According to World Health Organization estimates, more than 1 million patients aged less than 15 years develop tuberculosis (TB) each year worldwide. In some regions, up to 25% of new TB cases are caused by drug-resistant strains. Although Spain is considered a low-incidence country, several hundred children and adolescents develop TB each year. The importance of paediatric TB has been minimized for years due to the lack of microbiological confirmation in many patients and because these patients are not usually contagious. Nevertheless, in the past 15 years there have been major improvements in the epidemiological reporting of TB in children and adolescents, new immunodiagnostic tests have been developed, molecular methods that allow rapid microbiological diagnosis and detection of variants associated with drug resistance have become available, novel second-line antituberculosis drugs have been discovered, including for paediatric use, and the results of clinical trials have validated shorter courses of treatment for some patients. This document, developed by a group of experts from the Sociedad Española de Infectología Pediátrica and the Sociedad Española de Neumología Pediátrica, updates and complements the previous guidelines for the diagnostic and therapeutic management of children with TB in Spain based on the newly available scientific evidence.
Más de un millón de pacientes menores de 15 años desarrollan tuberculosis (TB) anualmente en el Mundo, según estimaciones de la Organización Mundial de Salud. La TB por cepas resistentes a los fármacos de primera línea alcanza al 25% de los nuevos casos en algunas regiones. Aunque España es considerada un país de baja incidencia, varios centenares de niños y adolescentes enferman de TB cada año. La importancia de la TB pediátrica ha sido minimizada durante años por la dificultad en confirmar el diagnóstico microbiológico y la escasa contagiosidad que asocia. Sin embargo, en los últimos 15 años, se han reportado mejoras relevantes en los informes epidemiológicos de la TB en niños y adolescentes, han surgido nuevos tests inmunodiagnósticos, se dispone de estudios de biología molecular que permiten un diagnóstico microbiológico y una identificación de mutaciones asociadas a resistencia rápidos, han surgido nuevos fármacos antituberculosos de segunda línea, también en pediatría, y se han publicado ensayos clínicos que validan tratamientos acortados en algunos pacientes. Este documento, realizado por un grupo de expertos de la Sociedad Española de Infectología Pediátrica y la Sociedad Española de Neumología Pediátrica, actualiza y complementa las recomendaciones previas para el manejo diagnóstico y terapéutico del niño con TB en España, en base a las nuevas evidencias científicas disponibles.
Tuberculosis (TB) continues to be one of the most important infectious diseases worldwide. The End TB strategy of the World Health Organization (WHO) established the objective of reducing the incidence of TB by 20% between 2015 and 2020, and Europe was the only region in the world that has achieved it. However, this decreasing trend halted in the wake of the COVID-19 pandemic, which had a strong negative impact on the control of TB. For the first time since 2005, the mortality associated to TB has increased.1,2
Based on the most recent data available, there were an estimated 7.8 cases of TB per 100 000 inhabitants in Spain in 2020.3 Children aged less than 15 years account for approximately 4% of cases of TB in Europe.4 Before the pandemic (data from 2019), the incidence of paediatric TB in Spain was of 6.1 cases/100 000 inhabitants in the group aged 0–4 years and 3.4/100 000 in children aged 5–14 years.3 The main risk factors were immigrant status and immunosuppression.5
Immunological diagnosis of tuberculosis infectionThe tuberculin skin test is based on the delayed hypersensitivity reaction that follows the intradermal injection of 0.1 mL of Mycobacterium tuberculosis complex (MTBC) antigens in the anterior surface of the forearm. The maximum diameter of the resulting induration is measured 48–72 h after the injection. This result is interpreted based on epidemiological and clinical factors (Table 1).5 A positive tuberculin test only indicates the presence of TB infection (TBI), and patients with a positive result need to be evaluated to rule out disease. The tuberculin test can yield false positive results in patients with infection by nontuberculous mycobacteria or vaccinated with bacillus Calmette-Guérin (BCG). It can also yield false negative results due to technical errors, in patients with weak or immature cellular immunity (newborns and infants, patients with primary or acquired immunodeficiency) and in patients with disseminated TB, recent viral infection or who have received an attenuated live vaccine in the past 6 weeks. Although they are not yet available, new intradermal tests have been developed based on specific antigens of MTBC (ESAT6 and CFP10) that are more specific and may be an alternative for diagnosis in the future.6,7
Thresholds used to define a positive result in the tuberculin test, independently of the history of vaccination with BCG.
| Induration ≥ 5 mm |
| • Children who are close contacts of an index case or suspected case of TB |
| • Children with clinical and/or radiographic features suggestive of TB |
| • Immunosuppressed children |
| • Children with tuberculin test conversion to positive after a previously negative result |
| Induration ≥ 10 mm |
| • All other cases: immigrant status or travel to endemic area, screening of healthy children |
BCG, bacillus Calmette-Guérin; TB, tuberculosis.
