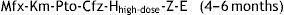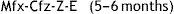Tuberculosis (TB) is the most important infectious disease all over the world, with a high morbidity and mortality. Paediatric tuberculosis has been a neglected epidemic, due to the difficulties in assessing its global impact, reduced incidence and lower infectivity compared to adults. In 2015, the WHO reported 1 million cases of paediatric TB and 169,000 deaths. In Europe, the emergence of MDR TB is a major concern, representing 16% of the new diagnosis in Eastern Europe. In 2014, it was estimated that about 219,000 children were infected by MDR-TB-strains in Europe, and 2120 developed the disease. Spain is the Western European country with more paediatric cases, with an incidence 4.3/100,000 inhabitants in 2014. Paediatric tuberculosis mortality in Spain is rare, but extra-pulmonary disease is associated with significant complications. The prevalence of paediatric drug resistant TB in Spain is over 4%, higher than the estimated incidence in adult population, representing mayor difficulties for therapeutic intervention. These data reveal that paediatric TB is still a Public Health priority in our country.
The difficulties in diagnosis and the lack of optimal paediatric drug formulations are the major challenges for controlling the childhood's tuberculosis epidemic. A group of national paeditric TB experts has reviewed the international guidelines and the most recent evidences, and has established new recommendations for the management of paediatric TB contacts, latent infection and active TB disease, especially focused in drug resistant cases. This document replaces the former national guidelines from the Spanish Society for Pediatric Infectious Diseases, although the prior recommendations on the diagnosis remain valid.
La tuberculosis (TB) es la enfermedad infecciosa más importante del mundo, asociando enorme morbimortalidad. La TB pediátrica ha sido una epidemia oculta por su escasa capacidad infectiva y menor incidencia comparada con adultos. El informe-OMS 2015 estimó un millón de niños enfermos de TB en el mundo y 169.000 fallecidos. En Europa, el problema acuciante es la tuberculosis multirresistente, con tasas del 16% en nuevos diagnósticos, especialmente en países del este. En 2014, 219.000 niños se infectaron por cepas-MDR en Europa, 2.120 desarrollaron enfermedad. España es el país de Europa con mayor número de casos pediátricos, con una incidencia en 2014: 4,3/100.000 habitantes. La mortalidad por TB pediátrica en nuestro país es excepcional, pero las formas extrapulmonares ocasionan importantes complicaciones. La TB resistente en niños en España presenta una prevalencia >4%, superando incluso la notificada en adultos. Estos datos reflejan que la TB en niños en nuestro medio continúa siendo un problema de salud pública prioritario.
Las dificultades diagnósticas del niño y la falta de formulaciones pediátricas óptimas son el mayor desafío para control de TB infantil. El Grupo de expertos de TB pediátrica realiza un análisis de las nuevas tendencias internacionales y guías terapéuticas de tuberculosis en niños, según nuevas evidencias disponibles; y considera una prioridad actualizar las guías pediátricas nacionales de exposición a TB, infección tuberculosa latente y enfermedad, y particularmente los casos de resistencia a fármacos. Este documento, por tanto, sustituye a todos los previos en cuanto a las pautas terapéuticas, aunque siguen estando vigentes las indicaciones diagnósticas.
Tuberculosis (TB) is the most important infectious disease worldwide and one of the 10 leading causes of death, although its incidence and associated mortality are experiencing a marked decline. In 2015, there were 104 million new cases, 480,000 of them of multidrug-resistant TB (MDR-TB), and 1.4 million deaths caused by this disease. Through its End TB strategy, the WHO intends to decrease the incidence of TB by 80% and its mortality by 90% by year 2030.
Paediatric TB has been a hidden global epidemic for decades due to the difficulty of estimating its true impact. In highly endemic countries, the main barriers are poverty and the limited accessibility of health care. International policies have neglected this population due to the lower incidence of TB in children compared to adults and the low infectivity of TB. Even the Stop TB strategy, based on the quantification and treatment of individuals with active disease, excluded children because they rarely have positive sputum-smear results. Since 2012, there has been an increasing awareness of the need to include children in these programmes.2 The latest reports of the WHO1 provide more accurate data on the impact of childhood TB, with an actual incidence that exceeds published data by 25%, an estimated one million diseased children worldwide, and 169,000 dying from TB in 2015.
In Europe, the overall prevalence of childhood TB has been decreasing each year, but TB continues to be a public health priority, with more than 40,000 cases reported in the past decade and an overall prevalence of MDR-TB of 16% in newly diagnosed cases and 48% in previously treated cases. Eastern European countries are the most important contributors to these figures and pose a serious threat to the overall control of the disease.3 It is estimated that in 2014 there were 219,000 children infected with MDR strains in Europe, of who 2120 developed the disease.4
Spain is the country in Western Europe with the highest number of paediatric cases, although the incidence is progressively decreasing. In 2014, 303 cases were reported in children, corresponding to an incidence of 4.3 cases per 100,000 inhabitants.3 The epidemiology of TB, marked by HIV infection in the last two decades of the 20th century, has shifted, with immigrant status5 and immune disorders being the most important risk factors at present.6 The difficulties of its detection in children and the lack of drug formulations suitable for paediatric use pose the greatest challenges in the control of childhood TB. Deaths due to childhood TB are rare in Spain, but extrapulmonary forms of disease cause significant complications and sequelae.7 Last of all, we do not know the burden of drug-resistant TB in children in Spain. A prevalence of more than 4% has been estimated, exceeding the prevalence reported in adults, and with a predominance of cases in large cities.8
Exposure to tuberculosisExposure to TB is defined as meeting all of the following criteria9,10:
- a.
Recent close contact (>4h a day in the same closed-off space), in the past 3 months, with an individual with confirmed/suspected active TB disease (pulmonary, laryngeal, tracheal or endobronchial).
- b.
Negative tuberculin skin test (TST) (<5mm). Negative results in the interferon-gamma release assay (IGRA) when performed.
- c.
Absence of clinical signs and symptoms compatible with TB.
- d.
In immunosuppressed patients or young children, normal findings in chest radiographs (frontal and lateral views), when performed.
In this situation, given the risk of developing TB disease during the window period if there is an undiagnosed primary infection, the Sociedad Española de Infectología Pediátrica (Spanish Society of Paediatric Infectious Disease [SEIP]) has recommended initiation of primary prophylaxis with isoniazid (H) in all children aged less than 18 years since 2006.9 In 2010, the American Academy of Pediatrics (AAP) and the American Thoracic Society (ATS) proposed that prophylactic treatment be given only to children aged less than 4 years.11 Since 2014, the WHO recommends prophylaxis in children aged less than 5 years,12 while the AAP maintains the age limit of 4 years.13 In 2016, the British Thoracic Society (BTS) further reduced the age range to less than 2 years.14 These recommendations are based on studies that show that initiation of prophylaxis does not offer any advantages over the close monitoring of patients while awaiting the results of a second TST or IGRA, save in very young or immunosuppressed children, who are at higher risk of TB infection or disease following exposure.
