionnaire between Visit 1 and 2 between those children (9–11 years of age) or their tutors (for children 4–8 years of age) who declared no change in asthma control at Visit 2. For the reliability of test–retest, we used interclass correlation coefficient (ICC), considered acceptable if ICC≥0.70.
Sensitivity to change was considered the difference observed between the Sc-ACT questionnaire administrated at Visits 1 and 3, and was analyzed by using Student's t-test calculating the additional effect size (defined as the difference in mean score between Visits 1 and 3 divided by the SD of the score at Visit 1, according to the change observed in asthma control at Visit 3). We calculated the minimum clinically important difference (MCID) based on the mean change observed in the questionnaire scores of those children who, for the question of change in asthma control, chose very good, somewhat good, or not too poor.
The cutoff analysis for the Sc-ACT questionnaire was calculated using different variables. We defined three clinical groups (clinical criteria of asthma control, % of predicted FEV1, and clinical change in treatment) and compared each of them with the global score of the questionnaire. An ANOVA or Kruskal–Wallis test, when appropriate, was used for comparing the scores among groups. The receiver operating characteristics (ROC) curves were used to determine how the different cutoffs affected the sensitivity and specificity of the ACT questionnaire to measure asthma control. The measurement criteria were the dichotomic classification of patients as either having controlled or uncontrolled asthma, by clinical criteria. We classified asthma status as controlled if the pediatrician responded with very good, somewhat good, or not too poor; and as uncontrolled if the pediatrician responded with neither good nor poor, fairly poor, somewhat poor, or very poor. The cutoff selection included the following factors: (1) balance between sensitivity and specificity; (2) greater sensitivity in the identification of patient with uncontrolled asthma; (3) greater area under the curve (ROC) and percentage of patients correctly classified; and (4) clinical validation. The cutoff selected was confirmed by the results obtained as a function of the three clinical groups.
A logistic regression multivariate analysis was performed to evaluate the association of potential factors implied in the asthma control. Since asthma control could be estimated by 3 models (by tutors, by clinical approach, or by Sc-ACT), we performed one multivariate analysis for each model where the dependent variable was asthma control.
The data analysis was performed using the statistical package SAS v. 9.1.3. A p-value of <0.05 was considered significant.
A sample size of 420 children was required to evaluate the psychometric properties of the questionnaire and to evaluate the sensitivity of a change in the questionnaire from Visit 1 to Visit 3 with a SD of 0.15, a significance level of 0.05, a statistical power of 0.80 assuming that 15% of the patients would be lost to follow-up.
ResultsBaseline demographic and clinical dataWe recruited 394 children; 382 were evaluated. The baseline demographic and clinical characteristics of the children are summarized in Table 2. The mean age was 7.9±2.4 years old (57% male); 187 (49%) had mild persistent asthma, 149 (39%) had moderate persistent asthma, and 46 (12%) has severe persistent asthma. The overall mean time since asthma diagnosis was 4.2±2.9 years. Almost half of the children (84, 48%) were taking inhaled corticoids, these children were mainly receiving budesonide alone (118, 64%). Other medications included long-acting ß2-agonists (27, 7%) or a combination of both drugs (113, 30%).
Baseline demographic and clinical characteristics by asthma severity.
| Total(N=382) | Mild persistent(N=187) | Moderate persistent(N=149) | Severe persistent(N=46) | |
|---|---|---|---|---|
| Age (years), mean (SD) | 7.9 (2.4) | 7.7 (2.4) | 8.2 (2.4) | 8.0 (2.3) |
| Gender (male), % | 219 (57.3) | 110 (58.8) | 84 (56.4) | 25 (54.3) |
| FEV1(L), mean (SD) | 1.7 (0.5) | 1.7 (0.5) | 1.7 (0.5) | 1.4 (0.5) |
| Presence of symptomsan (%) | ||||
| Coughing | 272 (71.2%) | 122 (65.2%) | 110 (73.8%) | 40 (87.0%) |
| Wheezing | 163 (42.8%) | 66 (35.3%) | 71 (48.0%) | 26 (56.5%) |
| Shortness of breath | 172 (45.0%) | 63 (33.7%) | 79 (53.0%) | 30 (65.2%) |
| Exacerbations | 172 (45.9%) | 75 (41.0%) | 68 (46.3%) | 29 (64.4%) |
FEV1=forced expiratory volume in the first second.