Interferon-gamma release assays (IGRAs) are assays used to detect the presence of interferon-gamma produced by T cells sensitised by specific MTBC antigens (ESAT6 and CFP10) in serum. Their sensitivity is similar to the sensitivity of the tuberculin skin test, but they offer a higher specificity, as these antigens are not present in the BCG vaccine strain of M bovis or in nontuberculous mycobacteria, with the exception of M kansasii, M marinum, M szulgai and M flavescens. As is the case of the tuberculin skin test, a positive IGRA result is only indicative of infection, and TB disease has to be ruled out in patients with positive results. Interferon-gamma release assays include a positive control that serves to assess the cellular immunity of the patient and do not require a second visit for their interpretation, but they require a blood draw and laboratory infrastructure and are expensive. The results can be positive, negative or indeterminate (in less than 5% of tests, especially in children aged less than 2 years).8 Several IGRAs are commercially available at the moment, of which the QuantiFERON-TB Gold-Plus assay (QIAGEN, USA) is the most widely used and the one for which there is the most evidence in the paediatric population.9,10 Recent studies have demonstrated that the diagnostic yield of the different IGRAs is very similar.9–11
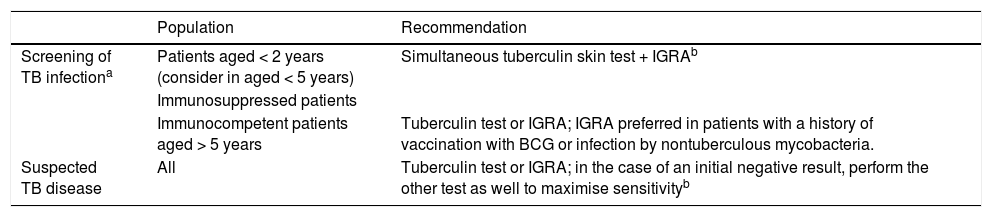
Recommendations on the use of the tuberculin skin test/IGRA (Table 2)The sensitivity of the tuberculin skin test and of IGRAs used in isolation for diagnosis of TB infection is estimated at 70%–80%, but the combination of both tests offers a sensitivity of 90%.10,12 The main international clinical practice guidelines disagree on the indications for the tuberculin skin test and IGRAs in the paediatric age group.13–16 Given the immaturity of the immune system in young children and the scarcer evidence on the use of IGRAs in paediatrics, guidelines generally recommend the tuberculin skin test in children under 5 years, alone or combined with an IGRA, but diverge as regards the use of IGRAs instead of the tuberculin skin test in children aged more than 5 years.13–16 In Spain, we recommend using both techniques for screening of TBI in patients at risk (patients of any age with immunosuppression and patients aged less than 2 years; also consider in patients aged less than 5 years, as the risk of developing TB is inversely proportional to age), and to use one or the other (tuberculin skin test or IGRA) in low-risk patients (age > 5 years and immunocompetent). When TB is suspected on account of the clinical presentation, we recommend maximizing the diagnostic sensitivity by using both techniques as needed (if the first one has turned out negative).
Recommendations for the use of the tuberculin skin test and interferon-gamma release assays in children and adolescents.
| Population | Recommendation | |
|---|---|---|
| Screening of TB infectiona | Patients aged < 2 years (consider in aged < 5 years) | Simultaneous tuberculin skin test + IGRAb |
| Immunosuppressed patients | ||
| Immunocompetent patients aged > 5 years | Tuberculin test or IGRA; IGRA preferred in patients with a history of vaccination with BCG or infection by nontuberculous mycobacteria. | |
| Suspected TB disease | All | Tuberculin test or IGRA; in the case of an initial negative result, perform the other test as well to maximise sensitivityb |
BCG, bacillus Calmette-Guérin; IGRA, interferon-gamma release assay.
