Paediatric tuberculosis (TB) disease continues to be a challenge. Difficulties in its diagnosis and limited experience on its treatment in children are some of the reasons to consider the need for specialized paediatric TB centres and to prioritize children in tuberculosis control programmes, particularly in low-incidence countries. We describe the paediatric tuberculosis cases managed in a specialized paediatric outpatient TB centre.
Patients and MethodsWe conducted a retrospective analysis of epidemiological and clinical data on TB cases in patients aged less than 18 years in the period ranging from January 2007 to June 2017.
ResultsWe identified 46 cases of TB. The median age of the patients was 5 years (IQR, 1.75−13.25). Thirty cases (65.2%) were identified through screening following exposure to TB. Thirty-six children (78%) presented with a median duration of symptoms during 2 weeks, the most frequent being cough (54%) and fever (48%). The findings of the chest radiograph were abnormal in 73.9% of patients, and a CT scan was performed in 82.2%, the findings of which contributed significantly to the decision to treat in 85.3%. Despite collection of different microbiological specimens, diagnostic confirmation was possible in only 12 cases (26.1%). The results of culture and/or nucleic acid amplification tests were positive in 33.3% of samples of sputum, 28.1% of bronchoalveolar lavage and 12.9% of gastric aspirates. The most frequent diagnosis was pulmonary TB (n=31), followed by pleuropulmonary TB (n=6), lymph node disease (n=3), uveitis (n=2), bone tuberculosis, disseminated TB, cerebellar tuberculoma and erythema nodosum (each n=1).
ConclusionsTuberculosis in children is an epidemiological indicator of recent transmission of Mycobacterium tuberculosis in the community. Efforts must be made to collect microbiological specimens before initiating treatment whenever possible. Management by an experienced paediatrics team allows an accurate diagnosis even when microbiologic confirmation is not possible.
La tuberculosis continúa siendo un reto en la edad pediátrica. Las dificultades en su diagnóstico y la escasa experiencia en su tratamiento en la infancia y adolescencia son algunas de las razones para considerar la necesidad de centros especializados en tuberculosis pediátrica y priorizar a la población pediátrica en los programas de control de tuberculosis, especialmente en países de baja incidencia. Descripción de casos de tuberculosis pediátrica tratados en un centro ambulatorio especializado en TB.
Pacientes y MétodosAnálisis retrospectivo de datos epidemiológicos y clínicos de casos de tuberculosis en pacientes menores de 18 años en el período de enero de 2007 a junio de 2017.
ResultadosSe identificaron 46 casos de tuberculosis, con una edad mediana de 5 años (RIC, 1,75−13,25). Treinta casos (65.2%) se identificaron en contexto de cribado por exposición conocida a la tuberculosis. En 36 pacientes (78%), la duración mediana de los síntomas fue de 2 semanas y los síntomas más frecuentes fueron tos (54%) y fiebre (48%). La radiografía de tórax fue anormal en el 73,9% de los pacientes y se realizó TC en el 82,2%, cuyos hallazgos proporcionaron claves importantes para la decisión de tratar en el 85,3%. A pesar de recogerse distintas muestras microbiológicas, solo se confirmó el diagnóstico en 12 casos (26,1%). Las pruebas de cultivo y/o de amplificación de ácidos nucleicos fueron positivas en el 33,3% de las muestras de esputo, el 28,1% de las de lavado broncoalveolar y el 12,9% de las de aspirado gástrico. La tuberculosis pulmonar fue el diagnóstico más frecuente (n=31), seguida de la tuberculosis pleural (n=6), linfadenitis tuberculosa (n=3), uveitis (n=2), tuberculosis ósea, tuberculosis diseminada, tuberculoma cerebelar y eritema nodoso (cada uno, n=1).
ConclusionesLa tuberculosis en la edad pediátrica es un marcador epidemiológico de transmisión reciente de Mycobacterium tuberculosis en la comunidad. Debe hacerse todo lo posible para recoger muestras microbiológicas antes de iniciar el tratamiento. Un equipo pediátrico especializado permite la realización de un diagnóstico preciso incluso en ausencia de confirmación microbiológica.
