The Advisory Committee on Vaccines of the Spanish Association of Paediatrics annually publishes the immunisation schedule considered optimal for children resident in Spain, according to available evidence on current vaccines.
Regarding funded immunisations, 2+1 strategy (2, 4, 11–12 months) with hexavalent (DTPa-IPV-Hib-HB) and 13-valent pneumococcal vaccines are recommended.
Administration of the 6-year booster dose with DTPa is recommended, and a poliomyelitis dose for children who had received the 2+1 scheme, as well as Tdap vaccine for adolescents and pregnant women in every pregnancy between 27 and 32 weeks’ gestation.
The two-dose scheme should be used for MMR (12 months and 2–4 years) and varicella (15 months and 2–4 years). MMRV vaccine could be applied as the second dose if available.
Coverage of human papillomavirus vaccination in girls aged 12 with a two dose scheme (0, 6 months) should be improved. Information and recommendation for male adolescents about potential beneficial effects of this immunisation should be provided as well. The new 9 genotypes vaccine is now available, expanding the coverage for both gender. Regarding non-funded immunisations, Committee on Vaccines of the Spanish Association of Paediatrics recommends meningococcal B vaccination, with a 3+1 schedule, and requests to be included in the National Immunisation Program. Tetravalent meningococcal vaccine (MenACWY) is recommended to adolescents (14–18 years) who are going to live in countries with systematic vaccination against ACWY serogroups, and people >6 weeks of age with risk factors or travellers to countries with very high incidence.
Vaccination against rotavirus is recommended in all infants.
El Comité Asesor de Vacunas de la Asociación Española de Pediatría publica anualmente el calendario de vacunaciones que estima idóneo para los niños residentes en España, teniendo en cuenta la evidencia disponible. En cuanto a las vacunas financiadas, se recomienda emplear el esquema 2+1 (2, 4 y 11-12 meses) con vacunas hexavalentes (DTPa-VPI-Hib-VHB) y con antineumocócica conjugada 13-valente. Se aconseja un refuerzo a los 6 años, preferentemente con DTPa, junto a una dosis de polio para aquellos que recibieron esquemas 2+1, así como vacunación con Tdpa en adolescentes y en cada embarazo, entre la 27 y 32 semanas. Se emplearán esquemas de dos dosis para triple vírica (12 meses y 2-4 años) y varicela (15 meses y 2-4 años). De haber disponibilidad, la segunda dosis se podría aplicar como vacuna tetravírica. Se deben incrementar las coberturas frente al papilomavirus en niñas de 12 años con dos dosis (0, 6 meses), así como informar y recomendar la vacunación de los varones, dados los beneficios potenciales de la misma. La nueva vacuna de 9 genotipos ya está disponible, ampliando la cobertura para ambos sexos. Respecto a vacunas no financiadas, se recomienda la antimeningocócica B, con esquema 3+1, solicitando su entrada en calendario. Se recomienda individualmente la vacuna antimeningocócica conjugada tetravalente (MenACWY) en adolescentes (14-18 años) que vayan a residir en países con vacunación sistemática frente a los serogrupos ACWY. También en mayores de 6 semanas de vida con factores de riesgo o viajeros a países de elevada incidencia.
Es recomendable vacunar a todos los lactantes frente al rotavirus.
The Advisory Committee on Vaccines of the Spanish Association of Paediatrics (CAV-AEP) annually updates its immunisation schedule taking into account the available evidence in order to offer the recommendations considered most appropriate for children residing in Spain.
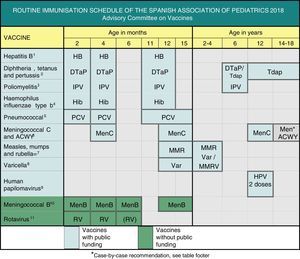
This year, the main changes involve the measles, mumps and rubella, varicella, meningococcal and human papillomavirus vaccines, as can be seen in Fig. 1. The CAV-AEP maintains the 2+1 schedule with hexavalent vaccine, which is now established in the Spanish childhood immunisation schedule,1 increasing its efficiency and uniformity.
Routine immunisation schedule of the Spanish Association of Pediatrics 2018.
