Febrile neutropenia is one of the main infectious complications experienced by paediatric patients with blood or solid tumours, which, despite the advances in diagnosis and treatment, are still associated with a significant morbidity and mortality. These patients have several risk factors for infection, chief of which are chemotherapy-induced neutropenia, the disruption of cutaneous and mucosal barriers and the use of intravascular devices. Early diagnosis and treatment of febrile neutropenia episodes based on the patient’s characteristics is essential in patients with blood and solid tumours to improve their outcomes. Therefore, it is important to develop protocols in order to optimise and standardise its management. In addition, the rational use of antibiotics, with careful adjustment of the duration of treatment and antimicrobial spectrum, is crucial to address the increase in antimicrobial drug resistance.
The aim of this document, developed jointly by the Spanish Society of Pediatric Infectious Diseases and the Spanish Society of Pediatric Hematology and Oncology, is to provide consensus recommendations for the management of febrile neutropenia in paediatric oncology and haematology patients, including the initial evaluation, the stepwise approach to its treatment, supportive care and invasive fungal infection, which each facility then needs to adapt to the characteristics of its patients and local epidemiological trends.
La neutropenia febril es una de las principales complicaciones infecciosas que sufren los pacientes pediátricos oncohematológicos, y a pesar los avances en diagnóstico y tratamiento, siguen condicionando una mortalidad y morbilidad significativa. Estos pacientes agrupan una serie de factores de riesgo de infección, donde destaca la neutropenia asociada a quimioterapia, la disrupción de barreras cutáneo-mucosas y el uso de dispositivos intravasculares. El abordaje diagnóstico y terapéutico precoz de los episodios de neutropenia febril en los pacientes oncohematológicos, ajustado a las características individuales de cada paciente, es fundamental para mejorar su pronóstico. Por ello, diseñar protocolos de abordaje, que sistematicen su atención, permite optimizar y homogeneizar su abordaje. Además, racionalizar el uso de los antimicrobianos, ajustando la duración y el espectro de los mismos, es crucial para hacer frente al incremento de resistencias a antimicrobianos.
El objetivo de este documento, elaborado entre la Sociedad Española de Infectología Pediátrica y la Sociedad Española de Hematología y Oncología Pediátrica, es dar recomendaciones de consenso sobre el manejo de la neutropenia febril en el paciente oncohematológico, respecto al abordaje inicial, terapia secuencial y de soporte e infección fúngica invasiva, que cada centro debe adaptar a las características de sus pacientes y epidemiología local.
Despite the advances in treatment and increased survival in childhood cancer, infections continue to be an important cause of morbidity and mortality in affected patients, especially those who receive chemotherapy and develop neutropenia, and fever may be the sole manifestation or it may be associated with other nonspecific signs and symptoms.
Paediatric patients who receive chemotherapy usually develop mucosal barrier injury, or mucositis,1 which facilitates infection by opportunistic microorganisms present in the microbiota of the skin and of the oral and gastrointestinal mucosa.2 On the other hand, the increase in antimicrobial drug resistance in health care-associated infections complicates the management of these patients.
The Working Groups on Bacterial Infection and Fungal Infection of the Sociedad Española de Infectología Pediátrica (Spanish Society of Paediatric Infectious Disease) and the Support Group of the Sociedad Española de Hematología y Oncológica Pediátrica (Spanish Society of Paediatric Haematology and Oncology) have developed a consensus document to update the recommendations for the diagnosis and treatment of febrile neutropenia (FN) in paediatric oncological/haematological patients, recognising that each facility must in turn adjust its protocols to the particular epidemiology of the centre and its available resources to improve diagnosis and treatment.
Questions and recommendationsTable 1 summarises the recommendations of the consensus group.
Summary of the consensus recommendations for the management of febrile neutropenia in paediatric oncological/haematological patients.
| 1. What is the definition of paediatric oncological/haematological patient with FN?? |
| - The definition of FN requires the presence of: |
| Neutropenia: absolute neutrophil count ≤500 mm3 or ≤1000 mm3 expected to drop below 500 mm3 in 24–48 h. |
| - Fever: single measurement of axillary temperature ≥38.3–38.5 °C (>38 °C in the guidelines of the United Kingdom and Australia) or axillary temperature ≥38 °C in at least 2 measurements or sustained for 1 h. |
| 2. Which are the microorganisms involved in episodes of FN in paediatric oncological/haematological patients? What is the prevalence of multidrug-resistant (MDR) microorganisms? |
| A bacterium is isolated from blood culture in 10%–30% of fever episodes in paediatric patients with FN. There are differences in the aetiology of bacteraemia between paediatric and adult patients, between geographical areas and between hospitals. There has been a progressive increase in the prevalence of MDR gram-negative bacteria. |
| 3. What is the appropriate risk stratification strategy for oncological/haematological paediatric patients with FN? |
| The use of risk stratification models validated in paediatric patients with FN allows the differentiation of groups of patients at increased risk of severe or protracted infection (Table 2) and patients with low risk. Their application also makes it possible to adapt the treatment to each situation and to consider outpatient treatment or early discharge. |
| Each facility has to choose the risk stratification criteria that it wants to apply, preferably models tested in populations of similar characteristics and, ideally, validating them at the local level. |
| 4. What should be the initial approach in the management of paediatric oncological/haematological patients with FN? |
| Neutropenia is associated with a decreased inflammatory response, and therefore fever in a patient with or at risk of severe neutropenia requires early and thorough evaluation to identify potential foci of infection and guide the aetiological diagnosis and the initiation of appropriate treatment. The evaluation must include blood culture, a complete blood count and assessment of serum inflammatory markers. The performance of other diagnostic tests (Table 3) will be based on the specific clinical presentation (signs and symptoms). |
| 5. What are the recommendations for empiric antibiotherapy in paediatric oncological/haematological patients with FN? |
| As soon as the initial evaluation is completed, paediatric oncological/haematological patients with FN should start empiric antibiotherapy with broad-spectrum agents covering P. aeruginosa within 60 min of the start of the care episode. |
| Different approaches are recommended depending on individual risk: |
| 5.1 High-risk patients |
| 5.1.1 Haemodynamically stable patients |
| - Empiric treatment with a β-lactam active against P. aeruginosa (cefepime, piperacillin–tazobactam or ceftazidime), adjusting the selection to the specific epidemiological and drug resistance trends of the centre. |