Contact investigation, screening of immigrant population, screening prior to immunosuppression therapy and in immunosuppressed patients.
Interpretation of discordant results. Patients at risk (age < 5 years, immunosuppressed or in whom immunosuppression therapy has been prescribed, with known, recent significant exposure, or suspected TB disease): always consider infection by Mycobacterium tuberculosis. Patients at low risk with positive tuberculin skin test/negative IGRA results: if there is a history of vaccination with BCG or infection by nontuberculous mycobacteria, consider repeating the IGRA 6–8 weeks later, and if it continues to be negative, interpret it as the patient not being infected. Patients at low risk with negative tuberculin skin test/positive IGRA results: confirm use of correct technique in tuberculin skin test.
Performance of frontal anteroposterior and lateral chest X-rays is indicated whenever TB is suspected, be it pulmonary or extrapulmonary.17,18 Chest X-rays are also indicated in children with a diagnosis of TBI (positive tuberculin skin test or IGRA) and for evaluation of at-risk patients (immunosuppressed patients of any age and children aged less than 2−5 years) exposed to TB. Although this continues to be the recommended imaging modality for the initial assessment of TB, it is nonspecific, does not always detect characteristic features and shows considerable interrater variability in its interpretation.16–18 Computer-aided detection software is already being used for interpretation of chest X-rays in the assessment of TB in adults, and several such systems are available.19,20 Some have been validated for the paediatric age group, but none yet for children aged less than 2 years.
Computed tomography (CT) is more sensitive than plain radiography, but its routine use is not recommended.21 It is useful in children with inconclusive radiographic findings, symptomatic patients with normal radiographic features, if the diagnosis is uncertain in at-risk groups, to assess for complications of endobronchial TB and in the follow-up of complicated cases.17,18,22 When it comes to the diagnosis of osteoarticular or central nervous system (CNS) TB, magnetic resonance imaging is more sensitive and allows early diagnosis.
Sonography is an increasingly used technique that can be performed at the bedside.23 An ultrasound examination of the lungs allows visualization of consolidation, cavitation and miliary nodules as long as these changes are directly in contact with the pleura, with no aerated lung in between. It can detect mediastinal lymphadenopathy and, with greater difficulty, hilar lymphadenopathy. In expert hands, it is more sensitive than plain radiography to detect mediastinal lymphadenopathy.24 It is also useful for detection of pleural effusion, abdominal lymphadenopathy or focal splenic lesions.25
In recent years, the use of nuclear imaging techniques, such as positron emission tomography (PET)/CT, which can detect active disease in most organs (with the exception of the kidneys and CNS), but entails greater exposure to radiation than CT.26,27 It allows differentiation of TB disease from TB infection and residual disease, assessment of disease extension, monitoring the response to treatment and identification of suitable biopsy sites.27
Microbiological diagnosis of tuberculosisSamples. In children unable to expectorate, the preferred option for diagnosis of pulmonary TB is collection of gastric lavage samples (minimum of 3−4 mL; the greater the volume, the greater the sensitivity) after an overnight fast on 3 consecutive days, or induced sputum samples, which offer a similar sensitivity and are obtained following administration of inhaled salbutamol followed by hypertonic 3% saline nebulised over 15 min. The optimal yield may be achieved with paired induced sputum and gastric lavage specimens collected on the same day.28 Nasopharyngeal aspirate samples offer a lower sensitivity but may be an acceptable alternative in children who are not hospitalised or in whom the aforementioned techniques are not possible or do not yield a viable sample. In adolescents or older children with productive cough, collection of sputum samples is preferred, and in intubated patients, bronchial aspirate or bronchoalveolar lavage samples. Recently, testing of stool samples has been included in the routine diagnostic workup of young children.29 In patients with disseminated or extrapulmonary disease, it is also possible to test cerebrospinal fluid or synovial fluid samples, biopsy specimens etc.
Acid-fast bacillus (AFB) smear microscopy. It involves the use of staining methods (Ziehl-Neelsen, auramine-rhodamine) that allow quick and easy identification of the bacillus. They offer a sensitivity of less than 15% in children with primary TB infection that increases in cases of congenital TB infection and cavitary TB in adolescents. A negative AFB smear does not rule out disease in any case. Direct staining is less specific in situations in which infection by nontuberculous mycobacteria is more likely (cystic fibrosis, bronchiectasis, lymphadenitis or immunosuppression).