(1) Hepatitis B vaccine (HB). 3 doses of hexavalent vaccine at 2, 4 and 11–12 months of age. Children of HBsAg-positive mothers will also be given one dose of monovalent HB vaccine at birth along with 0.5mL of hepatitis B immune globulin (HBIG), all within 12h of birth. When maternal serological status is unknown, the neonatal dose should be administered and maternal serology tested immediately, and should the mother test be positive, HBIG should be administered to the neonate as soon as possible, and in the first week of life. The administration of 4 doses of HB vaccine is generally acceptable and recommended in children of HBsAg-positive mothers vaccinated at birth, even in newborns with birth weights of less than 2000g, as the neonatal dose should not be included in the count in these cases. Unvaccinated children and adolescents should be given three doses of monovalent vaccine or the combined hepatitis A and B vaccine on a 0, 1 and 6 month schedule, at any age.
(2) Diphtheria, tetanus and acellular pertussis vaccine (DTaP/Tdap). 5 doses: primary vaccination with 2 doses, at 2 and 4 months, of DTaP (hexavalent) vaccine; booster at 11–12 months (third dose) with DTaP (hexavalent) vaccine; at 6 years (fourth dose) with the standard load vaccine (DTaP-IPV), preferable to the low diphtheria and pertussis antigen load vaccine (Tdap-IPV), and at 12–18 years (fifth dose) with Tdap, preferably at 12–14 years.
(3) Inactivated poliovirus vaccine (IPV). 4 doses: primary vaccination with 2 doses, at 2 and 4 months, and booster doses at 11–12 months and 6 years.
(4) Haemophilus influenzae type b conjugate vaccine (Hib). 3 doses: primary vaccination at 2 and 4 months and booster dose at 11–12 months.
(5) Pneumococcal conjugate vaccine (PCV). 3 doses: the first two at 2 and 4 months, with a booster dose at 11–12 months of age. The vaccine recommended in Spain by the CAV-AEP continues to be the PCV13.
(6) Meningococcal C conjugate vaccine (MenC) and meningococcal ACWY conjugate vaccine (MenACWY). 3 doses of monovalent MenC conjugate vaccine with a 1+1+1 schedule: one dose at 4 months, another at 12 months and one final dose at 12 years of age. The CAV-AEP recommends providing information on the MenACWY vaccine and its case-by-case administration in children and adolescents: from age 14 years, in those that are to live in countries where the vaccine is administered at this age, such as the United States or UK; children aged more than 6 weeks travelling to countries with a high incidence of IMD caused by vaccine serogroups; children aged more than 6 weeks with risk factors for IMD: anatomic or functional asplenia, complement component deficiency, treatment with eculizumab, prior episode of IMD caused by any serogroup, and contacts of an index case of IMD caused by serogroup A, W or Y. We recommend informing ≥14-year-old children and their parents of the availability of tetravalent meningococcal vaccines, that could extend individually the protection against IMD.
(7) Measles, mumps and rubella vaccine (MMR). 2 doses of measles-mumps-rubella vaccine (MMR). The first at age 12 months and the second at age 2–4 years, preferably at 2. If available, the tetravalent MMRV vaccine may be administered for the second dose. Susceptible patients outside the specified ages will be vaccinated with 2 doses of MMR at least 1 month apart.
(8) Varicella vaccine (Var). 2 doses: the first at age 15 months (age 12 months is also acceptable) and the second at age 2–4 years, preferably at 2. If available, the tetravalent vaccine (MMRV) may be used for the second dose. Susceptible patients outside the specified ages will be vaccinated with 2 doses of Var at least 1 month apart.
(9) Human papillomavirus vaccine (HPV). vaccination of all girls, preferably at age 12 years, to prevent cervical and anal cancer and precancerous lesions of the female genital tract. Information on the potential for vaccination should be provided and vaccination recommended in male patients for the HPV9, HPV4 and HPV2 vaccines, which are authorised for use in males, although there is little data on the latter for this sex. Administration of 2 doses at age 11–12 years. The vaccination schedule depends on the vaccine used: for the tetravalent vaccine, a 2-dose series (at 0 and 6 months) for girls aged 9–13 years and a 3-dose series (at 0, 2 and 6 months) in those aged ≥14 years; for the 2-valent and 9-valent vaccines, a 2-dose series (at 0 and 6 months) in girls aged 9–14 years and a 3-dose series (at 0, 1–2 and 6 months) in those aged ≥15 years. The HPV vaccine may be administered at the same time as the MenC, hepatitis A and B and Tdap vaccines. There are no data on its coadministration with the varicella vaccine, although it should not cause any problems.