| - Consider combination therapy with an antibiotic active against β-lactam-resistant GNB (aminoglycosides, fluoroquinolones or colistin) and/or an antibiotic active against methicillin-resistant GPC (vancomycin, teicoplanin or daptomycin) in specific situations, such as suspected catheter-related bloodstream infection or infection of the skin, mucosae and/or soft tissues (Table 4). |
| - We recommend avoiding the use of carbapenems (meropenem or imipenem–cilastatin), although they can be considered on a case-by-case basis in patients at high risk of infection by an MDR pathogen. |
| 5.1.2 Haemodynamically unstable patients |
| Empiric antibiotherapy should be initiated immediately with a carbapenem active against P. aeruginosa (meropenem or imipenem/cilastatin), and, in the case of septic shock, we recommend the addition of an antibiotic with activity against β-lactam-resistant GNB and an antibiotic with activity against methicillin-resistant GPC, taking into account the characteristics of the patient and local epidemiological trends. |
| 5.2 Low-risk patients |
| In stable patients considered to be at low risk in which adequate follow-up is feasible (adequate home environment, infrastructure of the medical facility and physical proximity allowing rapid reassessment), who have adequate oral tolerance and no features of malabsorption, consider outpatient oral antibiotherapy for empiric treatment after a 12–24 h monitoring period in hospital, ensuring close follow-up of the patient (Tables 4 and 5). |
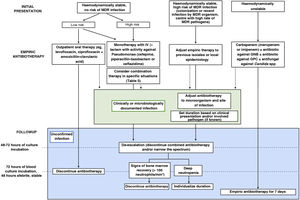
| 6. What is the appropriate antibiotic strategy beyond initial empiric therapy in paediatric oncological/haematological patients? (Fig. 1) |
| Once empiric treatment is initiated, the subsequent follow-up should be guided, ideally, by the results of culture or the identified focus of infection to establish targeted therapy against the isolated microorganism and for infection control at the focus, if applicable, taking into account the clinical condition and individual risk. |
| 6.1. Clinically or microbiologically documented bacterial infection |
| • In the case of isolation of a bacterium considered to be associated with the febrile episode, switch to targeted treatment with a narrower-spectrum antibiotic based on the susceptibility profile of the organism and the site of infection. Measures for infection control at the focus (e.g., abscess drainage, removal of infected catheters), whenever possible, are also important. |
| • The duration of treatment will depend on the clinical presentation, the course of disease and the isolated organism, if there is one, according to specific guidelines. In general, treatment can be discontinued if the signs and symptoms have resolved, the patient has been afebrile for at least 72 h and after at least 7 days of antibiotherapy. If severe neutropenia persists, we recommend maintaining the patient under observation for 24–48 h after discontinuation of antibiotherapy. |
| • In the case of a good clinical and microbiological response, if there is an antibiotic active against the isolated organism or appropriate for the clinical presentation, with adequate oral bioavailability and tolerance and in the absence of clinical features of malabsorption, de-escalation to oral antibiotherapy can be contemplated. |
| 6.2. Bacterial infection without clinically and/or microbiologically documented infection |
| • We do not recommend switching to a broader-spectrum antibiotic due to the persistence of fever if the patient remains stable, without development of additional signs or symptoms and without isolation of a pathogen in culture. |
| • In patients that remain clinically stable at 48–72 h receiving empiric combination therapy, the antibiotics combined with the β-lactam antibiotic (e.g., aminoglycosides, glycopeptides) should be discontinued unless their continued use is justified by microbial isolation. In the case of empiric therapy with carbapenems, de-escalation to cefepime, piperacillin–tazobactam or ceftazidime is possible under the same circumstances noted above. |
| • In patients who were clinically stable at presentation, empiric antibiotherapy can be suspended once the patient has been afebrile for 48 h, in the absence of microbial growth in blood culture after 72 h of incubation and with evidence of bone marrow recovery (e.g., >100 neutrophils/mm3), or irrespective of the neutrophil count in low-risk patients. In high-risk patients, the decision must be individualised if there is no evidence of bone marrow recovery, and it is safe to discontinue empiric antibiotherapy after 5–7 days of treatment if the patient has been afebrile for 48 h, keeping the patient under observation for 24–48 h after discontinuation. |
| For patients who were clinically unstable at admission (severe sepsis or septic shock), stabilised with empiric therapy and in whom cultures remain sterile, we recommend maintaining empiric antibiotherapy for a minimum of 7 days |
| 7. What is the significance of invasive fungal infection (IFI) in paediatric oncological/haematological patients with FN? |
| The incidence of IFI in paediatric patients with cancer or who have undergone HSCT ranges from 2% to 20%.33,35 Individual risk depends on the underlying disease of the patient and associated factors (Table 6), of which prolonged severe neutropenia is the most relevant. |
| 8. What is the appropriate strategy to assess for IFI in paediatric oncological/haematological patients with FN? |
| Assess for potential IFI in patients with persistent FN ≥ 96 h despite appropriate antibiotic treatment. We recommend performance of imaging tests (chest CT scan and possibly others depending on the presentation) in addition to cultures and tests to assess markers of fungal infection. |
| 9. When and how should antifungal therapy be initiated in paediatric oncological/haematological patients with FN? What is the appropriate management strategy after initiation of antifungal therapy? |
| If IFI is suspected, consider the immediate initiation of empiric antifungal therapy (caspofungin or liposomal amphotericin B) or pre-emptive therapy (based on the results of imaging tests and fungal biomarkers). Both strategies are valid in paediatric patients, and the choice will be based on the characteristics of the patient, the suspected aetiological agent and the resources and epidemiology in the given centre. It is also essential to control infection at the focus and correct the predisposing factors. Treatment duration and drug monitoring will depend on the isolated or suspected organism. |
| 10. Which non-antimicrobial treatments are indicated in the management of paediatric oncological/haematological patients? |
| The treatment of patients with FN includes general supportive care measures in addition to preventive measures aimed at reducing the incidence and severity of infections and their complications in neutropenic patients (Table 7). |
| Chief among them is the use of G-CSF in patients in patients with solid tumours or lymphoma, which reduces the duration of neutropenia. Many prevention strategies have been changing over time or been abandoned due to the lack of evidence in support of their use. |
CT, computed tomography; FN, febrile neutropenia; GNB, gram-negative bacilli; GPC, gram-positive cocci; HSCT, haematopoietic stem cell transplantation; IFI, invasive fungal infection; MDR, multidrug-resistant.