The clinical presentation of tuberculosis (TB) in the paediatric age group differs from that in adults, which makes TB a particular challenge in the former: a) the risk of developing disease following infection is greater in children, and the latency period is shorter compared to adults; b) children are more likely to developing extrapulmonary TB and severe disease; c) clinical and radiological manifestations are heterogeneous and often nonspecific; d) collection of samples for microbiological testing is more difficult in children, and the disease is often paucibacillary.1–4
Tuberculosis disease in children is an epidemiological indicator of recent transmission of Mycobacterium tuberculosis in the community, revealing a public health system failure in controlling TB.3–5 Screening of contacts of index cases and administration of prophylaxis to those susceptible of developing the disease (specially children) are recommended to minimise its spread.4,6
In Portugal, the incidence and notification of cases of TB has declined by approximately 40% in the last decade, and Portugal has been a low-incidence country since 2015.7 A public health policy of universal vaccination with Bacillus Calmette-Guérin (BCG) was in effect until 2016, when it shifted to selective vaccination of individuals in at-risk groups.8 In 2017, its incidence was estimated at 5.8 cases per 100 000 children aged 0–4 years and 1.8 cases per 100 000 children aged 5–14 years.9 This year, imported TB cases amount to 19.5% of all TB cases notified in Portugal, a percentage that is lower compared to other countries in the European Union and European Economic Area (33.1%).9
Given the epidemiological context, we considered the importance of improving health care facilities where TB patients are diagnosed and treated, particularly children. In Portugal, there is a network of TB Outpatient Centres within the National Health Service where patients with suspected or confirmed TB are evaluated and treated. These centres are linked to different hospitals to allow a full investigation until a diagnosis is made. Only 2 of these centres have paediatricians and a multidisciplinary team specialized in TB. The first one is the Reference Centre for TB in Children in North Portugal, which has been operative since 2010. The aim of our study was to describe the cases of TB in children and adolescents managed in this reference centre during a 10-year-period.
MethodsWe conducted a retrospective cross-sectional study at a TB centre, where children are managed by paediatricians specialized in TB. This centre managed 233 initial visits and 485 follow-up visits in 2017. We identified cases of TB disease in patients aged less than 18 years by consulting the records of the national TB surveillance system from 2007 through June 2017.10
We reviewed health records and collected data in an electronic database. We retrieved information on demographic characteristics (sex, age, enrolment in childcare facility or school), the medical history (previous diseases, vaccination status, use of medication), the reason and source of referral for consultation at the TB centre and the index case, when available. When it came to the cases of suspected TB, we collected data on clinical manifestations (symptoms and their duration), immunological test results (tuberculin skin test [TST] and interferon gamma release assay [IGRA]), chest radiographs, chest computed tomography (CT) scan, other imaging tests and bronchoscopy if performed. We considered a TST positive if the induration had a diameter of 5mm or greater in children that were immunocompromised or aged less than 5 years and not vaccinated, or of 10mm or greater in vaccinated children aged less than 5 years or children aged more than 5 years independently of vaccination status.6 We also recorded information on the collection of microbiological specimens (gastric secretion aspirate, sputum, bronchoalveolar lavage [BAL], cerebrospinal fluid [CSF], pleural fluid, biopsy), the results of acid-fast bacilli (AFB) smears, nucleic acid amplification tests (NAATs) and/or cultures and the presence of granulomas in the histological examination.
We classified TB by type as pulmonary or extrapulmonary, and cases as ‘possible’, ‘probable’ and ‘confirmed’ applying the definitions of the European Centre for Disease Prevention and Control (ECDC).11
Lastly, we collected data on the pharmacological treatment, including the agents used, dosage and duration of treatment, adverse events and adherence to treatment.
We collected data and performed the statistical analysis with the software IBM SPSS Statistics® version 24. We have expressed continuous variables as median and interquartile range (IQR) and categorical variables as absolute and relative frequencies. The study protocol was approved by the Ethical Committee and Administrative Council of the centre.