Culture. It is the gold standard of diagnosis. It allows identification of the species and performance of antimicrobial susceptibility testing. Its drawbacks are a low sensitivity (30%–50%) and a long turnaround time (2–4 weeks to get definitive results).30
Molecular techniques, polymerase chain reaction and next generation sequencing. Although they do not replace culture as the gold standard, their sensitivity is near that of culture, their specificity is high in children and the results are available in a few hours.31 The most widely used molecular test is the Xpert MTB/RIF Ultra assay (Cepheid, USA), which allows simultaneous testing of rifampicin (RMP) resistance. There are also next generation sequencing tests, such as the Deeplex Myc-TB assay (GenoScreen, France), which allows the identification of the species, prediction of drug resistance and phylogenetic tracing for better control of transmission in the population.32
Molecular detection of drug resistance. Culture is the gold standard for the assessment of sensitivity to anti-TB drugs. However, rapid molecular tests have been developed for the detection of genetic mutations associated with resistance that offer advantages such as their quickness, high sensitivity (90%–97% in the case of positive smear results, 67% in the case of negative smear results) and specificity (99%). In addition to Xpert MTB/RIF Ultra, the most widely used tests in Spain are those for detection of resistance to isoniazid (INH) and to RMP, such as BD MAX MDR-TB (BD, USA), Genotype MTBDRplus (Hain Lifescience, Germany) or FluoroType MTBDR (Hain Lifescience, Germany), and second-line drugs, such as Xpert MTB/XDR (Cepheid, USA) or GenoType MTBDRsl (Hain Lifescience, Germany).33
Novel diagnostic techniques. Novel diagnostic methods have been in development in recent years to test samples that are easier to obtain, such as molecular assays for capillary blood samples34 or detection of biomarkers in saliva35 and in urine,36 which are still not commercially available.
Preventive treatment of exposure to tuberculosis and tuberculosis infectionIn children aged less than 5 years and immunosuppressed patients of any age in contact with individuals with smear-positive active TB, once TB infection and disease have been ruled out, it is still recommended to administer post-exposure prophylaxis with INH until the immunological diagnostic tests can be repeated at the end of the window period (8–12 weeks). In the case of strains resistant to INH, we recommend prophylaxis with RMP. When it comes to exposure to multidrug-resistant TB (MDR-TB), the evidence is scarce, and prophylaxis should be personalised (none or administration of a quinolone) taking into account the risk of the patient and intensity of exposure.5,16 In immunocompetent patients aged more than 5 years, post-exposure prophylaxis is not recommended.
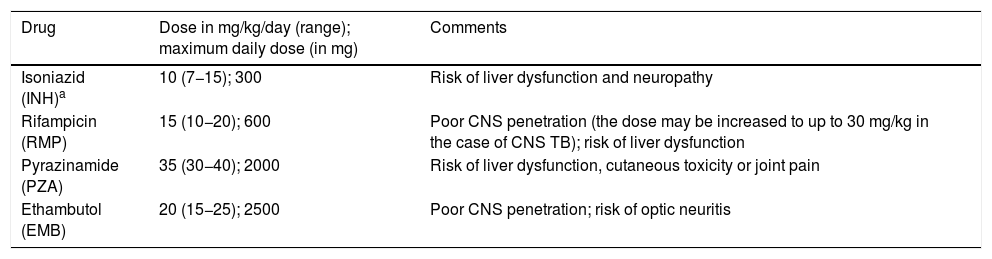
All children and adolescents with a diagnosis of TBI must be treated. In Spain, the recommended regimens are INH + RMP for 3 months, RMP for 4 months and INH for 6–9 months (Table 3).5,37 The efficacy is similar for all, but adherence is better and toxicity lower in shorter regimens. The WHO also recommends the combination of INH and rifapentine, but the latter is not yet available in Europe.38,39 In patients who are taking other medications, the risk of drug interactions must be assessed.37 In cases of TBI involving a multidrug-resistant strain, treatment regimen should be based on the results of antimicrobial susceptibility testing. The most common regimen is levofloxacin with or without ethambutol (EMB). The appropriate duration has yet to be clearly established, and a duration of 6–12 months is currently recommended.38,40
Recommended dosage of first-line antituberculous drugs.5,16
| Drug | Dose in mg/kg/day (range); maximum daily dose (in mg) | Comments |
|---|---|---|
| Isoniazid (INH)a | 10 (7−15); 300 | Risk of liver dysfunction and neuropathy |
| Rifampicin (RMP) | 15 (10−20); 600 | Poor CNS penetration (the dose may be increased to up to 30 mg/kg in the case of CNS TB); risk of liver dysfunction |
| Pyrazinamide (PZA) | 35 (30−40); 2000 | Risk of liver dysfunction, cutaneous toxicity or joint pain |
| Ethambutol (EMB) | 20 (15−25); 2500 | Poor CNS penetration; risk of optic neuritis |
CNS, central nervous system; HIV, human immunodeficiency virus; TB, tuberculosis.