The term FN entails the cooccurrence of fever and neutropenia.3–5 The definition of FN allows the establishment of validated recommendations for its management. The recommendations of this guideline may not be the most appropriate in other situations in which there is risk or suspicion of infection (e.g., fever without severe neutropenia), for which specific protocols should be established.
Which are the microorganisms involved in episodes of FN in paediatric oncological/haematological patients? What is the prevalence of multidrug-resistant (MDR) microorganisms?The relative frequency of isolation of gram-positive cocci (GPC) and gram-negative bacilli (GNB) depends on various factors, such as the use of central venous catheters and their care (coagulase-negative staphylococci), the emergence of Viridians streptococci in association with haematopoietic stem cell transplantation (HSCT), more aggressive induction chemotherapy regimens and the presence of mucositis,1,2,6,7 with a greater frequency of GPC in blood compared to solid tumours and in patients who undergo HSCT and aggressive induction therapy.
We are witnessing an increase in the prevalence of MDR pathogens, chief among them extended spectrum beta-lactamase (ESBL)-producing bacteria6,8–11 and carbapenem-resistant bacteria (especially Pseudomonas aeruginosa and Klebsiella pneumoniae),6–8,12 which is associated with an increased severity and mortality, and the prevalence of methicillin-resistance Staphylococcus aureus in Spain has remained unchanged. In consequence, empiric antibiotherapy must be selected taking into account individual risk as well as local epidemiological trends.
Viral detection is associated with less severe disease and a decreased length of stay,13,14 but its impact on the discontinuation of antibiotherapy in these patients remains small,14 probably due to the impossibility of ruling out bacterial coinfection with absolute certainty and the prolonged shedding of respiratory viruses in these patients, which complicates the interpretation of viral detection. On the other hand, the detection of a viral pathogen is useful for the purpose of implementing infection control measures and to initiate effective antiviral therapy in the case of viruses for which it is available. In addition, in patients at low risk who are stable and improving, viral detection could guide decision-making regarding antimicrobial de-escalation or discontinuation.5
What is the appropriate risk stratification strategy for oncological/haematological paediatric patients with FN?There are several risk stratification models validated in the paediatric population (Table 2) that allow the differentiation of groups of patients with FN with a probability of bacterial infection of less than 10% and a low risk of complications. Their application allows the implementation of outpatient care protocols and/or early discharge in patients with low-risk FN. Although many studies have excluded patients aged less than 1 year or who develop fever while hospitalised, the study by Haeusler et al. included these subsets of patients.15
Risk stratification strategies for paediatric oncological/haematological patients with febrile neutropenia.
| Rackoff (1996) | Santolaya (2001) | Alexander (2002) | Ammann (2004) | SPOG-SAE (Ammann 2010) | |
|---|---|---|---|---|---|
| Patient-related factors | Leukaemia relapse, CTX within last 7 days | AML, NHL, ALL, induction phase or BM relapse | BM involvement | CTX more intense than ALL maintenance = 4 points | |
| Episode-specific factors | MONO: 3; Temp ≥/<39 °C | CRP ≥90 mg/L | Hypotension, altered level of consciousness, tachypnoea, hypoxia (<94%), abnormal X-ray, severe mucositis, vomiting or abdominal pain, focal infection, hospital admission | Leucocytes ≤500 mm3; Temp >39.7 °C; presentation requiring admission | Leucocytes <300/mm3 = 3 points; Hb ≥ 9 g/dL = 5 points; platelets ≤ 50,000 mm3 = 3 points |
| Hypotension | |||||
| Platelets ≤50,000 mm3 | |||||
| LR/HR | LR: MONO ≥ 100 mm3/HR: MONO < 100 mm3 + Temp ≥ 39 °C | LR: no risk factor or only platelets/<7 days after CTX | LR: no risk factor | LR: no risk factor | LR: <9 points (applied at 8–24 h) |
| Bacteraemia | 20%: LR 0% (19%) HR 48% | 20% | 12.5%: LR 4 vs 22% HR | 24%: LR 4 vs 33% HR | 16% |
| Bacterial infection/AE | 22%: LR 4 vs 51% HR | 40%: LR 15 vs 60% HR | 21%: LR 4 vs 40% HR | Not analysed | AE: 29% (55% first 24 h) |
| % LR-[IR]-HR | 17%–65%–18% | 40%–60% | 53%–47% | 29%–71% | 35% LR at 8–24 h |
| Sen/Spe/PPV/NPV (%) | 42/88/48/85 | 90/65/75/87 | 91/65/41/96 | 95/36/4/96 | 92/52/43/94 |
| Region where validated | USA and Australia | Chile | UK and Australia | Europe and Australia | Europe and Australia |
| Validation and/or adaptation studies and validated applications. Sen/Spe/PPV/NPV (%) | ||||
|---|---|---|---|---|
| Haeusler et al., 202041 | HR: 91/26/16/95 LR: 35/83/23/90 | 64/44/39/69 | 96/18/15/96 | 72/45/44/73 |
| Sen/Spe/PPV/NPV (%) | ||||