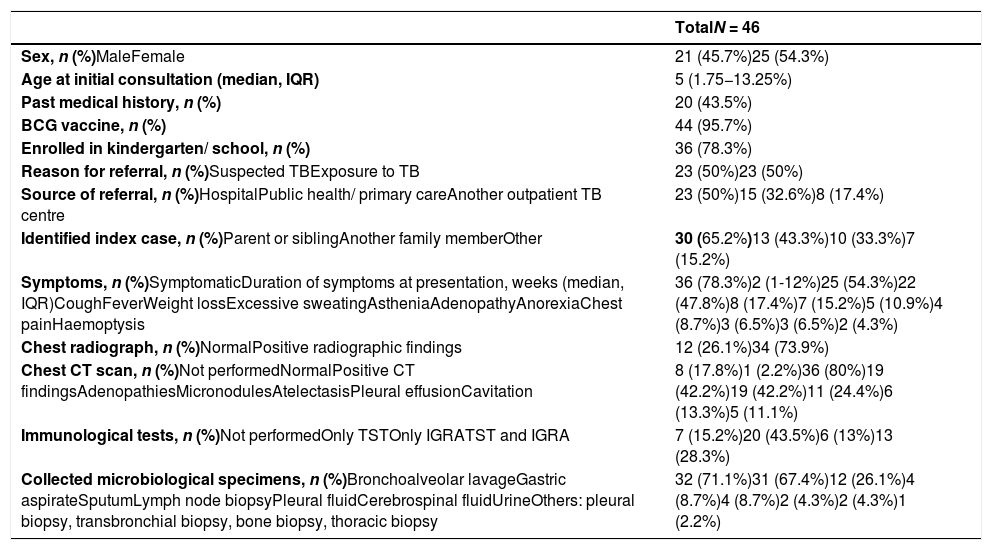
ResultsSampleWe identified a total of 46 cases of TB, 54.3% in female patients (Table 1). The median age at the time of the initial visit was 5 years, and 24 patients (52.2%) were aged less than 6 years. Almost all patients had been vaccinated with BCG (95.7%), the exceptions being an infant aged 11 months that did not meet the criteria for vaccination with BCG8 and an adolescent migrant from Guinea-Bissau. There was another adolescent who was a resident of Angola. All children were immunocompetent except 1 that had familial haemophagocytic lymphohistiocytosis.
Sample characteristics, symptoms, imaging findings and microbiological specimens that lead to diagnosis.
| TotalN = 46 | |
|---|---|
| Sex, n (%)MaleFemale | 21 (45.7%)25 (54.3%) |
| Age at initial consultation (median, IQR) | 5 (1.75−13.25%) |
| Past medical history, n (%) | 20 (43.5%) |
| BCG vaccine, n (%) | 44 (95.7%) |
| Enrolled in kindergarten/ school, n (%) | 36 (78.3%) |
| Reason for referral, n (%)Suspected TBExposure to TB | 23 (50%)23 (50%) |
| Source of referral, n (%)HospitalPublic health/ primary careAnother outpatient TB centre | 23 (50%)15 (32.6%)8 (17.4%) |
| Identified index case, n (%)Parent or siblingAnother family memberOther | 30 (65.2%)13 (43.3%)10 (33.3%)7 (15.2%) |
| Symptoms, n (%)SymptomaticDuration of symptoms at presentation, weeks (median, IQR)CoughFeverWeight lossExcessive sweatingAstheniaAdenopathyAnorexiaChest painHaemoptysis | 36 (78.3%)2 (1-12%)25 (54.3%)22 (47.8%)8 (17.4%)7 (15.2%)5 (10.9%)4 (8.7%)3 (6.5%)3 (6.5%)2 (4.3%) |
| Chest radiograph, n (%)NormalPositive radiographic findings | 12 (26.1%)34 (73.9%) |
| Chest CT scan, n (%)Not performedNormalPositive CT findingsAdenopathiesMicronodulesAtelectasisPleural effusionCavitation | 8 (17.8%)1 (2.2%)36 (80%)19 (42.2%)19 (42.2%)11 (24.4%)6 (13.3%)5 (11.1%) |
| Immunological tests, n (%)Not performedOnly TSTOnly IGRATST and IGRA | 7 (15.2%)20 (43.5%)6 (13%)13 (28.3%) |
| Collected microbiological specimens, n (%)Bronchoalveolar lavageGastric aspirateSputumLymph node biopsyPleural fluidCerebrospinal fluidUrineOthers: pleural biopsy, transbronchial biopsy, bone biopsy, thoracic biopsy | 32 (71.1%)31 (67.4%)12 (26.1%)4 (8.7%)4 (8.7%)2 (4.3%)2 (4.3%)1 (2.2%) |
BCG, Bacille Calmette-Guérin; CT, computed tomography; IGRA, interferon-gamma release assay; IQR, interquartile range; TB, tuberculosis; TST, tuberculin skin test.