Having reviewed the evidence, and in line with current updates, the SEIP puts forward new recommendations on post-exposure prophylaxis:
- –
Initiate prophylaxis with H (Table 1):
- ∘
All children aged less than 5 years.
- ∘
Children of any age undergoing treatment that leads to immunosuppression (prolonged corticosteroid therapy, TNF-alpha antagonists, immunosuppressive agents, etc.) or with comorbidities involving the immune system (HIV infection, chronic kidney failure, solid or blood tumours, primary immunodeficiencies, etc.).12–14
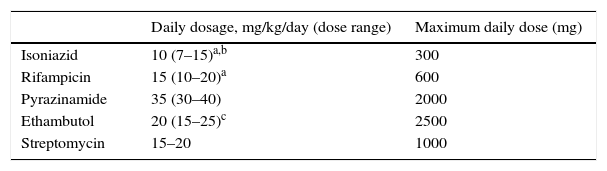
Table 1.Recommended doses of the first-line antituberculous drugs used most frequently in the paediatric age group.12
Daily dosage, mg/kg/day (dose range) Maximum daily dose (mg) Isoniazid 10 (7–15)a,b 300 Rifampicin 15 (10–20)a 600 Pyrazinamide 35 (30–40) 2000 Ethambutol 20 (15–25)c 2500 Streptomycin 15–20 1000 bAdd pyridoxine at 15–50mg/day (maximum 50mg/day) in children who are exclusively breastfed or follow a vegetarian diet, with changes in nutrition or with HIV, and in pregnant adolescents.
cIt is recommended that ethambutol be used at more potent doses (20–25mg/kg/day) during the initiation phase, decreasing the dose to 15–20mg/kg/day during the continuation phase.
Intermittent therapy with higher doses given 3 days a week can be considered in especial cases, always as directly observed therapy.
- ∘
- –
When 8–10 weeks13–15 have elapsed since the last exposure to a source of infection, another TST will be performed, whether treatment with H was initiated or not. Subsequent management will depend on its results:
- ∘
If the induration in the new TST is <5mm (or in case of a negative IGRA, when applicable) and there are no clinical manifestations: discontinue prophylaxis if it has been initiated. Exceptions: immunosuppressed children or infants aged less than 3 months with a high-risk contact (close contact with a noncompliant source case, a retreatment case, etc.) in who completion of a course of treatment for latent tuberculosis infection (LTBI) is recommended even in the absence of a positive TST.
- ∘
If the induration in the new TST ≥5mm (or the IGRA positive, if applicable): follow recommendations for LTBI.
- ∘
It is important to temporarily prevent further contact between the exposed child and the TB disease source, with the child isolated in a separate room in the home until confirmation that the source is not contagious13; which is estimated to occur starting from at least 2 weeks of adequate treatment.
In newborns of mothers with active TB disease, prophylaxis with H should be initiated after ruling out TB infection and disease. If the induration in the repeated TST is 0mm at 10–12 weeks and the decision is made to discontinue prophylactic treatment, the possibility of administering the BCG vaccine should be assessed, as the window period may not have concluded.16 Breastfeeding is not recommended against, except in cases of tuberculous mastitis.16 Maternal milk extraction and administration with a bottle are recommended to avoid mother–baby contact under the following circumstances16:
- –
The mother has been treated for fewer than 2 weeks.
- –
The mother continues to be contagious despite treatment.
- –
The causative TB strain is not susceptible to first-line antibiotics.
Latent tuberculosis infection (LTBI) in children and adolescents is defined as infection through contact with an individual with active disease that does not progress to TB disease and remains as an asymptomatic latent infection.
In clinical practice, LTBI is diagnosed in asymptomatic patients with normal chest radiographs (frontal and lateral) and with:
- –
A positive TST.
- –
Known contact with an individual with active TB (source).
- –
No known contact with an active TB source, but positive TST and/or IGRA results, especially in children aged less than 5 years or who are immunosuppressed.17
In the absence of known contacts and risk factors, if there is a history of vaccination with BCG combined with a positive TST and a negative IGRA, the TST reaction will be interpreted as an effect of the BCG vaccine. Such cases are not considered LTBIs.
Guidelines for the treatment of LTBIAll children and adolescents that receive a diagnosis of LTBI must start treatment immediately to prevent progression to disease, and it is essential that active TB disease be ruled out prior to treatment initiation. The term “LTBI treatment” is preferred over terms like “secondary prophylaxis” or “post-exposure treatment”. The following regimens have been proposed12,14,18,19 (Table 1):
- –
H for 6–9 months (6 H or 9 H): in case of poor adherence, immunosuppression or underlying chronic disease, consider extending treatment to 9 months.
- –
H and rifampicin (R) for 3 months (3 HR), or, in children aged more than 12 years, directly observed therapy with H-rifapentine at 1 dose a week for 12 weeks: this regimen is as efficacious as monotherapy with H, is very well tolerated and has low toxicity. It is especially recommended in adolescents or when poor adherence is suspected. In children with HIV coinfection, the use of R is discouraged, as it is an essential drug in case of disease progression and its earlier use may render it useless. Rifampicin interacts with antiretrovirals, especially with protease inhibitors, resulting in reduced antiretroviral concentrations and increased R toxicity.
- –
R for 4 months (4 R): indicated in patients experiencing H toxicity, in who H is contraindicated, or infected with Mycobacterium tuberculosis strains that are resistant to H and susceptible to R.
Due to the low risk of hepatotoxicity in children, routine monitoring of serum liver enzymes is not recommended during treatment of LTBI except in cases with manifestations suggestive of hepatotoxicity, underlying liver disease or with concomitant treatment with hepatotoxic drugs. In immigrant children from countries with a high prevalence of viral hepatitis or HIV, these diseases must be ruled out before initiating treatment.
Treatment of tuberculosis diseasePulmonary tuberculosisConsidering the 4% and higher rate of H resistance in Spain, the first-line treatment in cases where strain susceptibility is unknown will adhere to the following guidelines12–14,20 (Table 1):
- –
Initiation phase (HRZE 2 months): the most frequently used fourth drug is oral ethambutol (E), monitoring the patient for the potential development of optic neuritis (visual acuity and red-green colour vision tests). A possible alternative in exceptional cases is the use of aminoglycosides such as amikacin or streptomycin.
- –
Duration of treatment with the fourth drug: discontinue when the susceptibility of the strain (source case) becomes known. If it remains unknown, maintain fourth drug for 2 months. If aminoglycosides are used, consider discontinuation at 4–6 weeks.