The treatment of TB is the same for microbiologically confirmed cases and probable cases (diagnosis based on epidemiological circumstances, clinical and imaging findings, immunological test results and response to treatment). The treatment regimen will depend on the age, clinical factors and location of disease. First-line anti-TB drugs (Table 3) should be taken as a single dose on an empty stomach, usually in the morning. Intermittent courses of treatment are not recommended.5 The licensed formulations of anti-TB drugs currently available in Spain are not adequate for young children, except for RMP.41,42 The recommendations for the preparation and administration of anti-TB medication in the paediatric population (commercially available dosage forms and compounded preparations), monitoring of toxicity and directly observed treatment are summarised in the guidelines published in the framework of the Proyecto Magistral.41,43 Given the prevalence of isoniazid-resistant strains in Spain (> 4%), EMB must be added in the intensive phase if the sensitivity of the aetiological agent to first-line drugs has not been confirmed in the patient or the index case. Table 4 presents the indications for steroid therapy.
Indications for the use of steroid therapya in paediatric tuberculosis.
| - Tuberculous meningitis or central nervous system involvement. |
| - Spinal TB with spinal cord involvement. |
| - Tuberculous pericarditis. |
| - Severe airway obstruction secondary to extrinsic compression due to lymph node enlargement or endobronchial tuberculosis. |
| - Consider in case of paradoxical reaction in patients with peripheral or hilar lymphadenitis (newly developed atelectasis) and in patients with pleuritis (or other forms of serositis) with an important inflammatory component. |
Prednisone (or equivalent): dose of 2 mg/kg/day (maximum, 60 mg/day) for 4 weeks, taper over 2 weeks (total of 6 weeks).5,15
New short course based on the results of the SHINE trial,444 months of treatment: INH, RMP, pyrazinamide (PZA) ± EMB for 2 months (intensive phase), followed by INH and RMP for another 2 months (maintenance phase), in patients meeting the following criteria:
- •
Age 3 months to 16 years.
- •
Nonsevere disease: peripheral lymph node TB; intrathoracic lymph node TB without airway obstruction, noncavitary disease confined to one lobe of the lungs and without a miliary pattern, with or without uncomplicated pleural effusion.
- •
Negative AFB smear.
- •
Strain known or likely to be susceptible to first-line drugs.
- •
It must be taken into account that the trial excluded infants born preterm or with birth weights of less than 3 kg and pregnant women.
Traditional regimen, 6 months of treatment: INH, RMP, PZA ± EMB for 2 months, followed by INH, RMP for at least 4 more months.5,15,16,45 Indicated in patients who do not meet the criteria for the short course or with extrapulmonary forms of TB (with the exception of peripheral lymphadenitis, in which the short course may be used, CNS involvement, disseminated or miliary TB or osteoarticular TB).
In patients with TB meningitis or any other form of CNS involvement, we recommend INH, RMP, PZA ± EMB for 2 months, followed by INH, RMP for a minimum of 10 months, using the highest recommended doses. In cases of CNS TB caused by a susceptible strain, consider intensive treatment with INH, RMP, PZA and ethionamide for 6 months; this regimen is not recommended in patients with HIV infection.16 For osteoarticular TB, we recommend INH, RMP, PZA ± EMB for 2 months, followed by INH, RMP for at least 7–10 months.15,45 The treatment for disseminated/miliary TB (involvement of ≥2 noncontiguous organs, isolation of MTBC from blood or urine) is not well established. We recommend INH, RMP, PZA ± EMB for 2 months, followed by INH, RMP for at least 4–10 months, depending on the course of disease, whether the CNS is involved and whether the patient is immunosuppressed.5,46
The following are important aspects in the follow-up of treatment of TB in paediatric patients5,45:
- 1
Monitoring adherence and problems with treatment and ensuring an appropriate dosage in each visit. Poor adherence is the most common cause of treatment failure.47 In some cases, directly observed treatment or monitoring of drug serum levels may be appropriate to assess and ensure adherence.