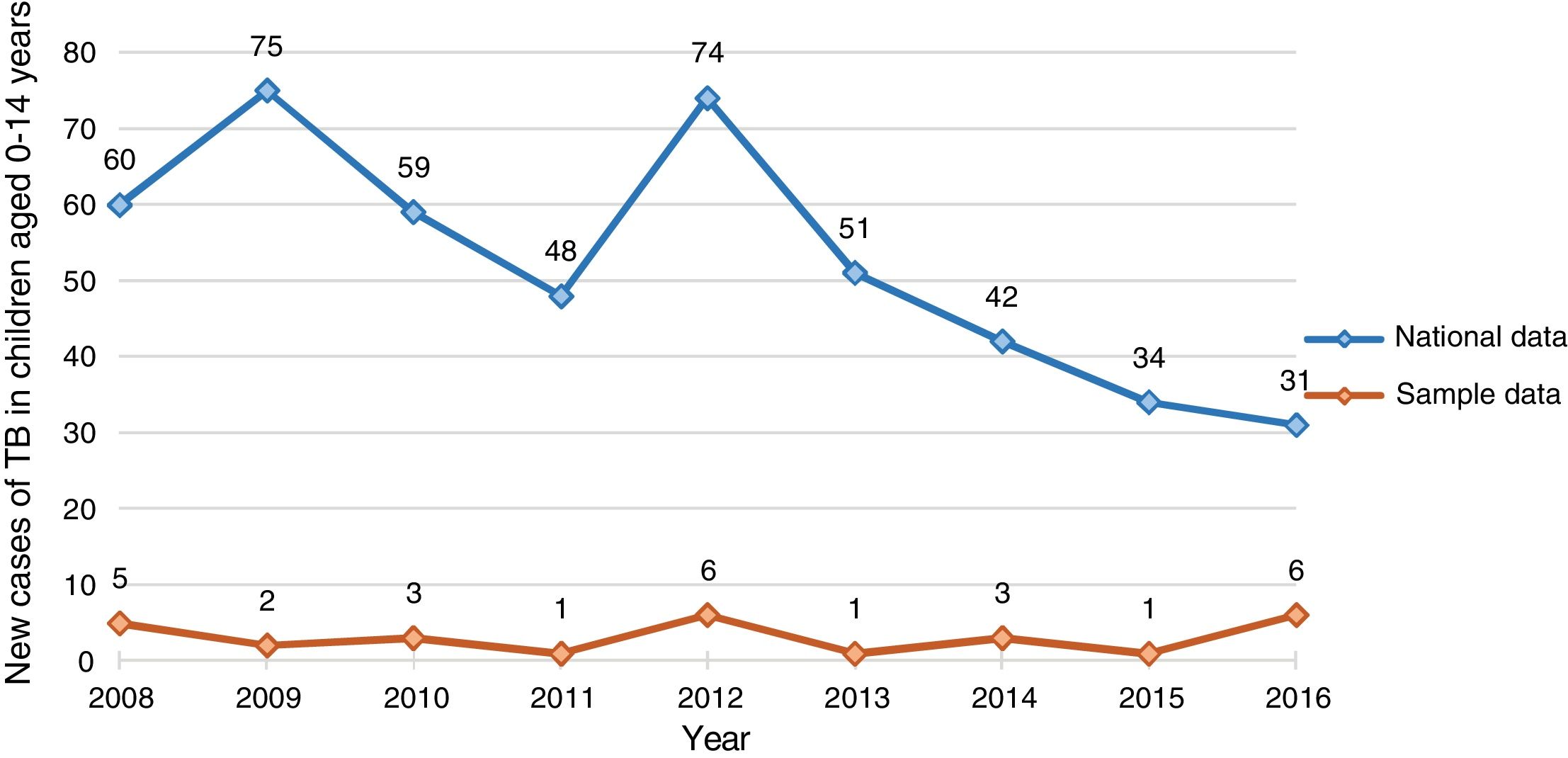
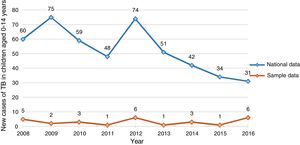
Fig. 1 compares the distribution of new cases in our sample of children aged 0–14 years with nationwide data for Portugal.
The reason for referral in 26 cases (50%) was suspicion of TB, while the remaining half of the patients were referred following a known exposure to TB. The index case was identified in 30 patients (65.2%) and corresponded to a close contact in the family in 76.6%.
DiagnosisThirty-six patients (78.3%) developed symptoms, with a median of 2 weeks’ duration (IQR, 1−12) at the time of the initial visit. The most frequent symptoms were cough (n = 25; 54.3%) and fever (n = 22; 47.8%), followed by weight loss in 8 patients (17.4%) and night sweats in 7 (15.2%).
The findings of chest radiograph were abnormal in 34 patients (73.9%); a chest CT scan was performed in 37 patients, with findings compatible for TB in 97.3%: adenopathy in 19 (51.4%), noncalcified nodules in 19 (51.4%), atelectasis in 11 (29.7%), pleural effusion in 6 (16.2%) and cavitation in 5 (13.5%).