A total of 380 (99.7%) children and their caregivers completed all the items of the Sc-ACT in a mean time of 5.3±4.4min.
ValidityThe analysis of the concurrent validity showed that the Sc-ACT score was correlated with the perception of asthma control by the physician, the patient, and the caregiver (−0.52, −0.53, and −0.51, respectively), and correlated with the PAQLQ and PACQLQ scores (0.56 and 0.55, respectively). The correlation results between the Sc-ACT score and the PAQLQ score were similar for the subgroups of children 6–8 years of age (0.46) and 9–11 years of age (0.61). The asthma classification and the number of exacerbations were the only clinical variables well correlated with Sc-ACT score (0.49 and −0.42, respectively). As for the longitudinal validity, only the change in asthma classification showed an acceptable positive correlation (0.41) with the change in Sc-ACT score over time.
ReliabilityThe internal consistency of the questionnaire was good (CCα=0.81). The test–retest reliability showed high ICC estimates (ICC≥0.85 in all the items) (Table 3).
Results of the test–retest reliability of Sc-ACT questionnaire measured between Visits 1 and 2.
| Question number of Sc-ACT questionnaire (Items) | n (ICC) |
|---|---|
| Item 1 | 80 (0.942) |
| Item 2 | 80 (0.885) |
| Item 3 | 80 (0.849) |
| Item 4 | 80 (0.879) |
| Item 5 | 80 (0.896) |
| Item 6 | 80 (0.962) |
| Item 7 | 80 (0.951) |
ICC: intraclass correlation coefficient. n: number of patients/tutors Sc-ACT test–retest reliability was calculated with patients/tutors who reported no variations in asthma control in Visit 2 (no changes in symptoms, exacerbations, treatment and variations in FEV1 <9%).
The effect size for the Sc-ACT scores was moderate (0.55), and the mean change in Sc-ACT score between baseline and Visit 3 was 3.0±5.7 points (18.5±5.3 and 21.5±4.6, respectively). The MCID was 3.8±5.4 points (4 points).
Selection of the cutoff pointsThe selection of a cutoff point was based on a balance between sensitivity and specificity, highest sensitivity for identifying uncontrolled patients, highest AUC and proportion of patients who were classified properly, and clinical validity. Patients were classified as controlled or uncontrolled according to the clinician's criteria, so patients whose specialist rated their asthma control as very good, somewhat good, or not too poor had their asthma controlled (52.1%, n=199, at baseline), and patients in the remaining categories of asthma control (fairly poor, somewhat poor, and very poor) had their asthma uncontrolled (41.6%, n=159 at baseline). The AUC was 0.598. The cutoff point scores of 20.5–22.5 had the best balance between sensitivity (ranging from 73% to 57.3%) and specificity (ranging from 47.1% to 60.9%).
Regarding the clinical validity, mean Sc-ACT scores were significantly different (p<0.001) among the seven categories created for defining the specialist's perception of asthma control (Table 4). The highest mean score was reported for children with very good control (23.6±3.6) and the lowest mean for children with very bad asthma control (17.3±3.8). Differences in mean Sc-ACT scores were also significant between the specialist-assessed change in therapy (p<0.001), with the highest scores (22.3±4.4) in children with no changes in therapy, and the lowest scores (20.4±4.9) in children with dose reductions. Finally, mean Sc-ACT scores were also significantly different (p<0.001) depending on the FEV1, so children with less than 75% of the FEV1 reference value had the lowest Sc-ACT scores (20.4±4.2), and children with more than 125% of the FEV1 reference value had the highest Sc-ACT mean scores (22.7±3.2) (Table 4).