- –
Continuation phase (HR 4 months): in cases of pulmonary TB caused by a susceptible or unknown strain and that respond well to treatment. For cases of extrapulmonary or drug-resistant TB, consult the specific section on the subject.
This treatment scheme achieves a cure in more than 95% of cases, with a low incidence of adverse events. All drugs should be taken at the same time and in a fasting state. We do not recommend the use of intermittent therapy, except in cases managed with directly observed therapy (DOT). For dosage, drug formulations and combination/fixed-dose formulations, see Tables 1 and 2. For recommendations on compounding,21 see Table 3. Patients with comorbidities or receiving concomitant treatment with other drugs should be referred to a specialty unit.14 In patients at risk of non-adherence, who are immunosuppressed or infected with resistant strains, supervised treatment or DOT is recommended.14
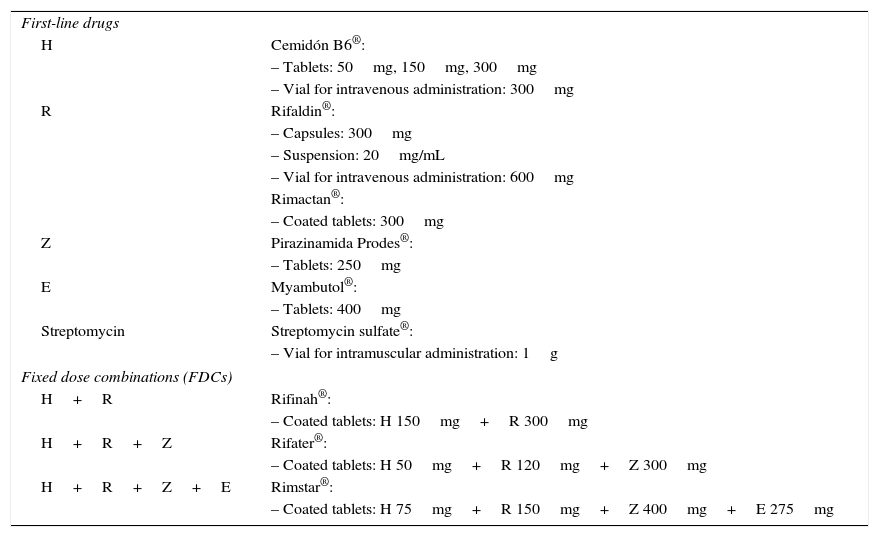
Antituberculous drug formulations commercially available in Spain (last updated: October 30, 2016).
| First-line drugs | |
| H | Cemidón B6®: |
| – Tablets: 50mg, 150mg, 300mg | |
| – Vial for intravenous administration: 300mg | |
| R | Rifaldin®: |
| – Capsules: 300mg | |
| – Suspension: 20mg/mL | |
| – Vial for intravenous administration: 600mg | |
| Rimactan®: | |
| – Coated tablets: 300mg | |
| Z | Pirazinamida Prodes®: |
| – Tablets: 250mg | |
| E | Myambutol®: |
| – Tablets: 400mg | |
| Streptomycin | Streptomycin sulfate®: |
| – Vial for intramuscular administration: 1g | |
| Fixed dose combinations (FDCs) | |
| H+R | Rifinah®: |
| – Coated tablets: H 150mg+R 300mg | |
| H+R+Z | Rifater®: |
| – Coated tablets: H 50mg+R 120mg+Z 300mg | |
| H+R+Z+E | Rimstar®: |
| – Coated tablets: H 75mg+R 150mg+Z 400mg+E 275mg | |
E, ethambutol; H, isoniazid; R, rifampicin; Z, pyrazinamide.
Source: Agencia Española del Medicamento y Productos Sanitarios (Spanish Agency of Medicines and Medical Devices [AEMPS]).38
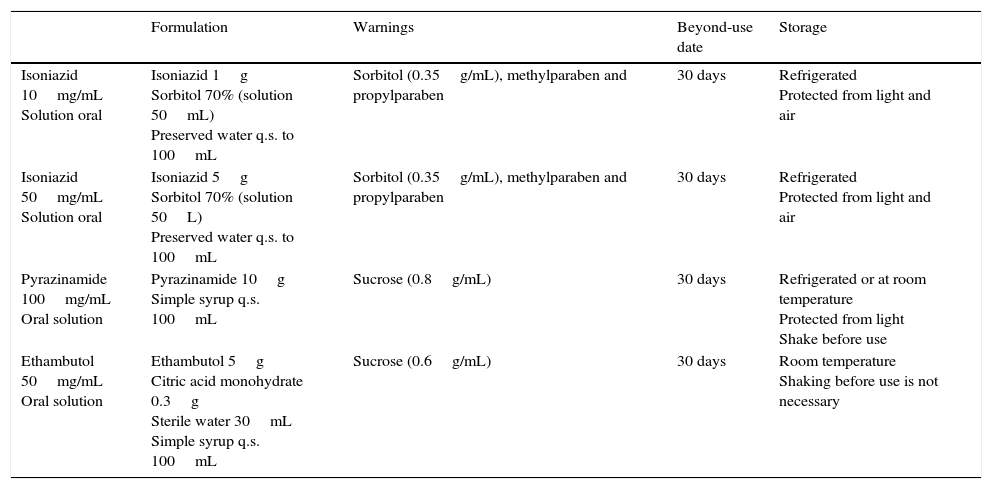
Compounded antituberculous drug formulations. Magistral Project.
| Formulation | Warnings | Beyond-use date | Storage | |
|---|---|---|---|---|
| Isoniazid 10mg/mL Solution oral | Isoniazid 1g Sorbitol 70% (solution 50mL) Preserved water q.s. to 100mL | Sorbitol (0.35g/mL), methylparaben and propylparaben | 30 days | Refrigerated Protected from light and air |
| Isoniazid 50mg/mL Solution oral | Isoniazid 5g Sorbitol 70% (solution 50L) Preserved water q.s. to 100mL | Sorbitol (0.35g/mL), methylparaben and propylparaben | 30 days | Refrigerated Protected from light and air |
| Pyrazinamide 100mg/mL Oral solution | Pyrazinamide 10g Simple syrup q.s. 100mL | Sucrose (0.8g/mL) | 30 days | Refrigerated or at room temperature Protected from light Shake before use |
| Ethambutol 50mg/mL Oral solution | Ethambutol 5g Citric acid monohydrate 0.3g Sterile water 30mL Simple syrup q.s. 100mL | Sucrose (0.6g/mL) | 30 days | Room temperature Shaking before use is not necessary |
Source: Piñeiro Pérez et al.21
Pre-treatment measurement of baseline serum transaminase levels, and subsequently, measurement at 2–3 weeks or before if the patient develops symptoms (Table 4).