A TST was performed in 33 patients (71.7%) and an IGRA in 19 (41.3%), most of them in the context of contact screening. The test used in the IGRA was the QuantiFERON-TB Gold® until May 2015 and the QuantiFERON-TB Gold Plus® thereafter. This assay was positive in 78.9% of tested patients, and the TST was positive in 72.7%, with 85% of patients in the sample having at least one positive test. Out of the 13 patients in whom both tests were performed, 6 (46.2%) tested positive for both, and 6 had discordant results (46.2%). Out of the 12 patients with disease confirmed by culture, 7 patients underwent a TST or an IGRA and had at least 1 positive result.
All patients underwent microbiological testing for M. tuberculosis, most frequently during the hospital stay (n = 38; 84.4%). Gastric fluid samples were collected in 31 patients (67.4%), and sputum samples in 12 (26.1%). Gastric aspirates were the predominant type of specimen collected for microbiological analysis in children under 5 years.
In addition, 32 patients (71.1%) underwent bronchoscopy with collection of a BAL sample. The macroscopic changes observed in the sample were: endobronchial mucosal inflammation in 15 (46.9%), mucopurulent secretions in 8 (25%), bronchial extrinsic compression in 4, granulation in 3, lymph node invasion in 3 and bronchial stenosis in 2.
Other sample collection procedures performed less frequently included nodal biopsy (n = 4), thoracocentesis (n = 4), collection of urine (n = 2), cerebrospinal fluid (n = 2) and, in a single patient each pleural, thoracic mass, bronchial and bone aspirate biopsies, all based on the location of disease.
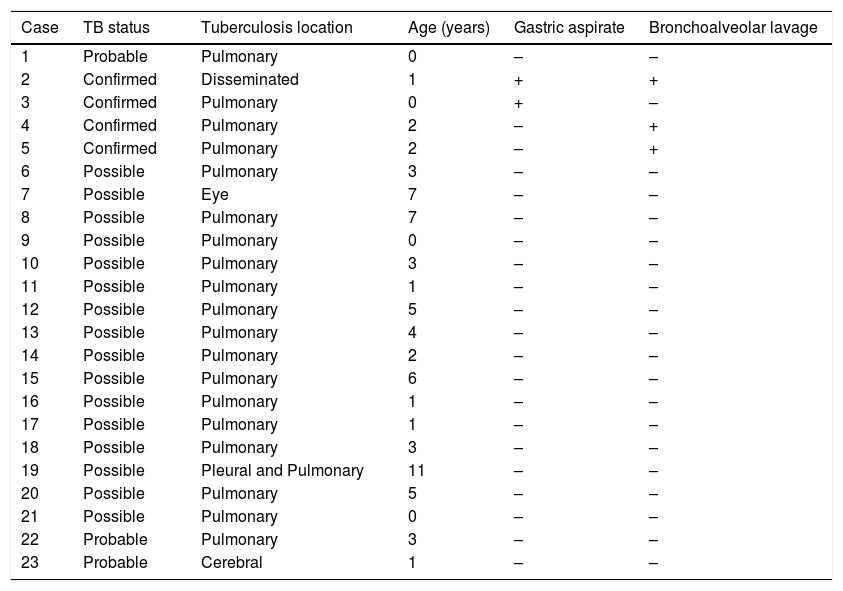
Table 1 details all the microbiological specimens collected in the patients. We analysed the distribution of positive results of the AFB smear, NAATs and cultures, and found that in some patients, M. tuberculosis was detected in more than 1 type of specimen. The culture and NAAT were positive, respectively, in 75% and 25% of pleural fluid samples, 33.3% and 16.6% of sputum samples, 21.9% and 18.8% of BAL samples, and 12.9% and 6.5% of gastric aspirate samples. The pathological examination of the biopsies performed in 8 patients revealed a granulomatous inflammatory process in 7 (87.5%). Table 2 presents the information on the 23 cases in which gastric aspirate and bronchoalveolar lavage samples were collected. In 2 of them, the invasive procedure used to collect the sample made it possible to confirm the diagnosis.
Cases with simultaneous collection of gastric aspirates and bronchoalveolar lavage, demonstrating and positive (+) and negative (-) results of nucleic acid amplification tests and cultures.