(10) Meningococcal B vaccine (MenB). 4 doses: the first 3 in the first year of life (2, 4 and 6 months) with a booster between 12 and 15 months of age, although administration at least 15 days apart from other injectable inactivated vaccines is recommended up to age 18 months to minimise potential reactogenicity and avoid coadministration with the MenC vaccine conjugated with tetanus toxoid. The separation by a 15-day interval is not necessary for the varicella, MMR and rotavirus vaccines.
(11) Rotavirus vaccine (RV). 2 or 3 doses of rotavirus vaccine: at 2 and 4 months with the monovalent vaccine or at 2, 4 and 6 months with the pentavalent vaccine. It is very important to start vaccination between 6 and 12 weeks of life in order to minimise risks, and to complete it before 24 weeks for the monovalent vaccine or 32 weeks for the pentavalent vaccine. Doses must be given at least 4 weeks apart. Both doses may be given at the same time as any other vaccine.
We recommend reading the expanded version of these recommendations at www.vacunasaep.org. Furthermore, the recommendations for special situations and risk groups can also be found in the CAV-AEP website at http://vacunasaep.org/documentos/manual/manual-de-vacunas.
The Spanish Association of Pediatrics (AEP) is pleased with the improvement of the national immunisation schedule through the incorporation of new vaccines, and that vaccines that are not funded by the state are now available in community pharmacies.
Ideally, scientific societies would be taken into account in the decision-making process, and the autonomous communities and the Ministry of Health would make a greater joint economic effort to fund a more comprehensive routine immunisation schedule. Alternative systems should be set up to assist families in paying for vaccines that are not funded by the state, as is done for other medications.
In order to prevent the re-emergence of vaccine-preventable diseases, we need to continue vaccinating all children, striving to maintain high vaccination coverage rates and to persuade parents that refuse vaccination.
Vaccination against hepatitis B2018 recommendation:We recommend the vaccination of infants with 3 doses of hexavalent vaccine at 2, 4 and 11–12 months of age. Four-dose schedules may be administered in infants that received a first dose at birth. Previously unvaccinated older children and adolescents will receive 3 doses of the monovalent vaccine in a 0, 1 and 6 months schedule.
In Spain, the annual incidence of hepatitis B remains under 2 cases per 100000 inhabitants. In 2015, a total of 690 cases (1.48 cases/100000 inhabitants) were reported to the National Network of Epidemiologic Surveillance of Spain.2
Since 2017, every autonomous community (AC), save for Castilla y León and the Community of Madrid, which maintain the neonatal dose in their immunisation schedules, initiate the hepatitis B series at age 2 months with the hexavalent vaccine. Vaccination at birth should continue to be performed in newborns of mothers who are HBsAg positive or of unknown serological status, always with the monovalent vaccine. Newborns of HBsAg-positive mothers should also be given hepatitis B immune globulin in the first 12h of life; while in those of mothers of unknown status, administration of immune globulin can be delayed for a maximum of 1 week while awaiting the results of maternal serological testing.
Vaccination against diphtheria, tetanus, pertussis, poliomyelitis and Haemophilus Influenzae type b2018 recommendation:We recommend a 2+1 schedule with the DTaP-IPV-Hib-HB vaccine at 2, 4 and 11–12 months. Children that have received the 2+1 series should be given DTaP-IPV, preferably, or Tdap-IPV at age 6 years and Tdap at age 12–18 years, preferably between ages 12 and 14. We recommend vaccination with Tdap of all pregnant women in each pregnancy starting at 27 weeks of gestation, and preferably before 32 weeks.