Comparison of Sc-ACT scores across clinical categories at Visit 3.
| Category | N | Mean Sc-ACT score | SD | p-value | |
|---|---|---|---|---|---|
| % predicted FEV1a | <75% | 18 | 20.4 | 4.2 | <0.001 |
| 75%≥FEV1>90% | 54 | 20.3 | 4.5 | ||
| 90%≥FEV1>110% | 101 | 21.5 | 4.8 | ||
| 110%≥FEV1>125% | 39 | 22.0 | 5.0 | ||
| ≥125% | 45 | 22.7 | 3.2 | ||
| Physician's assessment of asthma control | Very good | 45 | 23.6 | 3.6 | <0.001 |
| Somewhat good | 98 | 22.2 | 4.2 | ||
| Not too poor | 46 | 21.3 | 4.7 | ||
| Neither good nor poor | 22 | 21.1 | 5.0 | ||
| Fairly poor | 69 | 20.7 | 4.8 | ||
| Somewhat poor | 65 | 21.1 | 4.7 | ||
| Very poor | 8 | 17.3 | 3.8 | ||
| Physician's assessed change in child's therapy | Increase dose | 90 | 21.6 | 4.2 | <0.001 |
| No change | 159 | 22.3 | 4.4 | ||
| Decrease dose | 105 | 20.4 | 4.9 | ||
| Asthma severitya | Mild persistent | 183 | 19.8 | 4.4 | <0.001 |
| Moderate persistent | 146 | 18.4 | 5.3 | ||
| Severe persistent | 46 | 14.2 | 6.1 | ||
| Symptom intensitya | |||||
| Coughing | Mild | 172 | 18.8 | 4.9 | <0.001 |
| Severe | 95 | 15.3 | 5.1 | ||
| Wheezing | Mild | 101 | 17.1 | 5.0 | <0.001 |
| Severe | 56 | 13.5 | 4.9 | ||
| Shortness of breath | Mild | 109 | 16.7 | 4.9 | 0.004 |
| Severe | 56 | 14.4 | 5.2 | ||
| Symptom frequency* | |||||
| Coughing | <1/week | 42 | 19.7 | 5.7 | <0.001 |
| ≥1/week | 119 | 18.3 | 4.7 | ||
| Daily | 98 | 15.6 | 5.3 | ||
| Wheezing | <1/week | 36 | 18.5 | 4.6 | <0.001 |
| ≥1/week | 75 | 15.6 | 4.5 | ||
| Daily | 40 | 13.4 | 5.5 | ||
| Shortness of breath | <1/week | 21 | 16.4 | 5.9 | <0.001 |
| ≥1/week | 92 | 16.4 | 4.5 | ||
| Daily | 31 | 12.5 | 5.6 | ||
FEV1=forced expiratory volume in the first second.
Additional analysis showed that children with severe persistent asthma had the lowest mean Sc-ACT scores (14.2±6.1), whereas those with mild persistent asthma had the highest mean scores (19.8±4.4). Accordingly, the more intense and frequent the symptoms the higher the Sc-ACT scores that were obtained (Table 4).
DiscussionThis study validates the Sc-ACT by demonstrating its feasibility, validity, reliability, and sensitivity to change when assessing asthma control in Spanish-speaking children in Spain ages 4–11. Use of the Sc-ACT is highly feasible; 99.7% of children completed all items in the Sc-ACT taking an average time of 5.3min. The Sc-ACT was a reliable, valid, and sensitive tool to measure asthma control in children using a simple patient/caregiver perspective questionnaire, without needing pulmonary function testing.