| Haeusler et al., 202015 | Only MONO ≥/<15 mm3Baorto, 2001 (bacteraemia 6% LR vs 36% HR) 71/50/30/85 | HR (≥1): CTX, leukopenia or thrombopenia (from onset of episode) | ||
| Sen/Spe/PPV/NPV (%) | ||||
| Arif et al., 201417 | Initial LR + LR at 48 h (36%): 0% AE no initial LR: 5% serious AE, 53% AE no LR at 48 h: 0% serious AE, 30% AE | LR (51%): serious AE 0%, AE 28%/HR (49%): serious AE 4%, AE 51% | ||
| Sen/Spe/PPV/NPV (%) | ||||
AE, adverse event; ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; ANC, absolute neutrophil count; BC, blood culture; BM, bone marrow; CTX, chemotherapy; Hb, haemoglobin; HR, high risk; IR, intermediate risk; LR, low risk; MONO, absolute monocyte count; NHL, non-Hodgkin lymphoma (Burkitt); NPV, negative predictive value; PPV, positive predictive value; Rx, radiograph; Sen, sensitivity; Spe, specificity; Temp, temperature.
Adapted from Lehrnbecher et al.3.
These models have exhibited a good sensitivity and negative predictive value and therefore can be used to identify patients eligible for initiation of treatment at the outpatient level or for antimicrobial de-escalation and early discharge (after 24–48 h of clinical stability),16,17 with an admission/readmission rate of less than 10% and a very low probability of significant adverse events.
In Spain, the multicentre clinical trial e-STOP (EudraTC 2018-000775-32) is currently underway to assess the safety of early discontinuation of antibiotherapy in patients with FN and validate a clinical scoring system in the Spanish population.
More recently, an algorithm for risk stratification has been developed and published (DISCERN-FN) that has exhibited a high sensitivity (95%–98%) and an acceptable specificity (38%–58%) in the detection of severe infection, which has yet to be validated in Spain.18
What should be the initial approach in the management of paediatric oncological/haematological patients with FN?The initial assessment of paediatric oncological/haematological patients with FN must include an exhaustive history-taking, both general and focused on identifying potential foci of, an assessment of immunocompetence and associated risks, a careful and detailed physical examination—including vital signs and assessment of signs of sepsis—and blood tests including a complete blood count, blood chemistry panel and measurement of inflammatory markers, such as C-reactive protein (CPR)5 and procalcitonin (PCT),18 with the option of enhancing their sensitivity by expanding the assessment with additional markers (e.g., interleukin-6, interleukin-8, etc.).7Table 3 summarises the recommendations regarding the history-taking, physical examination and diagnostic tests.
History-taking, physical examination and diagnostic tests in the assessment of oncological/haematological patients with febrile neutropenia.
| General history | • Current symptoms. |
| • Past personal medical history and comorbidities. | |
| • Recent surgery. | |
| • Contact with pets. | |
| • Recent travel. | |
| • Epidemiological environment in/of the family. | |
| Oncological history | • Underlying disease. |
| • Devices (CVC, VP shunt, gastrostomy, etc.) and recent handlings of these devices. | |
| • Previous treatment (chemotherapy): regimen and dates. | |
| • Current or recent steroid therapy. | |
| • Previous infection and/or colonization by microorganisms resistant to antibiotics (multidrug resistance). | |
| • Results of most recent blood tests. | |
| • Recent transfusion. | |
| • Current treatment (including antibiotic prophylaxis). | |
| Physical examination | • Vital signs: temperature, heart rate, respiratory rate, blood pressure and oxygen saturation. |
| • Paediatric assessment triangle (early detection of sepsis). | |
| • Review of systems, including: neurologic assessment, perfusion, skin and mucosae (oral and perianal, avoid rectal palpation), any area with pain, scars, devices, etc. | |
| Blood tests | • Complete blood count. |
| • Blood chemistry panel including electrolytes, liver and kidney function (markers of sepsis, hydration status and deleterious effects of cytotoxic drugs that may require adjustments to the treatment). | |
| • Inflammatory markers: CRP ± PCT, IL-8, IL-6… | |
| Microbiological tests | • Blood culture: central line (all lumens) ± peripherical (differential). |
| • Urine culture (and sediment), non-invasive (low yield). | |
| • Nasopharyngeal aspirate/exudate (during epidemic season or multiplex PCR). | |
| • Collection of samples from cutaneous or mucosal lesions (herpes simplex, varicella-zoster, enterovirus, fungi). | |
| • Samples from identified possible foci of infection (stool, secretions, cerebrospinal fluid …). | |
| Other diagnostic tests | • Chest radiograph: in patients with respiratory symptoms. |
| • Abdominal ultrasound: in patients with abdominal pain, peritonism, etc. | |
| • Head CT/MRI ± lumbar puncture: in patients with focal neurologic deficits. |
CRP, C-reactive protein; CT, computed tomography; CVC, central vascular catheter; IL, interleukin; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; PCT, procalcitonin; VP, ventriculoperitoneal.