The incidence of pertussis has increased worldwide. Infants suffer the most severe and lethal forms of disease, so they are the most vulnerable group and warrant especial protection. Vaccination of pregnant women with Tdap in each gestation is safe, efficacious and the most effective and efficient means of preventing pertussis in infants.3 Vaccination in the second trimester of gestation increases neonatal antibody levels and widens the immunisation opportunity window,4 and consequently countries such as the United Kingdom (UK) recommend administration of the vaccine starting at 20 weeks’ gestation.5 There is evidence in the literature of a potential maternal antibody interference with the immune response to various vaccine antigens in infants of mothers vaccinated against pertussis during pregnancy,6 with the immune response usually normalising after administration of the booster dose, and no evidence up to date of an association of this phenomenon with negative changes in epidemiology.7
The 2+1 schedule is used in many European countries and was recommended by the CAV-AEP in 2017,5 and also by the Interterritorial Council of the Spanish National Health System (Consejo Interterritorial del Sistema Nacional de Salud [CISNS]).1 This schedule, which is safe and immunogenic, optimises the use of available doses. Earlier administration of the first dose at 6 weeks post birth is allowed.5
The DTaP vaccine is preferred over low-antigen load formulations (Tdap) for the booster at age 6 years, because it confers a longer-lasting protection.5
Children that received a 2+1 primary vaccination series with hexavalent vaccine as infants must receive a polio booster at age 6 years, preferably with the DTaP-IPV vaccine.5
Vaccination against pneumococcal disease2018 recommendation:Vaccination against pneumococcal disease is recommended for all children younger than 5 years and children that are at risk due to underlying disease at any age. A 2+1 series (2, 4 and 11–15 months) is recommended for routine vaccination of infants. On account of the current epidemiology of pneumococcal infections in Spain, the CAV-AEP continues to recommend the use of the 13-valent pneumococcal conjugate vaccine (PCV13).
We maintain the 2017 recommendations for vaccination against pneumococcal disease.5
Pneumococcal conjugate vaccines (10-valent [PCV10] and PCV13) reduce the incidence of invasive pneumococcal disease (IPD) by vaccine serotypes, both through direct protection of vaccinated individuals and indirect protection (herd immunity) of those who are unvaccinated. The final impact of vaccination depends to a great extent on the vaccine coverage of circulating serotypes. The CAV-AEP considers that coverage against serotype 19A is essential to achieve the maximum impact at the population level, since by 2010 this serotype had grown to cause more than 20% of cases of IPD in children8–10 and 10% in adults,8,11 including those aged more than 65 years, in who it reached a proportion of 15%.12 In the same period, this serotype was the most frequent aetiological agent in cases of pneumococcal meningitis.10,13
Approximately 50% of invasive 19A strains exhibit a reduced susceptibility to penicillin,8 and more than 50% of the strains isolated in meningitis cases may be resistant to cefotaxime.13
The PCV10 does not induce herd immunity against serotypes 19A and 6A. While it provides significant cross-protection against these serotypes, this only occurs in vaccinated individuals and does not seem to last more than two years. In fact, there has been an increase in the incidence of IPD caused by serotype 19A in several countries where this vaccine has been used.14,15
The impact of both vaccines on non-invasive disease (pneumonia and acute otitis media) has been generally greater than expected, especially for otitis media.5
Vaccination against group A, C, W and Y meningococcal disease2018 recommendation:We continue to recommend 3 doses of the monovalent meningococcal conjugate vaccine (MenC) with a 1+1+1 schedule at 4 months, 12 months and 12 years. We recommend the use on a case-to-case basis of the tetravalent meningococcal conjugate vaccine (MenACWY) in adolescents (14–18 years) who are going to live in countries with systematic vaccination against ACWY meningococcal serogroups. It is also recommended in children aged more than 6 weeks with risk factors or travelling to endemic regions.
There is ample evidence of the effectiveness of the monovalent MenC vaccine. The rate of invasive meningococcal disease (IMD) by serogroup C in Spain remained low in the 2015–2016 season (0.03 cases/100000 inhabitants).
In 2000, there was an IMD outbreak of group W meningococcal disease during Hajj (the annual pilgrimage to Mecca), which subsequently spread to several African countries.16 This outbreak was caused by serotype ST-11CC, a particularly virulent one, which spread through Latin America and from there to the UK and other European countries.17
Given the gradual increase of IMD by serogroup W, the UK decided to include the MenACWY in its immunisation schedule in 2015, with administration at age 14 years in substitution of the MenC dose previously given at age 12 years,18 which achieved a 69% reduction in cases of IMD by serogroup W despite a vaccine coverage of only 36%.19 Other European countries (Austria, Greece and Italy) and the United States have also replaced the MenC dose given to adolescents with a dose of MenACWY.
On the other hand, in other European regions, and particularly in Nordic countries, there had been an increase in cases of IMD caused by serogroup Y since 2007 that was controlled without the introduction of vaccination.20
In Spain, there has been a progressive increase in IMD caused by serogroup W since 2015,5 with a fourfold increase in incidence in the 2015/2016 season compared to the preceding season. However, the latest data reported to the Centro Nacional de Epidemiología (National Epidemiology Centre) through week 36 of 2017 show a plateau in the number of cases,21 discontinuing the explosive growth trend observed in the previous season.