| Case | TB status | Tuberculosis location | Age (years) | Gastric aspirate | Bronchoalveolar lavage |
|---|---|---|---|---|---|
| 1 | Probable | Pulmonary | 0 | – | – |
| 2 | Confirmed | Disseminated | 1 | + | + |
| 3 | Confirmed | Pulmonary | 0 | + | – |
| 4 | Confirmed | Pulmonary | 2 | – | + |
| 5 | Confirmed | Pulmonary | 2 | – | + |
| 6 | Possible | Pulmonary | 3 | – | – |
| 7 | Possible | Eye | 7 | – | – |
| 8 | Possible | Pulmonary | 7 | – | – |
| 9 | Possible | Pulmonary | 0 | – | – |
| 10 | Possible | Pulmonary | 3 | – | – |
| 11 | Possible | Pulmonary | 1 | – | – |
| 12 | Possible | Pulmonary | 5 | – | – |
| 13 | Possible | Pulmonary | 4 | – | – |
| 14 | Possible | Pulmonary | 2 | – | – |
| 15 | Possible | Pulmonary | 6 | – | – |
| 16 | Possible | Pulmonary | 1 | – | – |
| 17 | Possible | Pulmonary | 1 | – | – |
| 18 | Possible | Pulmonary | 3 | – | – |
| 19 | Possible | Pleural and Pulmonary | 11 | – | – |
| 20 | Possible | Pulmonary | 5 | – | – |
| 21 | Possible | Pulmonary | 0 | – | – |
| 22 | Probable | Pulmonary | 3 | – | – |
| 23 | Probable | Cerebral | 1 | – | – |
TB, tuberculosis.
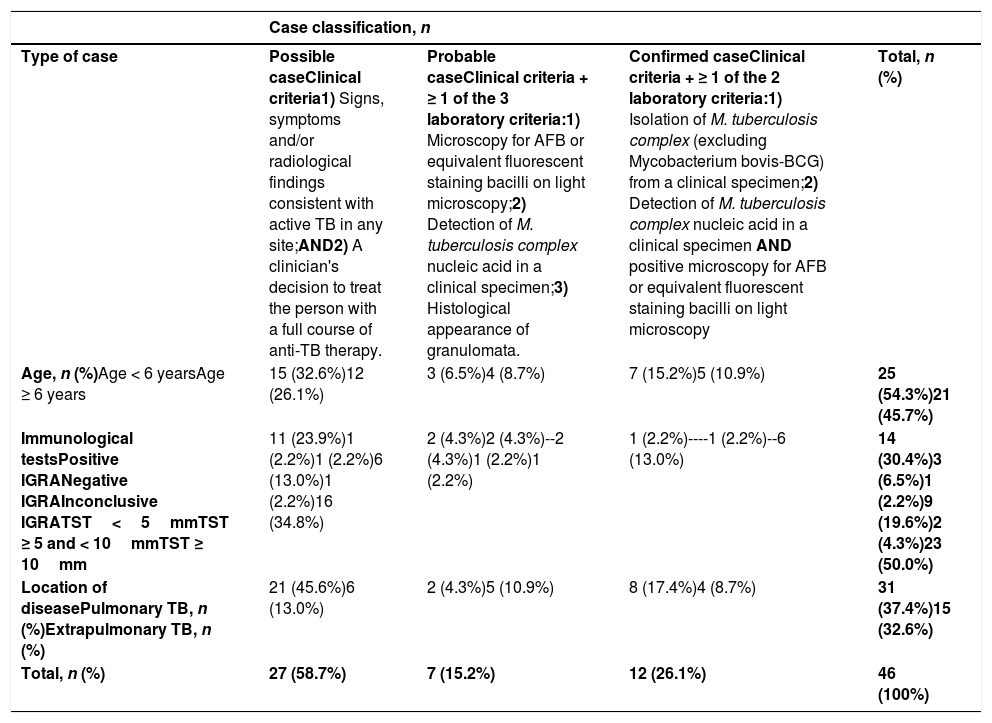
Of all cases, 12 were classified as confirmed (26.1%), 6 as probable (13%) and 28 as possible (60.9%) (Table 3). The most frequent type of TB by location was pulmonary (n = 31; 67.4%); extrapulmonary types included pleuropulmonary disease (n = 6), lymph node disease (n = 3), uveitis (n = 2) and bone TB, disseminated TB, cerebellar tuberculoma and erythema nodosum (each n = 1). Table 3 presents the distribution of cases by age, immunological test results and location of disease.
Distribution of identified cases according to the classification of the European Centre for Disease Prevention and Control11 and type of tuberculosis.