- 2
Assess for potential liver dysfunction. We recommend liver function tests with measurement of serum transaminase levels prior to treatment initiation; consider repetition of the tests within a few weeks of initiation and in the case of adverse events. Liver function should also be monitored in patients with past or current liver disease or receiving other hepatotoxic drugs.
- 3
In patients with smear-positive active disease (usually adolescents), the AFB smear should be repeated periodically (every 1−2 weeks) until the smear turns out negative, which allows discontinuation of airborne precautions.5,16
- 4
A follow-up clinical evaluation must be performed at least at 2 weeks of treatment, at 2 months to switch to the maintenance phase and every 2 months thereafter until treatment is completed.
Drug-resistant TB is always a microbiological diagnosis.48 It is classified as confirmed if the strain is isolated in the patient, probable if it is only isolated in the index case and possible if there is no improvement despite appropriate first-line treatment and adequate adherence when the susceptibility pattern of the index case is unknown, in case of previous treatment failure or retreatment or if the patient dies of TB during treatment despite adequate adherence.
In children, infection by a drug-resistant strain is usually transmitted by a contagious adult, resulting in primary resistance in the child.48 However, paediatric patients can also develop secondary resistance due to inadequate initial treatment or suboptimal adherence. The current definitions of drug-resistant TB are49,50:
- •
Rifampicin-resistant TB (RR-TB): strains resistant to RMP (by molecular detection) that may or may not be resistant to other drugs. In these cases, we recommend considering treatment with an MDR-TB regimen.
- •
Rifampicin-monoresistant TB (RMR-TB): strain resistant to RMP and susceptible to INH using phenotypic (not only genotypic) methods. In these cases, we recommend considering treatment with an MDR-TB regimen, although INH may be included.
- •
Isoniazid-monoresistant TB: strains resistant to INH alone. In these cases, we recommend 6 months of combination therapy with RMP, PZA, EMB and levofloxacin, with or without INH.51
- •
Polyresistant TB: strains susceptible to RMP, but resistant to 2 or more other anti-TB drugs. The prescribed regimen should include RMP.
- •
MDR-TB: strains resistant to at least INH and RMP.
- •
Pre-XDR TB: strains that fulfill the definition of MDR-TB and are also resistant to any fluoroquinolone.
- •
XDR-TB: strains that fulfill the definition of MDR-TB and are also resistant to any fluoroquinolone and at least one additional Group A drug (bedaquiline or linezolid).
Decisions regarding the treatment regimen to be administered will be based on the judgment of the clinician in charge of the patient and the preferences of the patient and the family, taking into account the following aspects: location and severity of TB, antimicrobial susceptibility results, treatment history of the patient and/or index case, potential adverse effects and availability, feasibility and appropriateness for use in children of potentially active drugs.16 Consultation with an expert is always recommended.
Basic guidelines for the treatment of MDR-TB16,48,52,53:
- •
Attempt to achieve bacteriological confirmation of TB caused by a drug-resistant strain in the patient; alternatively, use the antimicrobial susceptibility profile of the index case to build the regimen.
- •
In the intensive phase, use at least 4 or 5 active, oral second-line drugs based on the results of phenotypic drug susceptibility testing; in the maintenance phase, use at least 3 active drugs.
- •
Agents will be selected according to their activity against MTBC (Table 5). Injectable agents (aminoglycosides) will only be used as a last resort.