The sale in pharmacies of two MenACWY vaccines was authorised in Spain in September 2017.22 In light of the current epidemiology of IMD in Spain and the moderate emergence of serogroups W and Y in the past year, the CAV-AEP recommends providing information and administering the MenACWY vaccine on a case-by-case basis in children and adolescents:
- ∘
Adolescents ≥14 years old (14-18 years) that are going to live in countries where the vaccination is included in the systematic calendar at this age, such as the United States or the UK.
- ∘
Aged more than 6 weeks who are travelling to countries with a high incidence of IMD caused by vaccine serotypes.
- ∘
Aged more than 6 weeks with risk factors for IMD:
- •
Anatomic or functional asplenia.
- •
Complement component deficiency.
- •
Ongoing treatment with eculizumab.
- •
Prior history of IMD by any serogroup.
- •
Contacts of an index case of IMD caused by serogroup A, W or Y.
- •
We recommend informing ≥14-year-old children and their parents of the availability of tetravalent meningococcal vaccines, that could extend individually the protection against IMD.
The inclusion of the MenACWY vaccine in the routine immunisation schedule should be considered if the incidence of IMD caused by these serogroups continues to increase.
Vaccination against measles, mumps and rubella (MMR)2018 recommendation:We recommend the administration of a first dose of MMR vaccine at 12 months of age, with a second dose given between 2 and 4 years of age. When available, the tetravalent vaccine (MMRV) may be used for the second dose.
The tetravalent vaccine carries an increased risk of febrile seizure, especially following the first dose and in children aged less than 2 years, so it is recommended that the MMR and varicella vaccines be administered separately before that age.23
The prevalence of measles in the European Region of the WHO continues to decrease, although it is still unacceptably high (12439 cases between August 2016 and July 2017, predominantly in Romania and Italy [157 in Spain], including 25 deaths [1 in Spain]).24 There are also still outbreaks of mumps. Maintenance of high vaccination coverage rates and rigorous epidemiologic surveillance are essential for the purpose of eradicating these diseases. One dose at 12 months achieves seroconversion rates of 95% or higher for all three viruses, which reach nearly 100% after the second dose.
Vaccination against varicella2018 recommendation:We recommend vaccination with 2 doses, administered at ages 15 months and 2–4 years; when available, the MMRV vaccine should be used for the second dose. We also recommend catch-up vaccination with a 2-dose series for all children and adolescents that did not have the disease (or completion of the 2-dose series when applicable).
Since 2016, vaccination against varicella has been included in the immunisation schedule of every AC with a 2-dose series (at 15 months and 3–4 years).1 There are two monovalent and two tetravalent vaccines with evidence of a high effectiveness (92–97.3%) on the vaccinated and the unvaccinated populations,25 and excellent safety profiles.26
After 20 years of use in the United States, there is evidence of a sustained decline in the incidence of disease, with no shift of disease towards older ages27 or changes in the incidence of herpes zoster, a decisive factor in the cost-effectiveness of this childhood immunisation.26 In this regard, it is very important to guarantee completion of the 2-dose series in adolescents, with administration of catch-up doses as needed.
Vaccination against human papillomavirus (HPV)2018 recommendation:Routine vaccination against HPV is recommended for all girls, preferably at age 12 years, as a means to prevent cervical and anal cancer and precancerous lesions in the female genital tract. Health professionals should also provide information and recommend vaccination to male patients.
The optimal age for vaccination is 12 years, with administration of a 2-dose series (Fig. 1), the objective being to achieve the maximum possible benefit prior to sexual debut as well as the broadest possible vaccination coverage. The recommendation also extends to older ages if vaccination has been delayed, given the benefits that it may continue to provide.
Vaccination against HPV is showing a high efficacy and effectiveness in the prevention of persistent HPV infection, genital warts and precancerous cervical lesions, with a reduction of up to 85% in the incidence of high-grade dysplasias, even 10 years after its performance.5,28 In a few years, data will probably be available on the actual prevention of cervical cancer and other types of cancer associated with HPV.