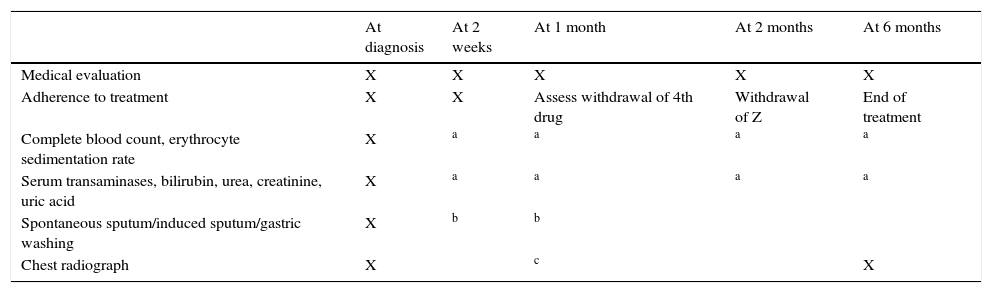
Follow-up of children undergoing treatment for pulmonary TB.
| At diagnosis | At 2 weeks | At 1 month | At 2 months | At 6 months | |
|---|---|---|---|---|---|
| Medical evaluation | X | X | X | X | X |
| Adherence to treatment | X | X | Assess withdrawal of 4th drug | Withdrawal of Z | End of treatment |
| Complete blood count, erythrocyte sedimentation rate | X | a | a | a | a |
| Serum transaminases, bilirubin, urea, creatinine, uric acid | X | a | a | a | a |
| Spontaneous sputum/induced sputum/gastric washing | X | b | b | ||
| Chest radiograph | X | c | X |
Diagnostic tests and additional visits would be made as needed based on clinical manifestations and patient characteristics.
Will only be repeated in special cases (based on clinical manifestations and previous laboratory abnormalities, or in patients with immunosuppression, infants, and patients undergoing concomitant treatment with potentially toxic drugs).
Cases that respond well and without extensive pulmonary disease: radiologic evaluation at the end of the initiation phase and at the end of treatment. Cases with hypoventilation, focal wheezing, extensive disease, cavitation, effusion, non-adherence or drug resistance: perform imaging tests or fibrobronchoscopy as needed. In the first 2 months of treatment, 5–10% of patients may exhibit a paradoxical reaction, characterised by clinical and radiologic worsening22; treatment in these cases will be supplemented with oral corticosteroids, with administration of 2mg/kg/day of prednisone or an equivalent drug for 3–4 weeks, with tapering off over 2 weeks.20,22
Following discharge home and once adherence has been confirmed, a few days of recovery should be allowed before the patient resumes normal activities. In adolescents with active disease, await the results of a sputum-smear culture in 2 weeks.
Extrapulmonary tuberculosisTables 5 and 6 present the recommendations of the SEIP for the treatment of extrapulmonary forms of TB and drug dosage.20 We do not recommend schedules based on the administration of 3 doses a week due to the scarcity of the supporting evidence.20 For adults, the preferred fourth drug is E, even in cases with central nervous system (CNS) involvement.20
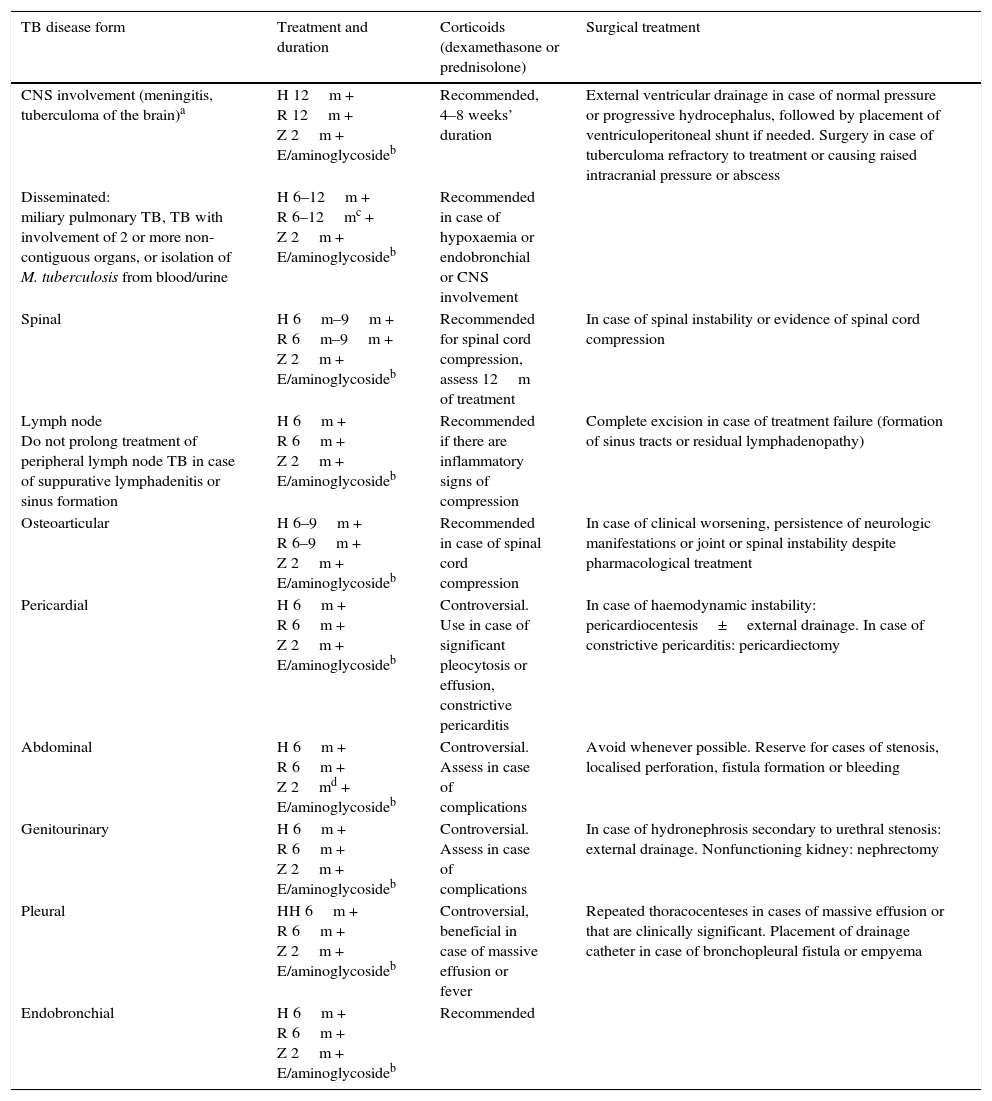
Recommendations for treatment of the main extrapulmonary forms of tuberculosis.