The Sc-ACT is reliable having a CCα=0.81 and retest reliability ≥0.85. Additionally, we calculated for first time the MCID of Sc-ACT (4 points). To date, no MCID for c-ACT has been reported.24–26
Since there is no gold standard for measuring asthma control in children, the construct validity of the Sc-ACT was assessed by demonstrating appropriate correlations with other measures of asthma control, such as clinical variables and questionnaires.25,27 We expected to find a low or moderate (r≤0.4) correlation between the Sc-ACT and PAQLQ/PACQLQ, indicating that the later measures quality-of-life more than asthma control. A coefficient ranging from 0.4 to 0.7 is considered a measure of good correlation.28 However, we expected that the variables that measured similar concepts to those on PAQLQ/PACQLQ would have a high correlation (r>0.8).
The results of this analysis showed that the Sc-ACT scores not only correlated well with the clinical perception of asthma control (by patient, caregiver, and physician: −0.53, −0.51, and −0.52, respectively), but also with some clinical variables, such as asthma classification of severity (0.49) and number of exacerbations (−0.42), as well as with the asthma-related quality of life (0.56 and 0.55 for PAQLQ and PACQLQ scores, respectively). Additional analyses demonstrated that mean Sc-ACT scores were also related to the intensity of asthma symptoms. As expected, these analyses showed that higher Sc-ACT scores correlated to the worst classification of asthma, higher number of exacerbations, more intense asthma symptoms, and a worse perception of asthma control.
The Sc-ACT tool also showed good performance in discriminating between groups of patients differing in the pediatrician's rating of asthma control, the need for change in a patient's therapy, and the percentage of predicted FEV1. Additionally, the Sc-ACT tool had good reliability and responsiveness, since the tool was internally consistent from one test administration to the next and had a moderate effect size between visits. The reliability of test–retest in the present study was >0.85 for all of the Sc-ACT items. In contrast, Sekerel et al. reported a test–retest reliability of 0.71 in the Turkish version of c-ACT 29; Chen et al. reported a test–retest reliability of 0.537 in the Chinese version of c-ACT.30 In addition to the above mentioned properties, the Sc-ACT questionnaire was completed by most participants (99.7%) in a short amount of time. Therefore it is a feasible and valuable complementary source of information in clinical practice.
Overall, the Sc-ACT questionnaire showed similar properties to the original English version (c-ACT) 27 and to the adult version (ACT).31 Previous studies that validated a Spanish ACT in Uruguayan adults showed a CCα 0.79.32 A study in Mexican children living in California reported a CCα 0.76 for children 6–11 years of age.33 A Chinese version of c-ACT performed in children 4–11 years of age showed a CCα 0.759.27 A Turkish version of c-ACT showed a good CCα for c-ACT item responses (0.82, 0.83, 0.82, 0.82 and 0.80 for first c-ACT to the fifth c-ACT) with a cutoff point of 21 as the highest ROC curve (80.3%); even sensitivities and specificities of the cutoff points of 18–21 were comparable.29 Nonetheless, our results suggest a higher cutoff point than was originally proposed (above 19) by Liu et al. among children in the US.9 In fact, in our study the mean Sc-ACT scores obtained across the categories defined for the specialist assessed child's therapy, and the specialist assessment of asthma control, as well as the balance between the sensitivity and specificity of the scale, suggested that a cutoff point of 21would be adequate. That is in accordance with a recent study performed in children of Mexican descent in California suggesting a c-ACT cutoff point of 22 for children 6–11 years of age.33 In a recent retrospective analysis, Liu et al. reported a second cutoff point of 12 or less on the c-ACT for children with the lowest level of asthma control. Hence, their group has now established a 3-level asthma control classification: score 20 or greater=well controlled; score of 13–19=not well controlled; and score of 12 or less=very poorly controlled.27 We are looking at our population for a cutoff for those with poorly controlled asthma.