With more than 300 million doses having been administered worldwide, these vaccines have proven to be safe and to have a very favourable risk–benefit ratio.28 Research has ruled out an association between these vaccines and the development of autoimmune and neurologic diseases.29 Still, the average coverage in Spain continues to be considerably low compared to all other vaccines in the routine immunisation schedule. This low coverage precludes the general population from deriving the indirect protection that the vaccine has been proven to confer. Health professionals must have adequate knowledge of its effectiveness and safety and reinforce the positive messages regarding this vaccine to improve its acceptance in the population
The 9-valent HPV vaccine (6/11/16/18/31/33/45/52/58 [HPV9]) is now available in Spain.22 It is safe and effective, increases the overall prevention of HPV-related cervical malignancies from 70% to 90% and may also prevent 85% to 95% HPV-related vulvar, vaginal and anal cancers,30 making it the best available option for both sexes, although this Committee recommends administration of whichever HPV vaccine has been selected by each AC. There are published expert opinions on the possibility of administering the HPV9 vaccine to individuals already vaccinated with HPV2 and HPV4.30
Vaccination of male individuals is included in the schedules of several countries, such as the United States, Australia or Canada. There are relevant data on the role of HPV in the aetiology of certain types of cancer affecting both sexes but with a higher incidence in males, such as rectal cancer and head and neck cancer. In Europe, there has been no evidence of male individuals benefitting indirectly from vaccination programmes in adolescent girls.31 In the UK, while the JCVI has not recommended the routine implementation of this strategy to prioritise the use of economic resources in more efficient public health interventions, it has acknowledged the benefits it would provide male individuals.32 For all the above reasons, the CAV-AEP considers that information on this vaccine should be provided and vaccination recommended to male adolescents, preferably starting at age 12 years.
Vaccination against group b meningococcal disease2018 recommendation: This vaccine exhibits the profile of a routine vaccine to be administered to all children starting at 2 months of age.
Although there are two vaccines for the prevention of IMD by group B meningococcus (MenB), the only one authorised for use in Europe is the 4CMenB vaccine, indicated for use starting at 2 months of age. Clinical trials have demonstrated its immunogenicity, but there is no definitive evidence on the duration of bactericidal antibodies and, therefore, on the need or lack thereof of a booster dose.33
The vaccine can be administered simultaneously with the rest of the vaccines included in the schedule, although this may increase reactogenicity. A recent study confirmed its compatibility with the meningococcal C-CRM vaccine.34
In 2015, the UK included it in its official immunisation schedule with a 2+1 schedule (at 2, 4 and 12–13 months).5 Data from the first 10 months of the programme showed an effectiveness of 83% against group B meningococcal B strains overall, and of 94.6% against vaccine strains. Cases of IMD by this bacterium decreased by 50% in the vaccine-eligible population.35
Recently, it has been confirmed that reduced schedules (2+1) like the one used in the UK are immunogenic and safe.36
The vaccine has also been included in the immunisation schedules of Ireland (with the same schedule used in the UK), Italy, Austria and the Czech Republic (with a 3+1 schedule in all three). Thus, we expect that more detailed information on its effectiveness, safety and reactogenicity will soon become available.
Vaccination against rotavirus2018 recommendation:Vaccination against rotavirus (RV) is an advisable health intervention for all infants.
In the more than 10 years elapsed since the introduction of this vaccine in the market, 88 countries have included it in their schedules, leading to vast public health benefits, with a considerable decrease in the morbidity and mortality associated with rotavirus gastroenteritis (RV-AGE) in infants and young children worldwide.
In the UK, the incidence has decreased by up to 77% of RV-AGE episodes.37 In Finland, the number of hospital admissions due to RV-AGE decreased by 94.4%.38 In Spain, the observed efficacy of vaccination in reducing hospital admissions in Valencia was of 85%.39
The benefits of vaccination extend to both vaccinated infants and, indirectly, to the unvaccinated environment, significantly increasing its public health impact.37 There is a growing body of evidence on the extraintestinal manifestations of RV infection and its association with systemic diseases.5
The benefits of vaccination vastly exceed the risks of intussusception, the only associated serious adverse effect, which is very rare (between 1 and 5 cases per 100000 vaccinated children).40
Two RV vaccines are available in pharmacies, a pentavalent and a monovalent vaccine. Their dosages can be found in Fig. 1.
FundingThe development of these recommendations (analysis of the published data, debate, consensus and publication) has not been supported by any funding source outside of the logistic support provided by the AEP.
Conflicts of interest (past 5 years)DMP has collaborated in educational activities funded by Astra, Pfizer, GlaxoSmithKline and Sanofi Pasteur MSD, as consultant on a GlaxoSmithKline advisory board and as a researcher in clinical trials by Novartis.