| TB disease form | Treatment and duration | Corticoids (dexamethasone or prednisolone) | Surgical treatment |
|---|---|---|---|
| CNS involvement (meningitis, tuberculoma of the brain)a | H 12m + R 12m + Z 2m + E/aminoglycosideb | Recommended, 4–8 weeks’ duration | External ventricular drainage in case of normal pressure or progressive hydrocephalus, followed by placement of ventriculoperitoneal shunt if needed. Surgery in case of tuberculoma refractory to treatment or causing raised intracranial pressure or abscess |
| Disseminated: miliary pulmonary TB, TB with involvement of 2 or more non-contiguous organs, or isolation of M. tuberculosis from blood/urine | H 6–12m + R 6–12mc + Z 2m + E/aminoglycosideb | Recommended in case of hypoxaemia or endobronchial or CNS involvement | |
| Spinal | H 6m–9m + R 6m–9m + Z 2m + E/aminoglycosideb | Recommended for spinal cord compression, assess 12m of treatment | In case of spinal instability or evidence of spinal cord compression |
| Lymph node Do not prolong treatment of peripheral lymph node TB in case of suppurative lymphadenitis or sinus formation | H 6m + R 6m + Z 2m + E/aminoglycosideb | Recommended if there are inflammatory signs of compression | Complete excision in case of treatment failure (formation of sinus tracts or residual lymphadenopathy) |
| Osteoarticular | H 6–9m + R 6–9m + Z 2m + E/aminoglycosideb | Recommended in case of spinal cord compression | In case of clinical worsening, persistence of neurologic manifestations or joint or spinal instability despite pharmacological treatment |
| Pericardial | H 6m + R 6m + Z 2m + E/aminoglycosideb | Controversial. Use in case of significant pleocytosis or effusion, constrictive pericarditis | In case of haemodynamic instability: pericardiocentesis±external drainage. In case of constrictive pericarditis: pericardiectomy |
| Abdominal | H 6m + R 6m + Z 2md + E/aminoglycosideb | Controversial. Assess in case of complications | Avoid whenever possible. Reserve for cases of stenosis, localised perforation, fistula formation or bleeding |
| Genitourinary | H 6m + R 6m + Z 2m + E/aminoglycosideb | Controversial. Assess in case of complications | In case of hydronephrosis secondary to urethral stenosis: external drainage. Nonfunctioning kidney: nephrectomy |
| Pleural | HH 6m + R 6m + Z 2m + E/aminoglycosideb | Controversial, beneficial in case of massive effusion or fever | Repeated thoracocenteses in cases of massive effusion or that are clinically significant. Placement of drainage catheter in case of bronchopleural fistula or empyema |
| Endobronchial | H 6m + R 6m + Z 2m + E/aminoglycosideb | Recommended |
CNS, central nervous system; E, ethambutol; H, isoniazid; R, rifampicin; TB, tuberculosis; Z, pyrazinamide.
Shorter regimens are sometimes contemplated, and have been used with very good outcomes in South Africa.39 Furthermore, ethionamide/prothionamide penetrate the CNS better and are considered alternative drugs. The doses used for TB with CNS involvement should be in the highest recommended ranges.
Used as the fourth drug until results of drug susceptibility testing become available. Consider maintenance for 2 months (ethambutol) or 4–6 weeks (aminoglycoside) if the strain is not isolated.
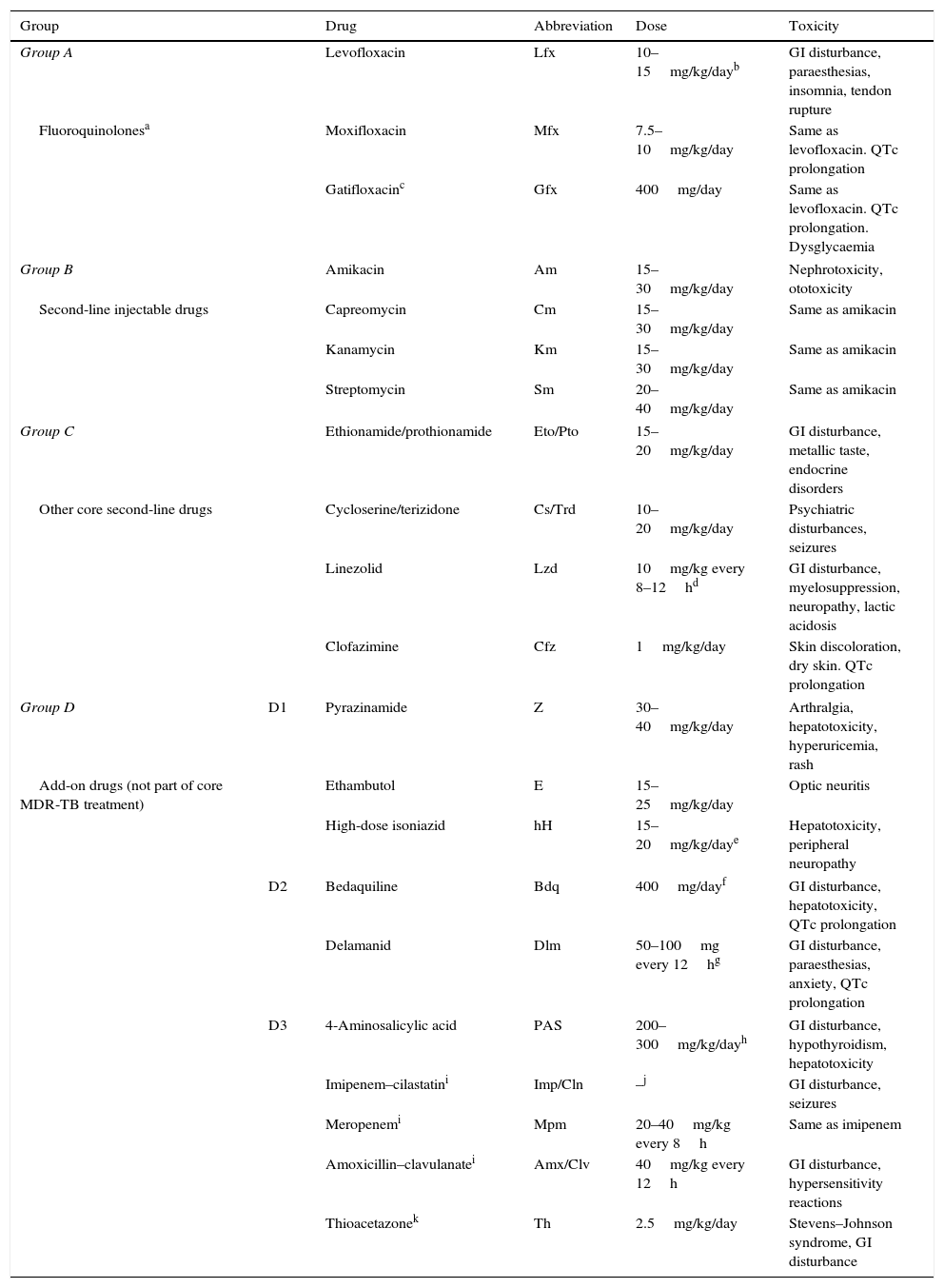
WHO grouping of essential drugs recommended for the treatment of RR-TB and MDR-TB and their doses.