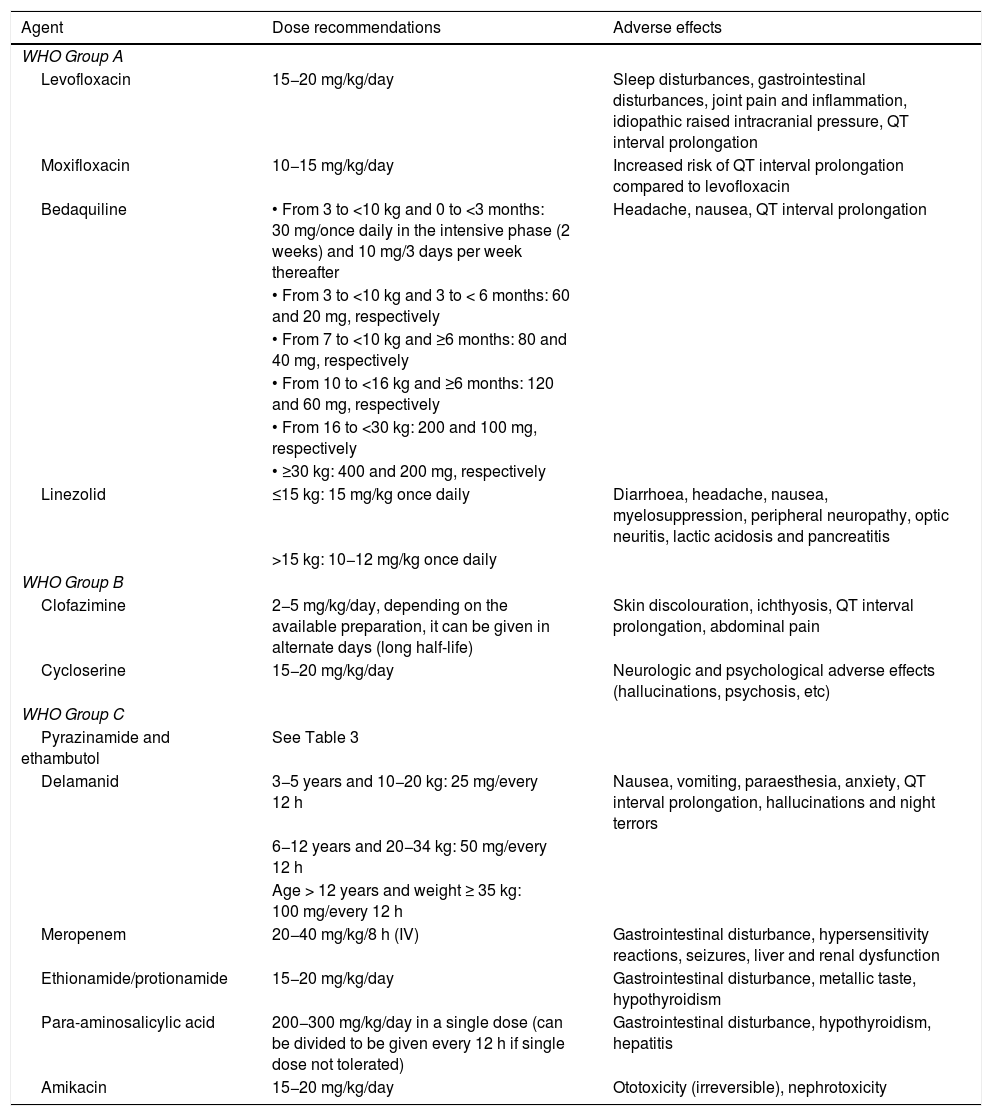
Table 5.Classification of second-line anti-tuberculosis drugs based on the priority given to their inclusion in MDR-TB treatment regimens.48,52
Group A 1. Include all 3 drugs Levofloxacin or moxifloxacin Bedaquiline Linezolid Group B 2. Add one or both drugs Clofazimine Cycloserine or terizidone Group C 3. Add as needed to complete the regimen if any of the drugs in groups A or B cannot be used Delamanid EMB PZA Imipenem or meropenem Ethionamide or protionamide Para-aminosalicylic acid Amikacin MDR-TB, multidrug-resistant tuberculosis.