FJAG has collaborated in educational activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD and as a consultant on GlaxoSmithKline, Novartis and Pfizer advisory boards.
JAF has collaborated in educational activities funded by Astra, GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, and as a consultant on GlaxoSmithKline and Pfizer advisory boards.
MJCO has collaborated in educational activities funded by GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD, as a researcher in clinical trials conducted by GlaxoSmithKline and Pfizer, and as a consultant on GlaxoSmithKline, Novartis, Pfizer and Sanofi Pasteur MSD advisory boards.
MGS has collaborated in educational activities funded by Astra, GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, as a consultant on GlaxoSmithKline and Novartis advisory boards, and as a researcher in clinical trials conducted by GlaxoSmithKline, Janssen and Sanofi Pasteur MSD.
NGS has collaborated in educational activities funded by Sanofi Pasteur MSD, and attended educational activities funded by Novartis and Pfizer.
AHM has received funding to attend domestic educational activities, and has participated in educational activities funded by Pfizer.
MMH has collaborated as a researcher in clinical trials conducted by GlaxoSmithKline and Novartis.
MMM has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, as a researcher in clinical trials conducted by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD and as a consultant on a Novartis advisory board.
AMM has received funding from Pfizer to attend educational activities in Spain and abroad.
JRC has collaborated in educational activities funded by GlaxoSmithKline, Pfizer and Sanofi Pasteur MSD, and as a researcher in clinical trials conducted by GlaxoSmithKline and Pfizer.
We thank Javier Arístegui, Jose María Corretger and Luis Ortigosa, for their expert advisory support with the Committee on the elaboration of these recommendations.
Composition and professional affiliation of the members of the Advisory Committee on Vaccines of the Spanish Association of Paediatrics:
David Moreno-Pérez. Paediatric Infectious Diseases and Immunodeficiencies, Paediatrics Clinical Management Unit, Hospital Materno-Infantil, Hospital Regional Universitario de Málaga. IBIMA Research Group. Department of Paediatrics and Pharmacology, School of Medicine, Universidad de Málaga.
Francisco José Álvarez García. Paediatrician. Centro de Salud de Llanera, Asturias. Associate Professor of Health Sciences, School of Medicine, Universidad de Oviedo.
Javier Álvarez Aldeán. Paediatrician. Chief of the Department of Paediatrics, Hospital Costa del Sol, Marbella, Málaga.
María José Cilleruelo Ortega. Department of Paediatrics, Hospital Universitario Puerta de Hierro-Majadahonda, Madrid. Department of Paediatrics, School of Medicine, Universidad Autónoma de Madrid.
María Garcés-Sánchez. Paediatrician. Centro de Salud Nazaret, Valencia. Researcher affiliated to the Section on Vaccines, FISABIO, Valencia.
Nuria García Sánchez. Paediatrician. Centro de Salud Delicias Sur, Zaragoza. Associate Professor of Health Sciences, Department of Paediatrics, School of Medicine, Universidad de Zaragoza.
Ángel Hernández Merino. Paediatrician. Centro de Salud La Rivota, Alcorcón, Madrid.
María Méndez Hernández. Paediatrician. Coordinator of the Infectious Disease Unit, Hospital Germans Trias i Pujol, Badalona, Barcelona. Associate Professor of Paediatrics, School of Medicine, Universidad Autónoma de Barcelona.
Manuel Merino Moína. Paediatrician. Centro de Salud El Greco, Getafe, Madrid. Lecturer, School of Medicine, Universidad Europea, Madrid.
Abián Montesdeoca Melián. Paediatrician. Centro de Salud de Guanarteme, Las Palmas de Gran Canaria.
Jesús Ruiz-Contreras. Department of Paediatrics, Hospital Universitario 12 de Octubre, Madrid. Department of Paediatrics, School of Medicine, Universidad Complutense de Madrid.
More information about the Advisory Committee on Vaccines of the Spanish Association of Paediatrics (CAV-AEP) is available in Appendix 1.
Please cite this article as: Moreno-Pérez D, Álvarez García FJ, Álvarez Aldeán J, Cilleruelo Ortega MJ, Garcés Sánchez M, García Sánchez N, et al. Calendario de vacunaciones de la Asociación Española de Pediatría (CAV-AEP): recomendaciones 2018. An Pediatr (Barc). 2018;88:53.e1–53.e9.