| Group | Drug | Abbreviation | Dose | Toxicity | |
|---|---|---|---|---|---|
| Group A | Levofloxacin | Lfx | 10–15mg/kg/dayb | GI disturbance, paraesthesias, insomnia, tendon rupture | |
| Fluoroquinolonesa | Moxifloxacin | Mfx | 7.5–10mg/kg/day | Same as levofloxacin. QTc prolongation | |
| Gatifloxacinc | Gfx | 400mg/day | Same as levofloxacin. QTc prolongation. Dysglycaemia | ||
| Group B | Amikacin | Am | 15–30mg/kg/day | Nephrotoxicity, ototoxicity | |
| Second-line injectable drugs | Capreomycin | Cm | 15–30mg/kg/day | Same as amikacin | |
| Kanamycin | Km | 15–30mg/kg/day | Same as amikacin | ||
| Streptomycin | Sm | 20–40mg/kg/day | Same as amikacin | ||
| Group C | Ethionamide/prothionamide | Eto/Pto | 15–20mg/kg/day | GI disturbance, metallic taste, endocrine disorders | |
| Other core second-line drugs | Cycloserine/terizidone | Cs/Trd | 10–20mg/kg/day | Psychiatric disturbances, seizures | |
| Linezolid | Lzd | 10mg/kg every 8–12hd | GI disturbance, myelosuppression, neuropathy, lactic acidosis | ||
| Clofazimine | Cfz | 1mg/kg/day | Skin discoloration, dry skin. QTc prolongation | ||
| Group D | D1 | Pyrazinamide | Z | 30–40mg/kg/day | Arthralgia, hepatotoxicity, hyperuricemia, rash |
| Add-on drugs (not part of core MDR-TB treatment) | Ethambutol | E | 15–25mg/kg/day | Optic neuritis | |
| High-dose isoniazid | hH | 15–20mg/kg/daye | Hepatotoxicity, peripheral neuropathy | ||
| D2 | Bedaquiline | Bdq | 400mg/dayf | GI disturbance, hepatotoxicity, QTc prolongation | |
| Delamanid | Dlm | 50–100mg every 12hg | GI disturbance, paraesthesias, anxiety, QTc prolongation | ||
| D3 | 4-Aminosalicylic acid | PAS | 200–300mg/kg/dayh | GI disturbance, hypothyroidism, hepatotoxicity | |
| Imipenem–cilastatini | Imp/Cln | –j | GI disturbance, seizures | ||
| Meropenemi | Mpm | 20–40mg/kg every 8h | Same as imipenem | ||
| Amoxicillin–clavulanatei | Amx/Clv | 40mg/kg every 12h | GI disturbance, hypersensitivity reactions | ||
| Thioacetazonek | Th | 2.5mg/kg/day | Stevens–Johnson syndrome, GI disturbance | ||
GI, gastrointestinal.
Levofloxacin: children aged <5 years, 7.5–10mg/kg every 12h; children aged >5 years, 10–15mg/kg every 24h.
Delamanid: 14 days for children weighing 20–34kg: 50mg every 12h; children weighing more than 35kg: 100mg/12h.
A more rigorous terminology on drug-resistant TB (DR-TB) in children, established by consensus, is currently used.23
- –
Monoresistant TB: TB by a strain resistant to a single first-line drug.
- –
Polyresistant TB: TB by a strain resistant to more than one drug, other than both H and R.
- –
MDR-TB: TB by a strain resistant to at least H and R.
- –
Pre-extensively drug-resistant TB (pre-XDR-TB): MDR-TB with resistance to fluoroquinolones (FQs) or injectable second-line TB drugs but not both.
- –
Extensively drug resistant TB (XDR-TB): MDR-TB with resistance to FQs and injectable second-line TB drugs.
- –
R-resistant TB (RR-TB): strain with any type of R resistance, including monoresistant, polyresistant, MDR- and XDR-TB.
Updates to the WHO guidelines for treatment of drug-resistant TB24:
- –
RR-TB: follow treatment for MDR-TB; resistance to R is a predictor of MDR with a sensitivity exceeding 95%. Routine performance of a line-probe assay is recommended.25 If the strain eventually proves susceptible to H, add H to the regimen and extend treatment to 9–12 months.
- –
Regrouping of TB medicines (Table 6):
- ∘
Group A: FQs.
- ∘
Groups B and C: essential medicines that, along with FQs, constitute the “core” second-line agents.
- ∘
Group D: add-on agents.
- •
Group D1 add-on agents: first-line drugs pyrazinamide (Z), H and E, which are included by default in every case unless complete resistance is confirmed. Weak correlation of in vivo data with in vitro resistance to Z and E.
- •
New group D2: bedaquiline and delamanid.
- •
Group D3: alternative drugs: PAS.
- •
Definitely excluded: macrolides.
- •
- ∘
- –
Short MDR-TB treatment regimen: 9–12 months (still not indicated in children, except for exceptional select cases).
- –
Surgery indicated in select cases.
As is done in cases of susceptible TB, prophylactic treatment will be given to children aged less than 5 years or with immunosuppression. Children exposed to H-resistant strains susceptible to R will receive R, and children exposed to RR-TB strains will be treated with H.
Treatment of latent tuberculosis infectionLatent TB infection by an H-resistant strain should be treated with 4 months of R. patients with LTBI by a strain susceptible to H and resistant to other drugs will receive a standard course of H lasting 6–9 months.
Treatment of isoniazid-resistant tuberculosis diseaseTreatment with RZE or RZE+FQ for 6–9 months or RZ+FQ for 9–12 months, with maintenance of the drugs used in the initiation phase during the continuation phase, or a 2 RZE+7–10 RE regimen.
Treatment of rifampicin-resistant tuberculosis diseaseThese children will receive the same treatment as those with MDR-TB, regardless of H-resistance status, until the latter can be confirmed; in case this is not possible, the strain should be considered MDR. Treat with aminoglycosides as described in the next section.24
Management of multidrug-resistant tuberculosisProphylaxis post exposure to multidrug-resistant tuberculosisTwo valid options supported by scant evidence, based on expert opinion:
- –
Clinical observation without initiation of prophylaxis14,24,25 (UK and WHO guidelines).
- –
Administration of 1 or 2 drugs to which the strain is known to be susceptible26,27 (AAP and Dubai consensus).