The performance of other diagnostic tests, which should not delay initiation of treatment, may include collection of samples for blood culture from all central line lumens. In addition, consider collection of peripheral blood samples for culture, especially in the case of suspected catheter-related bloodstream infection (for differential quantitative or qualitative culture, in this case, ensuring collection of the same volume and culture incubation time), non-invasive urine sample and sample for detection of respiratory viruses during epidemic seasons and/or in patients with respiratory symptoms. The performance of additional diagnostic tests will be based on the signs and symptoms.3–5
Furthermore, monitoring of clinical manifestations, haemodynamic status and laboratory values helps identify previously undetected foci of infection and complications and assess the response to treatment, which can then be adjusted as needed.7
What are the recommendations for empiric antibiotherapy in paediatric oncological/haematological patients with FN?Early initiation of broad-spectrum antibiotherapy in these patients is a crucial aspect that has an impact on patient outcomes.3,4 Given the high risk of infection by P. aeruginosa and the high associated morbidity and mortality, we recommend selecting agents active against this pathogen for empiric antibiotherapy. Fig. 1 presents the algorithm to select treatment based on individual risk, and Table 4 the dosage of the most frequently used antimicrobial agents.
Algorithm for the management of episodes of febrile neutropenia in paediatric oncological/haematological patients.
GNB, gram-negative bacilli; GPC, gram-positive cocci; MDR, multidrug-resistant.
Source: adapted from Lehrnbecher et al., 2014.4
Dosage of the main antibiotics used for treatment of febrile neutropenia in paediatric oncological/haematological patients.a
| Drug | Paediatric dose | Adult dose | Maximum dose | Comments |
|---|---|---|---|---|
| Antibiotic | ||||
| Cefepime | 150 mg/kg/day every 8 h | 2 g/8 h | 6 g/day | |
| Piperacillin–tazobactam | Piperacillin 300–400 mg/kg/day every 6–8 h | Piperacillin/tazobactam 4/0.5 g every 6–8 h | 16 g/day of piperacillin | In the case of severe infection, high MIC or infection by P. aeruginosa, prioritize administration of piperacillin 400 mg/kg/day every 6 h in continuous infusion over 4 h. |
| Ceftazidime | 150–200 mg/kg/day every 8 h | 1–2 g every 8 h | 8 g/day | In the case of severe infection, consider 200–300 mg/kg/day every 8 h. |
| Meropenem | 60 mg/kg/day every 8 h | 1−2 g/8 h | 6 g/day | In the case of severe infection, prioritize administration of 120 mg/kg/day every 8 h in continuous infusion over 4 h. |
| Imipenem | 60–100 mg/kg/day every 8 h | 0,5–1 g every 6 h | 4 g/day | Prioritize administration of the high dose in the case of severe infection or a high MIC. |
| Vancomycinb | 45–80 mg/kg/day every 6–8 h | 45–80 mg/kg/day every 8 h | 4 g/day | Prioritize administration of the high dose in the case of severe infection or a high MIC. |
| Teicoplanin | 10 mg/kg/dose every 12 h (3 doses) and every 24 h thereafter | 6 mg/kg/dose every 12 h (3 doses) and every 24 h thereafter | 400 mg/dose | In the case of severe infection, osteoarticular involvement or endocarditis, the dose can be increased to 12 mg/kg/dose every 12 h (3–5 dose) and every 24 h thereafter (maximum 800 mg/dose). |
| Amikacinc | 15–22.5 mg/kg/day every 24 h | Same dose as in children | None (adjust based on plasma concentration) | Consider 22–30 mg/kg/day every 24 h in patients with septic shock and/or pulmonary infection. |
| Antifungals | ||||
| Caspofungin | Loading dose of 70 mg/m2 on day 1, followed by 50 mg/m2/day thereafter | Loading dose of 70 mg on day 1, followed by 50 mg/day thereafter | 70 mg/day | Less active than liposomal amphotericin B against Aspergillus spp. |
| Liposomal amphotericin B | 3–5 mg/kg/day | Same dose | 600 mg/day | 5–10 mg/kg/day in the case of non-Aspergillus filamentous fungal pathogens. |
MIC, minimum inhibitory concentration.
Consult the recommended dose of each antibiotic based on the susceptibility of the microorganism in the following site: https://www.seipweb.es/dosisantibioticos/.42
Combination therapy has not been found to improve clinical outcomes in stable patients. Therefore, treatment with a β-lactam agent combined with a second antibiotic should be reserved for specific scenarios, such as septic shock and/or a high probability of infection by MDR microorganisms based on local epidemiology and drug resistance trends (Table 5).
Situations that warrant consideration of empiric combination therapy.
| Antibiotic with extended activity against β-lactam-resistant gram-negative bacilli (e.g., aminoglycosides, fluoroquinolones, colistin): |
| • Haemodynamically unstable patient. |
| • Recent colonization or history of infection by gram-negative bacilli resistant to first-line therapy (individualise treatment). |
| • Isolation in blood culture of a gram-negative bacillus, pending identification and antibiotic susceptibility testing, especially if the patient has not responded to empiric antibiotherapy and/or is clinically unstable. |
| • Recent treatment with broad-spectrum antibiotic. |
| Antibiotic with extended activity against methicillin-resistant gram-positive cocci (e.g., vancomycin, teicoplanin, daptomycin): |
| • Haemodynamically unstable patient. |
| • Colonization or previous history of infection by methicillin-resistant Staphylococcus aureus or cephalosporin-resistant Streptococcus pneumoniae. |
| • Isolation in blood culture of a gram-positive coccus, pending identification and antibiotic susceptibility testing, especially if the patient has not responded to empiric antibiotherapy and/or is clinically unstable. |
| • Severe mucositis (grade III–IV) or chemotherapy regimen that could induce it (high doses of cytarabine or fludarabine) in centres with a high prevalence of β-lactam resistance in viridans streptococci. |
| • Signs of vascular catheter infection or fever after handling of the catheter. |
| • Signs of local infection of skin or soft tissues. |
| • Centre with a high prevalence of methicillin-resistant Staphylococcus aureus. |
Certain considerations can guide the choice of one β-lactam over another. Piperacillin–tazobactam offers good activity against anaerobes, activity against Enterococcus spp. and is still active against some ESBL-producing GNB strains. Cefepime can cross the blood–brain barrier in sufficient amounts and is active against most AmpC β-lactamase-producing GNB strains. Ceftazidime exhibits only moderate activity against GPC and there is evidence of an increasing prevalence of resistance against it. Therefore, it is essential to consider current local epidemiological trends to guide the selection of empiric antibiotherapy. As regards carbapenems, it is important to take into account their environmental impact on local drug resistance trends, and they are drugs to be reserved for special cases.19,20
In oncological patients, colonization or recent infection by MDR pathogens is a risk factor for infection by an MDR agent,21 and therefore it is important to consider the drug susceptibility profile of the identified pathogens in the selection of antibiotics (Table 6).