| Case classification, n | ||||
|---|---|---|---|---|
| Type of case | Possible caseClinical criteria1) Signs, symptoms and/or radiological findings consistent with active TB in any site;AND2) A clinician's decision to treat the person with a full course of anti-TB therapy. | Probable caseClinical criteria + ≥ 1 of the 3 laboratory criteria:1) Microscopy for AFB or equivalent fluorescent staining bacilli on light microscopy;2) Detection of M. tuberculosis complex nucleic acid in a clinical specimen;3) Histological appearance of granulomata. | Confirmed caseClinical criteria + ≥ 1 of the 2 laboratory criteria:1) Isolation of M. tuberculosis complex (excluding Mycobacterium bovis-BCG) from a clinical specimen;2) Detection of M. tuberculosis complex nucleic acid in a clinical specimen AND positive microscopy for AFB or equivalent fluorescent staining bacilli on light microscopy | Total, n (%) |
| Age, n (%)Age < 6 yearsAge ≥ 6 years | 15 (32.6%)12 (26.1%) | 3 (6.5%)4 (8.7%) | 7 (15.2%)5 (10.9%) | 25 (54.3%)21 (45.7%) |
| Immunological testsPositive IGRANegative IGRAInconclusive IGRATST<5mmTST ≥ 5 and < 10mmTST ≥ 10mm | 11 (23.9%)1 (2.2%)1 (2.2%)6 (13.0%)1 (2.2%)16 (34.8%) | 2 (4.3%)2 (4.3%)--2 (4.3%)1 (2.2%)1 (2.2%) | 1 (2.2%)----1 (2.2%)--6 (13.0%) | 14 (30.4%)3 (6.5%)1 (2.2%)9 (19.6%)2 (4.3%)23 (50.0%) |
| Location of diseasePulmonary TB, n (%)Extrapulmonary TB, n (%) | 21 (45.6%)6 (13.0%) | 2 (4.3%)5 (10.9%) | 8 (17.4%)4 (8.7%) | 31 (37.4%)15 (32.6%) |
| Total, n (%) | 27 (58.7%) | 7 (15.2%) | 12 (26.1%) | 46 (100%) |
BCG, Bacille Calmette-Guérin; IGRA, interferon-gamma release assay; TB, tuberculosis; TST, tuberculin skin test.
The disease responded to pharmacological treatment in the 12 confirmed cases, although susceptibility tests were not performed; in most of the cases, information on susceptibility tests results of the index case were available.
TreatmentThe median time elapsed from the initial contact with the healthcare system (hospital or outpatient clinic) to initiation of treatment was 11 days (IQR, 6–17 days) for the children that presented with symptoms suggestive of TB, and 29 days (IQR, 16.5–81 days) for children that underwent evaluation in the context of contact screening, a difference that was statistically significant.
Since 73.9% of cases (n = 34) were probable or possible, the decision to initiate treatment in these cases was based on criteria other than microbiological confirmation. The epidemiological context was considered in 26 (76.5%), signs and symptoms compatible with TB in 27 (79.4%), abnormal imaging findings in 29 (85.3%) and positive immunological tests in 25 (73.5%). In 5 of these patients (14.7%), the AFB smear (n = 1) or NAAT (n = 4) results were positive, which supported the decision to treat.
In patients with known exposure to TB, treatment was guided by the drug susceptibility test results in the index case. Treatment consisted of isoniazid, rifampicin and pyrazinamide (HRZ) in 59% of patients, with the addition of ethambutol (E) in the remaining 41%. The duration of treatment varied depending on the clinical and radiological evolution of the patient and the type of disease, with a median duration of 7 months (IQR, 6–12).
In some cases, the duration of treatment exceeded the standard 6-month regimen12,13: treatment lasted 12 or more months in 9 patients with severe forms of disease, such as bone, disseminated and cerebral TB (in adherence with current recommendations), but also in cases of extensive pleuropulmonary disease, severe adverse reactions or paradoxical reaction to the initial treatment or if the index case was resistant to isoniazid.
Adverse reactions to antituberculosis drugs were rare (15.2%) and, in most cases, mild. Two patients developed severe adverse reactions and required prescription of alternative regimens: one patient that developed drug-induced autoimmune hepatitis and required second-line treatment with levofloxacin, amikacin, ethionamide, cycloserine and corticosteroids,14 and an adolescent that developed drug reaction with eosinophilia and systemic symptoms (DRESS) in response to rifampicin.15 In the latter case, the decision was made to discontinue treatment altogether for the following reasons: classification as possible case of TB (history of contact with an active case resistant to isoniazid, immunological tests positive and abnormal imaging findings), the severity of adverse reaction, and clinical improvement and absence of signs of active tuberculosis in subsequent years in the follow-up period.
Two infants (4.3%) with a diagnosis of pulmonary tuberculosis developed a paradoxical reaction at a mean of 9.5 weeks of treatment, with radiological worsening, enlargement of lymph nodes and bronchial invasion with pulmonary tuberculoma in one of them, despite an improvement in symptoms. In both patients, treatment was switched to prednisolone, starting with a maximum dose of 2mg/kg/day that was tapered down over 2–5 weeks, which achieved a clear clinical improvement.
All patients received support through regular visits and directly observed therapy to ensure adherence to treatment.