- –
Other European guidelines: either option is valid; decide on a case-to-case basis based on: (a) risk of progression, (b) drug resistance profile, and (c) risk of adverse reactions. Treatment is recommended for all children aged <5 years or who are immunosuppressed.28
Regimens recommended in children: FQ (levofloxacin or moxifloxacin); there is evidence on their bactericidal activity and safety profiles.29
Children exposed to MDR-TB with a negative result in the initial test (TST and/or IGRA): it is possible to opt for post-exposure prophylaxis with a combination of a FQ and E or ethionamide (Eto), avoiding monotherapy with FQs that could give rise to resistance in cases in which FQ treatment could eventually become necessary; some authors even recommend the addition of high-dose H until the second TST and/or IGRA at 10–12 weeks is confirmed to be negative.30 The option of maintaining the patient under observation without prophylactic treatment is equally acceptable, especially in children aged more than 5 years with no risk factors.
Children exposed to a XDR-TB or pre-XDR-TB resistant to FQs: strict monitoring without treatment is recommended, as there is not a suitable therapeutic option.
The follow-up should last a minimum of 2 years, with evaluations every 2–3 months at first, and subsequently every 6 months.
Treatment of latent MDR-TB infectionThe most widely accepted regimen is a FQ for 6–9 months (moxifloxacin can only be used in ages >12 years) in combination with another drug (E or Eto), assessing the potential addition of high-dose H.
There is no suitable treatment for cases of LTBI by an XDR or pre-XDR strain resistant to FQs; and close monitoring without treatment is recommended for these patients. In children aged less than 5 years or who are immunosuppressed, consider treatment with 2 drugs to which the strain is known to be susceptible for 9–12 months.
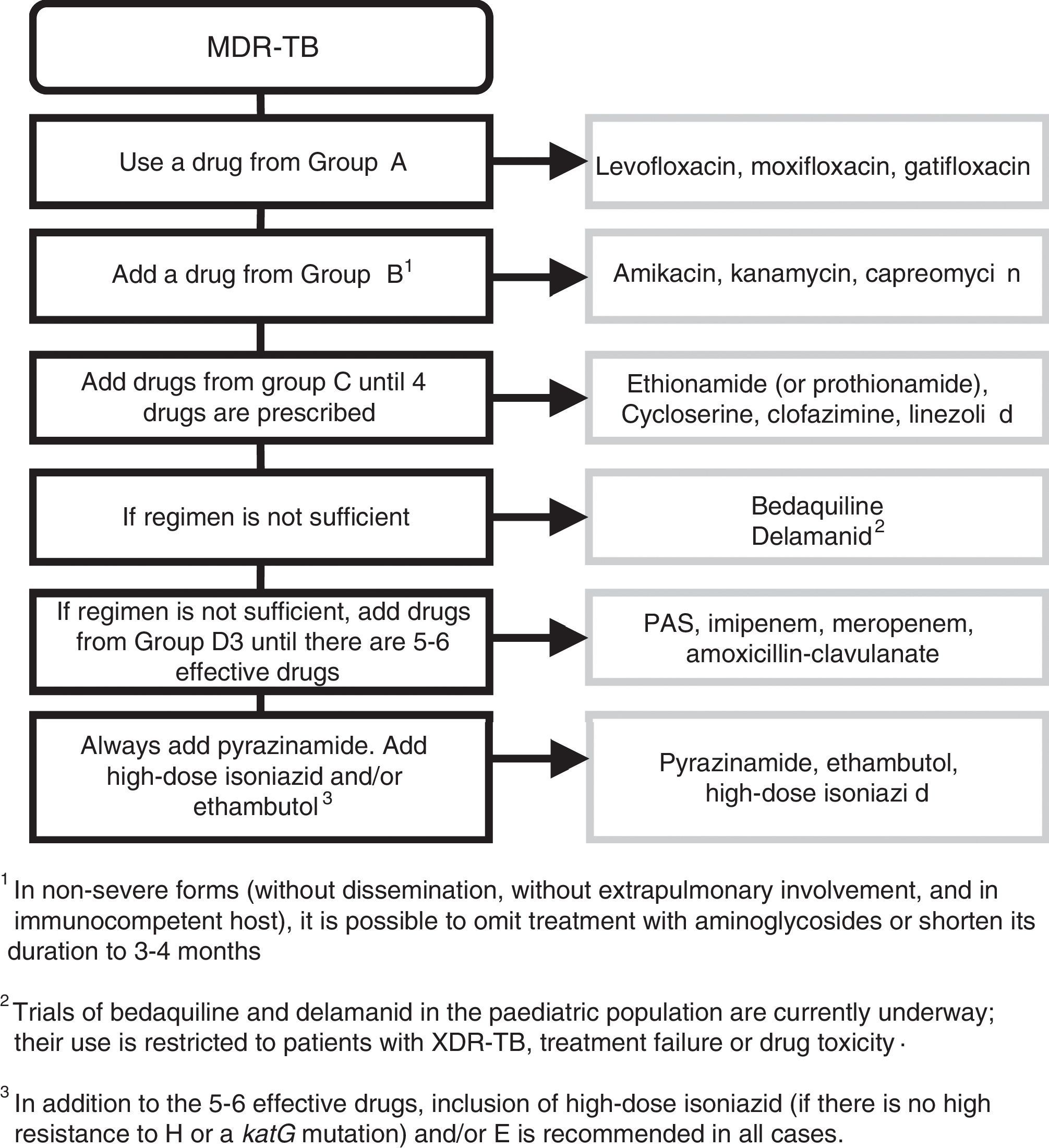
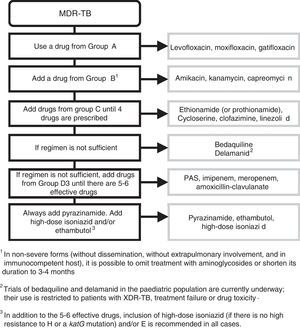
Treatment of multidrug-resistant tuberculosisThe standard treatment of MDR-TB must be overseen by a specialist in paediatric infectious disease. Treatment will last 18–24 months. The initial phase lasts 6–8 months, and it is only possible to contemplate cutting it down to 4–6 months in cases of mediastinal lymphadenopathy with the regimen including a minimum of 5 drugs of known effectiveness. Continuation phase: administration of at least 3 effective drugs.
The regimen is designed according to the following steps (Fig. 1): include every possible first-line add-on agent from group D1 (Z+high-dose H+E) and:
- –
One drug from group A (FQ).
- –
One drug from group B (second-line injectable aminoglycoside).
- –
At least 2 drugs from group C.
- –
Z: little agreement between in vitro testing and in vivo performance. Add routinely.
Recommendations41 for constructing a treatment regimen for MDR-TB and XDR-TB.