Stratification of the risk of invasive fungal infection and risk factors.
| Risk of IFI | Underlying disease | |
|---|---|---|
| High risk | ≥10% | AML |
| High-risk and/or relapsed ALL | ||
| Allogeneic HSCT | ||
| Low risk | 2%–9% (usually <5%) | Standard/intermediate-risk ALL |
| Non-Hodgkin lymphoma | ||
| Autologous HSCT | ||
| Sporadic | ≤1% | Solid organ tumour |
| Hodgkin lymphoma |
| Other factors that increase the risk of IFI |
|---|
| Clinical factors: |
| • Severe and prolonged neutropenia (≤500 neutrophils/mm3 for ≥7–10 days). |
| • Severe mucositis. |
| • Central venous catheter. |
| • Previous fungal colonization. |
| • Severe and prolonged lymphopenia. |
| • Graft versus host disease in HSCT recipient. |
| • Cytomegalovirus infection in HSCT recipient. |
| Pharmacological factors: |
| • High-dose steroid therapy (≥0.3 mg/kg/day of prednisone or equivalent) for ≥3 weeks. |
| • TNF inhibitors and other monoclonal antibodies (e.g., alemtuzumab). |
| • Nucleoside analogues (e.g., cytarabine). |
| • Chimeric antigen receptor (CAR) T-cell therapy. |
| • Tyrosine-kinase inhibitors (e.g., ibrutinib). |
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; HSCT, hematopoietic stem cell transplantation; IFI, invasive fungal infection; TNF, tumour necrosis factor.
In the case of patients with an identified focus of infection, the selection of empirical antibiotherapy will be based on which pathogens are most frequently involved at that location, ensuring adequate control of infection at the site (e.g., removal of infected catheters, abscess drainage, etc.).
Haemodynamically unstable patientsThe mortality associated with septic shock in oncological patients is high. One of the main factors associated with mortality in these cases is inadequate empiric antibiotherapy with antimicrobials that are not active against the causative agent.22 Therefore, we recommend broadening the antimicrobial spectrum to increase the chances of including an agent with activity against the involved microorganism, contemplating subsequent adjustments based on the course of disease and microbial isolation.
Recently developed agents (e.g., ceftazidime–avibactam, ceftolozane–tazobactam, meropenem–vaborbactam, cefiderocol), which are at different stages in their marketing authorization process for their use in children at the time of this writing, offer new treatment options.23 Their use should be restricted to haemodynamically unstable patients with recent infection or colonization by pathogens resistant to carbapenems in the case of empiric therapy and to cases in which the isolated pathogen is resistant to first-line antibiotics, chiefly in severe infections.24
Patients with β-lactam allergyIn patients with β-lactam allergy and a previous allergy evaluation, empirical antibiotherapy should be selected according to the results of said evaluation. In patients without a previous allergy evaluation, consider the use of aztreonam (combined with an agent with activity against GPC) or meropenem.
Low-risk patientsA systematic review did not find significant differences in the frequency of treatment failure or the mortality associated with infection in patients at low risk treated with oral versus intravenous empiric antibiotherapy.3 Different options have been proposed (e.g., levofloxacin, amoxicillin–clavulanic acid combined with ciprofloxacin, etc.), the selection of which should be based on local epidemiological trends and the previous history of infection.
It is important to monitor the patient closely. In the case of clinical worsening, oral intolerance, signs of focal infection associated with a high risk of progression or isolation of a pathogen that cannot be treated with oral antibiotherapy or in which intravenous delivery is considered a better option, the patient should be admitted to hospital. If the facility has the capability to offer at-home intravenous antibiotherapy, this option can be considered if the patient meets the established eligibility criteria.
What is the appropriate antibiotic strategy beyond empiric therapy in paediatric oncological/haematological patients?The mean time to positivity for blood cultures in patients with FN is of approximately 12 h, with a majority turning positive in the first 24 h.25 A time to positivity greater than 24–48 h is usually indicative of infection by slow-growing microorganisms (Candida spp., anaerobes or CoNS) or contamination.
In adults with blood tumours and high-risk FN of unknown aetiology, the discontinuation of empiric antibiotherapy 72 h after the patient becomes afebrile and all signs of symptoms of infection have resolved, as opposed to the standard approach of maintaining antibiotic treatment until the neutrophil count recovers, has been found to be safe, and it allows to reduce the duration of antimicrobial exposure with no impact on mortality. The updated guidelines for adult patients of the Fourth European Conference on Infections in Leukemia (ECIL-4) established that in patients with FN and no clinical or microbiological evidence of infection, empiric antibiotherapy can be discontinued after a minimum of 72 h of intravenous treatment in patients who have been haemodynamically stable from onset and afebrile for at least 48 h, independently of the neutrophil count or the expected duration of neutropenia.