- •
Table 6 presents the recommended dosage of anti-TB drugs; we recommend consulting updated dosage recommendations in the Sentinel Project Field Guide.48
Table 6.Recommended dosage of second-line anti-tuberculosis drugs. We recommend consulting updated dose recommendations in the Sentinel Project Field Guide.48
Agent Dose recommendations Adverse effects WHO Group A Levofloxacin 15−20 mg/kg/day Sleep disturbances, gastrointestinal disturbances, joint pain and inflammation, idiopathic raised intracranial pressure, QT interval prolongation Moxifloxacin 10−15 mg/kg/day Increased risk of QT interval prolongation compared to levofloxacin Bedaquiline • From 3 to <10 kg and 0 to <3 months: 30 mg/once daily in the intensive phase (2 weeks) and 10 mg/3 days per week thereafter Headache, nausea, QT interval prolongation • From 3 to <10 kg and 3 to < 6 months: 60 and 20 mg, respectively • From 7 to <10 kg and ≥6 months: 80 and 40 mg, respectively • From 10 to <16 kg and ≥6 months: 120 and 60 mg, respectively • From 16 to <30 kg: 200 and 100 mg, respectively • ≥30 kg: 400 and 200 mg, respectively Linezolid ≤15 kg: 15 mg/kg once daily Diarrhoea, headache, nausea, myelosuppression, peripheral neuropathy, optic neuritis, lactic acidosis and pancreatitis >15 kg: 10−12 mg/kg once daily WHO Group B Clofazimine 2−5 mg/kg/day, depending on the available preparation, it can be given in alternate days (long half-life) Skin discolouration, ichthyosis, QT interval prolongation, abdominal pain Cycloserine 15−20 mg/kg/day Neurologic and psychological adverse effects (hallucinations, psychosis, etc) WHO Group C Pyrazinamide and ethambutol See Table 3 Delamanid 3−5 years and 10−20 kg: 25 mg/every 12 h Nausea, vomiting, paraesthesia, anxiety, QT interval prolongation, hallucinations and night terrors 6−12 years and 20−34 kg: 50 mg/every 12 h Age > 12 years and weight ≥ 35 kg: 100 mg/every 12 h Meropenem 20−40 mg/kg/8 h (IV) Gastrointestinal disturbance, hypersensitivity reactions, seizures, liver and renal dysfunction Ethionamide/protionamide 15−20 mg/kg/day Gastrointestinal disturbance, metallic taste, hypothyroidism Para-aminosalicylic acid 200−300 mg/kg/day in a single dose (can be divided to be given every 12 h if single dose not tolerated) Gastrointestinal disturbance, hypothyroidism, hepatitis Amikacin 15−20 mg/kg/day Ototoxicity (irreversible), nephrotoxicity WHO, World Health Organization.
Source: adapted from Howell et al.53
- •
Administer treatment daily.
- •
Offer directly observed treatment. Otherwise, monitor adherence meticulously.
- •
Follow-up during treatment should include clinical and radiological monitoring and smear and culture monitoring until they become negative, if applicable.
- •
Monitor adverse effects of prescribed drugs.
In Spain, the treatment of drug-resistant TB in children has to be individualised according to the basic guidelines provided above. At present, all of the second-line drugs with the exception of pretomanid can be used in any paediatric age group (Tables 5 and 6). The WHO has established a minimum duration of 4–6 months for the intensive phase.16 The minimum total duration of treatment is 9 months for mild forms of TB disease if the following criteria are fulfilled: 1) verified susceptibility to fluoroquinolones; 2) mild forms of pulmonary TB (noncavitary, confined to a single lobe) or isolated peripheral lymphadenopathy; and 3) no past exposure to the drugs included in the regimen for more than 1 month. In all other cases, the minimum duration of treatment is 12–18 months, and will depend on the antimicrobial susceptibility profile and the severity of disease.
In the treatment of CNS or disseminated/miliary TB involving a multidrug-resistant strain, it is important to take into account the capacity of the selected agents to cross the blood-brain barrier, the most efficient blood-brain barrier penetration has been observed with fluoroquinolones, linezolid, cycloserine, ethionamide, meropenem and pyrazinamide. Delamanid and high-dose INH (in cases of resistance due to variants that confer a low level of resistance to INH) can also be useful.
FundingAlicia Hernanz-Lobo is funded by the Ministry of Science and Innovation fo Spain-Instituto de Salud Carlos III and a European Regional Development Fund (ERDF) grant (Río Hortega contract CM20/00128).
Paula Rodríguez-Molino is funded by the Ministry of Science and Innovation fo Spain-Instituto de Salud Carlos III and a European Regional Development Fund (ERDF) grant (Río Hortega contract CM21/00174).
Conflicts of interestFernando Baquero-Artigao has participated in research projects funded by Qiagen and Cepheid. Begoña Santiago-García has participated in research projects funded by Cepheid. The rest of the authors have no conflicts of interest to disclose.
We thank Dr. María José (Pepa) Mellado for her long-standing leadership and expertise in the study of paediatric TB in Spain.