If the resulting combination has fewer than 5 drugs, choose 1 drug from group D2, and as many as needed from group D3. The use of bedaquiline and delamanid (D2) in the paediatric population is still being investigated and limited in clinical practice to confirmed XDR-TB strains, cases of treatment failure or cases of drug toxicity.31 In addition to the 5 effective drugs, inclusion of high-dose H (as long as the strain is not highly resistant to H, that is, has a katG mutation) and/or E is recommended in all patients.
In mild forms of TB (without dissemination or extrapulmonary involvement, immunocompetent host), treatment with aminoglycosides can be excluded or cut down to 3–4 months due to the risk of ototoxicity and the difficulties involved in its parenteral administration. Observational studies have found that these patients had favourable outcomes.
In patients with primary pulmonary TB (identified in the MDR-TB contact investigation) who are asymptomatic and presumed to have a low bacterial load, it is possible to exclude injectable aminoglycosides if there are 2 effective bactericidal drugs (FQ+linezoid [Lzd]) that can be combined with 2 other effective drugs (Cs, Eto/prothionamide [Pto], clofazimine [Cfz]) with the further addition of Z+high-dose H+E. This patient profile is common in paediatrics, and early discontinuation or even exclusion of injectable drugs is advisable in these cases.
The WHO has proposed a new short regimen lasting 9–12 months that can be used in patients, including children, under the following circumstances:
- –
The patient has not previously received the second-line drugs included in the regimen for more than 1 month.
- –
The strain is not resistant to the drugs included in the regimen.
- –
The patient is not pregnant.
- –
Absence of extrapulmonary involvement.
The regimen consists of an intensive phase with 7 drugs lasting 4 months, which will be extended to 6 months if the patient continues to be contagious or is not improving adequately, and a continuation phase lasting 5–6 months with the following scheme:
This regimen is based on the STREAM clinical trial,32 which will be completed in 2018. Observational studies have shown that this regimen is more effective and safe than longer treatment regimens32–35; the WHO recommends it for both adults and children. There are concerns regarding its applicability in Europe, where there are countries with a high proportion of pre-XDR-TB and XDR-TB cases.36,37 While we await further data on the paediatric population, either regimen may be used in Spain, always under the supervision of an expert.
Care and follow-up of children with MDR-TBChildren with MDR-TB will be managed in appropriate units with experience in this disease. Even if they are not contagious, children will remain hospitalised in a negative pressure room until 3 consecutive sputum samples, taken at least 1 week apart, are negative. Visitors and health care staff will wear FFP3 masks until it is confirmed that the patient not contagious. Efforts will be made to mitigate the psychosocial impact of a prolonged hospital stay, offering social and psychological support.
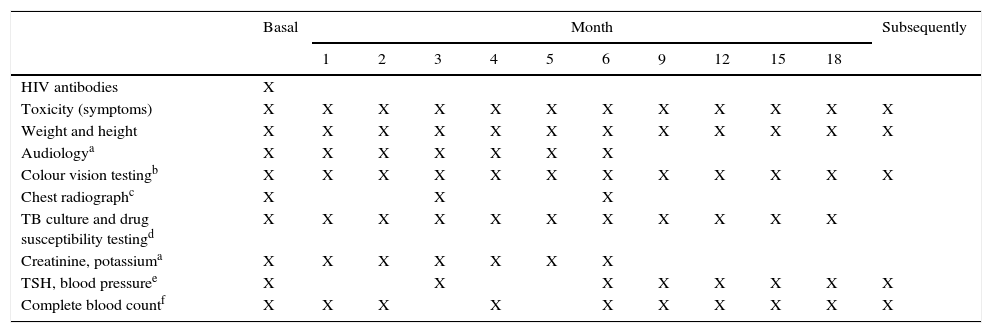
Table 7 presents the schedule for the follow-up, monitoring and early detection of adverse events in these cases.
Schedule of visits and monitoring for adverse events in MDR-TB.
| Basal | Month | Subsequently | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 9 | 12 | 15 | 18 | |||
| HIV antibodies | X | |||||||||||
| Toxicity (symptoms) | X | X | X | X | X | X | X | X | X | X | X | X |
| Weight and height | X | X | X | X | X | X | X | X | X | X | X | X |
| Audiologya | X | X | X | X | X | X | X | |||||
| Colour vision testingb | X | X | X | X | X | X | X | X | X | X | X | X |
| Chest radiographc | X | X | X | |||||||||
| TB culture and drug susceptibility testingd | X | X | X | X | X | X | X | X | X | X | X | |
| Creatinine, potassiuma | X | X | X | X | X | X | X | |||||
| TSH, blood pressuree | X | X | X | X | X | X | X | X | ||||
| Complete blood countf | X | X | X | X | X | X | X | X | X | X | ||
In case of negative culture, repeat only when clinically indicated. If the initial culture is positive and the child is able to expectorate, repeat sputum smear microscopy every month. If the initial culture is positive and the child is unable to expectorate, repeat only when clinically indicated. If the initial culture is positive and the child is able to expectorate, repeat sputum-smear microscopy every month. If the initial culture is positive and the child is unable to expectorate, repeat monthly until culture becomes negative, and every 3 months thereafter.
The authors have no conflicts of interest to declare.
Coordinator:
- –
María José Mellado Peña (Hospital Universitario La Paz-Carlos III, Madrid).
Members:
- –
Fernando Baquero Artiago (Hospital Universitario La Paz-Carlos III, Madrid).
- –
María José Cilleruelo Ortega (Hospital Universitario Puerta de Hierro, Majadahonda, Madrid).
- –
Lola Falcón (Hospital Universitario Virgen del Rocío, Seville).
- –
Laura Ferreras Antolín (St. George's Hospital, London).
- –
Ana Méndez Echevarría (Hospital Universitario La Paz-Carlos III, Madrid).
- –
David Moreno Pérez (Hospital Materno-Infantil, Hospital Regional Universitario de Málaga. Instituto de Investigación Biomédica de Málaga [IBIMA]).
- –
Antoni Noguera de Julián (Hospital Sant Joan de Déu, Barcelona).
- –
Roi Piñeiro Pérez (Hospital General de Villalba, Collado-Villalba, Madrid).
- –
Begoña Santiago García (Hospital General Universitario Gregorio Marañón, Madrid).
- –
Antoni Soriano Arandes (Hospital Vall d’Hebron, Barcelona).
The members of the Working Group on Tuberculosis and Other Mycobacterial Infections of the Sociedad Española de Infectología Pediátrica are presented in Annex 1.
Please cite this article as: Mellado Peña MJ, Santiago García B, Baquero-Artigao F, Moreno Pérez D, Piñeiro Pérez R, Méndez Echevarría A, et al. Actualización del tratamiento de la tuberculosis en niños. An Pediatr (Barc). 2018;88:52.e1–52.e12.
This project is endorsed by all the signing societies and institutions. In the case of RITIP and TEDDY, there is no endorsement as such, since the document was requested by these research networks. The SENP has also agreed to endorse the project.