When it comes to the paediatric population, Kobayashi et al.26 analysed 170 episodes of FN in which antibiotherapy had been discontinued before neutrophil recovery to assess the risk of recurrence. In the multivariate analysis, a neutrophil count of less than 100 cells/mm3 at the time of discontinuation was an independent risk factor for recurrent fever. Similarly, an observational study in children with FN concluded that discontinuation of empiric antibiotherapy in those who did not have bacterial infection and with neutrophil counts greater than 100 mm3 after 24 h of being afebrile was safe.27 An international paediatric guideline considers discontinuation of empiric antibiotherapy in low-risk patients with no documented pathogen safe if they have been afebrile for 24 h and received antibiotherapy for 72 h, irrespective of the neutrophil count, and gave no recommendation for the specific scenario of persistent severe neutropaenia.3
Based on the available evidence, we recommend reassessing the need for empiric antibiotherapy 48–72 h after its initiation, considering de-escalation and discontinuation of combination therapy. Once empiric antibiotherapy is initiated, subsequent adjustments should be based, preferably, on culture results or the identified focus of infection to establish targeted therapy against the isolated microorganism and for infection control at the focus, if applicable (Fig. 1).
When it comes to the use of inflammatory markers in the follow-up, the current evidence is not sufficient to support their use in guiding decisions regarding the duration of antibiotherapy.28
What is the significance of invasive fungal infection in paediatric oncological/haematological patients with FN?The incidence of invasive fungal infection (IFI) in paediatric patients with cancer or who have undergone HSCT ranges from 2% to 20%.29,30 Individual risk depends on the underlying disease of the patient and associated factors (Table 6), of which prolonged severe neutropenia is the most relevant.
The fungi isolated most frequently in these patients are Candida spp. and Aspergillus spp., which are associated with a mortality of 10%–25% and of 20%–50%, respectively.29
What is the appropriate strategy to assess for IFI in paediatric oncological/haematological patients with FN?Invasive fungal infection should be considered and suspected in patients with FN that persists for 96 h or longer despite appropriate antibiotherapy, especially those classified as being at high risk of IFI. Collection of samples, including blood and from the site of infection, is essential.31,32
We recommend performance of a serum galactomannan antigen test in patients with prolonged FN and risk of aspergillosis (cut-off point, 0.5). It is also possible to assess the presence of galactomannan in bronchoalveolar lavage and cerebrospinal fluid samples (cut-off point, 1).31–33 At present, routine testing for other biomarkers is not recommended for diagnosis of IFI in paediatric patients.31 Molecular tests (specific or panfungal [18S] PCR assays, T2MR) offer some advantages compared to traditional methods and need to be interpreted by an experienced microbiologist.31,34
A chest CT scan is indicated in paediatric patients at high risk of IFI with FN of more than 96 h’ duration or with respiratory manifestations and an inconclusive chest radiograph. In the case of abnormal findings in the chest CT scan, it is crucial to obtain bronchoalveolar lavage or lung biopsy samples for microbiological testing.31,35 Abdominal imaging should be considered to rule out invasive candidiasis if a focus is not identified, in addition to other imaging tests depending on the clinical presentation and level of risk. Based on the findings, IFI is classified as possible, probable or proven (criteria of the European Organization for Research and Treatment of Cancer [EORT]/ Mycoses Study Group [MSG]).36
When and how should antifungal therapy be initiated in paediatric oncological/haematological patients with FN? What is the appropriate management strategy after initiation of antifungal therapy?Two strategies have been established for the management of IFI in paediatric oncological/haematological patients with FN:
Empiric antifungal therapy consists in initiating antifungal treatment as soon as fungal infection is suspected, without waiting for the results of the applicable diagnostic tests. Consider caspofungin or liposomal amphotericin B (standard of care if there is a high risk of filamentous fungal infection), and possibly switching to a different class if the patient had received antifungal prophylaxis in the past (especially in the case of azoles).
Pre-emptive therapy consists in carrying out the tests first (imaging and fungal biomarkers) and initiating antifungal treatment once one of the results has turned out positive based on the findings.31,37
Both strategies have proven safe in paediatric care,38 so the choice will depend on the characteristics of the patient, the suspected microorganism and the resources and epidemiological trends of the centre. In clinically unstable patients with additional risk factors for candidaemia, administration of empiric therapy against yeasts should be prioritised, while in clinically stable patients with prolonged FN or clinical suspicion of filamentous fungal infection, we recommend waiting for tests results if the turnaround time is short.
If fungal infection is confirmed, it is important to control the focus of infection, either by removing indwelling devices or surgical resection, if necessary, as well as predisposing factors.
Once treatment has been initiated and all necessary diagnostic tests have been done to identify the aetiological agent and assess disease extension, we recommend monitoring the response to treatment (clinical, laboratory and imaging findings). In the case of candidaemia, a follow-up blood culture should be performed every 48 h until resolution of fever and one or more cultures turn out negative.