The clinical manifestations of asthma vary in frequency and intensity and require a standardized approach to assessment via validated tools.25 However, in addition to having a tool that is easy to use and provides valid results, a minimal level of change in score consistent with real benefit is needed. The MCID is the smallest change in a score on a questionnaire that is clinically meaningful and would lead a clinician to consider a change in treatment.25,26 A search of the medical literature finds no previously reported MCID for the c-ACT. We report a MCID of 3.8 points for the Sc-ACT.
The number of patients able to be recruited was lower than expected, limiting study results. This validation occurred in Spanish-speaking children in Spain; therefore, it may or may not be appropriate for other Spanish-speaking countries due to cultural nuances of Spanish spoken in different countries. Hence, a validation process in each potential country of use needs to be completed.
In conclusion, the Spanish version of the pediatric ACT is a reliable, valid, and sensitive instrument to assess asthma control in patients 4–11 years of age in Spanish-speaking asthmatic children living in Spain, both in a research or clinical setting. A cutoff point of 21 (with a MCID of 4) is adequate.
Author contributionsEGPY, JRVA, and JGG contributed to conception and design of the study. EGPY, JRVA, JGG, and FJHB were involved in analysis and interpretation of data. EGPY and JACR drafted the article. All authors revised the article critically for important intellectual content and approved this version of the manuscript.
Conflict of interestEGPY has consulting arrangements with Abbott and Merck Sharp & Dohme; has received grant support from Abbott, GlaxoSmithKline, and Merck Sharp & Dohme; and is on the speaker bureau for Abbott. JACR has participated as a lecturer and speaker in scientific meetings and courses under the sponsorship of AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, and Sanofi. JRVA has participated as a lecturer and speaker in scientific meetings and courses under the sponsorship of AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, and Novartis. JGG declares to have no conflict of interest. FJHB is a full-time employee of GlaxoSmithKline.
The study was sponsored by GlaxoSmithKline S.A.
The authors would like to acknowledge the role of Nicolás Cobos, M.D., as a member of VESCASI study Steering Committee. Moreover, the authors wish to thank all the investigators who participated in the study. Their names and affiliations are listed in the Appendix. Barbara Rinehart, MS of PRA Health Sciences (RPS) assisted in manuscript preparation.
Alcántara M (Jaén), Aloy G (Barcelona), Antelo C (Madrid), Asensio O (Barcelona), Bandrés F (Pontevedra), Bartolomé M (Burgos), Cabanes L (Madrid), Canseco A (Barcelona), Carrasco M (Huelva), Casanovas JM (Barcelona), Cortell I (Valencia), Corcuera P (San Sebastián), Corzo JL (Málaga), Cubero M (Jaén), Martin A (Sevilla), De Mir I (Barcelona), Echavarri F (Madrid), Edo A (Castellón), Escribano A (Valencia), Ferrés J (Barcelona), García-Hernández G (Madrid), Gartner S (Barcelona), Girón F (Granada), Herrera L (Barcelona), Korta-Murua J (San Sebastián), Larco JI (Madrid), López-Silvarey A (La Coruña), Lozano D (Ciudad Real), Marco N (Alicante), Martín-Mateos A (Barcelona), Martínez-Cañavate AM (Granada), Moya M (Ciudad Real), Navarro M (Sevilla), Oliva C (Tenerife), Pérez-Frías J (Málaga), Plaza AM (Barcelona), Rueda S (Madrid), Salcedo A (Madrid), Sánchez T (Barcelona), Sardón O (San Sebastián), Toral T (Alicante), Torres J (Córdoba), Valverde-Molina J (Los Arcos, Murcia), Vázquez C (Baracaldo), Vela C (Toledo), Velasco R (Toledo).
Please cite this article as: Pérez-Yarza EG, Castro-Rodriguez JA, Villa Asensi JR, Garde Garde J, Hidalgo Bermejo FJ, en representación del Grupo VESCASI. Validación de la versión en español de la prueba de control del asma infantil (sc-act) para su uso en españa. Anales de Pediatría. 2015;83:94–103.