When it comes to imaging for monitoring, the EORTC/MSG recommendation is carrying out imaging tests at 4–6 and 12 weeks of treatment. However, in some patients it may be necessary from the second week of treatment.31,32,34
Monitoring of plasma drug concentrations is essential in the case of voriconazole, posaconazole (oral suspension) and itraconazole, and should also be considered for isavuconazole until more data are available for the paediatric population.34,37
In the case of infection by Candida spp., treatment must be maintained for a minimum of 14 days after the first negative culture in patients with candidaemia, and longer in the case of infection of a solid organ.39 In the case of a catheter-related infection, the catheter must be removed. In the case of infection by filamentous fungi, we recommend a minimum duration of treatment of 6 weeks, to be prolonged as needed until the full clinical/laboratory/radiological resolution of the infection.31,32,34
Which non-antimicrobial treatments are indicated in the management of paediatric oncological/haematological patients?Supportive care (Table 7) encompasses general measures, such as fluid replacement therapy, analgesic and antipyretic drugs, any other ongoing treatment the patient is receiving, consideration of the need of stress doses of hydrocortisone (patients with current or recent steroid therapy) and measures aimed at reducing the incidence of infection in neutropenic patients.40
Supportive care and prevention measures for febrile neutropenia.
| Supportive care in FN | • Fluid replacement therapy: ensure adequate fluid and electrolyte balance. |
| • Analgesics and antipyretics (avoid NSAIDs). | |
| • Ongoing treatment: analgesia, prophylaxis, supplementation… use antihypertensive drugs with caution. | |
| • Usually, discontinuation of oral chemotherapy and consideration in consultation with oncologist/haematologist of completion (or discontinuation) of intravenous cycles in stable patients. | |
| • Consider need of stress dose of hydrocortisone: patients receiving steroids or that have received high-dose steroids in recent months. | |
| • Continue/initiate G-CSF in patients with solid tumours or lymphomas. | |
| Prevention | • G-CSF in patients with solid tumours receiving intensive chemotherapy (>20% FN), who need to maintain cytotoxic dose/chemotherapy intensity and/or with previous history of FN. |
| • Personal protective equipment (gown, gloves…) in patients with contagious infection or colonization by MDR microorganisms. | |
| • Annual vaccination against seasonal flu of patients and their household contacts and health care workers. | |
| • Aseptic technique in CVC handling. | |
| • Hand, surface and food preparation hygiene measures. | |
| • Avoid contact with pets associated with a high risk: turtles, cat’s litter, stables and new pets. This does not apply to other pets that are correctly vaccinated, in regard to which the sole precaution is to avoid contact with their faeces. | |
| Prevention of IFI (high risk patients/situations) | • Avoid flowers and plants in hospital rooms and the bedroom of the patient. |
| • Hospital rooms with HEPA filters and positive pressure (>12 room air changes/h). | |
| • Use of FPP2 masks in closed spaces without HEPA filters (except the usual place of residence, where ventilation, avoiding plants, moisture and construction work). | |
| • Avoid pools, especially public or indoor ones. | |
| Recommendation not supported by evidence | • Low-bacteria diet: not proven to reduce the incidence or severity of infection. |
| • Personal protective equipment for patients in absence of microbial isolation that justifies it. |
CVC, central venous catheter; FN, febrile neutropenia; G-CSF, granulocyte colony-stimulating factor; IFI, invasive fungal infection; MDR, multidrug-resistant; NSAID, nonsteroidal anti-inflammatory drug.
Treatment with granulocyte colony-stimulating factor (G-CSF) shortens the duration of neutropenia, in addition to the duration of antibiotherapy and the length of stay, but there is no evidence that it reduces the frequency or severity of infection. It is generally recommended in patients with solid tumours or lymphoma, but only occasionally in patients with leukaemia.3
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Coordinator: Leticia Martínez Campos (Hospital Universitario Torrecárdenas, Almería).
Secretary: Jesús Saavedra Lozano (Hospital Gregorio Marañón, Madrid).
Members: David Aguilera Alonso (Hospital Gregorio Marañón, Madrid); Cristina Calvo Rey (Hospital la Paz, Madrid); Jaime Carrasco Colom (Hospital Universitario Son Espases, Majorca); Elena Colino Gil (Hospital de las Palmas, Gran Canaria); David López Martín (Hospital Costa del Sol, Marbella); Ana Isabel Menasalvas Ruiz (Hospital Virgen de la Arrixaca, Murcia); Esmeralda Núñez Cuadros (Hospital Carlos Haya, Malaga) and Carlos Rodrigo Gonzalo de Liria (Hospital Vall d’Hebrón, Barcelona).
Coordinator: Peter Olbrich (Hospital Virgen del Rocío, Seville).
Secretary: Begoña Carazo Gallego (Hospital Carlos Haya, Malaga).
Members: Laura Ferreras Antolín (St. George’s Hospital, London); Carlos Daniel Grasa Lozano (Hospital La Paz, Madrid); Natalia Mendoza Palomar (Hospital Vall d’Hebron, Barcelona); Marisa Navarro Gómez (Hospital Gregorio Marañón, Madrid); Olaf Neth (Hospital Virgen del Rocío, Seville); José Tomás Ramos Amador (Hospital Clínico San Carlos, Madrid); Elena Rincón López (Hospital Gregorio Marañón, Madrid); Jesús Ruiz Contreras (Hospital 12 de octubre, Madrid) and Pere Soler Palacín (Hospital Vall d’Hebrón, Barcelona).
Coordinator: Paula Pérez-Albert (Hospital Vall d’Hebron, Barcelona).
Members: Laia Ferres Ramis (H. Universitario Son Espases, Majorca); Jorge Huerta Aragonés (Hospital Universitario Gregorio Marañón); Silvia López Iniesta (Complejo Universitario del Hospital de León); Raquel Olivas Mazón (Hospital Clínico Universitario de Valencia); Raquel Portugal Rodríguez (Hospital Universitario de Burgos); Alexandra Regueiro García (Hospital Clínico Universitario de Santiago); Susana Riesco (Hospital Clínico Universitario de Salamanca) and José Antonio Villegas Rubio (Hospital Universitario Central de Asturias).
Appendix A lists the members of the Working Group on Bacterial Infection of the Sociedad Española de Infectología Pediátrica (SEIP), Support Group of the Sociedad Española de Hematología, Oncología Pediátrica (SEHOP), Working Group on Invasive Fungal Infection of the Sociedad Española de Infectología Pediátrica (SEIP).