Only 1 case was lost to follow-up, a patient that returned to his country of origin. All other patients remained in follow-up, completed treatment and were cured. Only one patient aged 10 years that presented with multiple cavitary lesions developed significant sequelae, specifically residual calcified pulmonary nodules, pulmonary cavitation, traction bronchiectasis and an obstructive pattern of moderate severity in lung function tests.
DiscussionPaediatric tuberculosis reference centres play a key role in the early identification, screening and diagnosis of children exposed to tuberculosis. This specialized care allows children to be diagnosed in the early stages of disease or even when they are still asymptomatic, as was the case of 10 of our patients (21.7%).1,4
Twelve of the cases in our sample (26.1%) were confirmed. In the group aged less than 5 years, 23.8% of patients had a positive culture, which was consistent with the proportion reported in a study conducted in Northern Portugal between 2000 and 2009.16 The percentage of confirmed cases in patients aged 0–14 years reported for the same period in Europe was slightly lower (19.2%).17 There is evidence that collection of different microbiological specimens in addition to gastric aspirate samples is associated with a higher yield for TB confirmation, and can also guide the management.18,19
In our sample, the diagnostic yield of culture of BAL samples for detection of M. tuberculosis was higher compared to the yield for gastric aspirate samples (in one third of confirmed cases, cultures of BAL samples were positive and cultures of gastric aspirate samples negative). Most of the studies in the literature report a higher overall yield with the combination of both.18
Bronchoscopy should be considered as an additional tool to collect microbiological samples (BAL, endobronchial tissue, transbronchial needle-aspiration biopsy samples) when less invasive methods are ineffective.1,19 Bronchoscopic images can provide additional clues about TB, of which extrinsic compression of airway, identified in 12.5% of our cases, is the one reported most frequently (19%-24%). Other macroscopic changes described in the literature and observed in our sample are the presence of intraluminal caseous material, tissue granulation, endobronchial mucosal inflammation and mucosal erosion and ulceration.19
Despite positive detection in 85% of immunological tests, a negative IGRA or TST result does not rule out disease, and the sensitivity of these tests is reduced in immunocompromised patients.1,4,18,20 In almost half of the patients in our sample that had both tests done, the results of IGRA and TST were discordant, which was consistent with the literature.18
At present, there is no accurate test for diagnosis of paediatric TB, so while it is important to carry out the microbiological investigation, it is equally important to take the clinical manifestations into account and consider other available methods.4 An European survey of doctors/researchers with experience in paediatric TB found that the criterion used most frequently to initiate antituberculosis treatment without microbiological confirmation was the epidemiological context (89%), followed by signs and symptoms (86%), abnormal imaging findings (77%), the age of the patient (48%) and the results of immunological tests (45%).21 These percentages are similar to those found in our study (73.5% – 85.3%).
The selection of the pharmacotherapy regimen was based on the results of antimicrobial susceptibility testing of the M. tuberculosis isolate of the patient or the index case and the guidelines of the World Health Organization (WHO).13 Rapid molecular tests were not performed, as the study refers to years when they were not used routinely, but rather in case of risk of multidrug-resistant TB. In Portugal, the estimated prevalence of isoniazid resistance in 2018 was 5.3% (according to the most recent report, pending publication). The decision to prescribe a three-drug regimen (HRZ) was based in the known M. tuberculosis susceptibility of the index cases.
Corticosteroids were needed in cases with paradoxical reactions.12 These responses are thought to occur when treatment reverses a process of immune dysregulation, with worsening of clinical or radiological findings following initiation of appropriate antituberculosis drugs in the absence of evidence of recurrence or another diagnosis. These reactions are not rare, and the proportion of HIV-negative children with paradoxical reactions found in our study was within the range reported in larger studies (3.3%-10.2%).22,23
The regimens used in children have an excellent safety profile, which explains the low incidence of adverse reactions in our sample; routine biochemical monitoring is not necessary in children, unless clinically justified.1,12
The non-existent mortality and low morbidity observed in our sample could be explained by several factors: there were no children infected with HIV; only one patient had central nervous system disease; the potential impact of low socioeconomic status was minimised by free health care and medication, and the use of daily observed therapy and a client-centred approach.
Other important factors were the follow-up by a team of paediatricians specialized in TB and the advantages offered by a paediatric TB reference centre fully integrated in the national TB programme. However, it is important to continue educating the broader community of clinicians and the general population to guarantee early diagnosis of TB in children.
ConclusionsThe identification of children at risk of TB infection, early diagnosis of the disease and successful treatment of paediatric cases are key for the purpose of reducing future cases in adults, community spread and the development of sequelae. Although many cases are not confirmed by testing, in the presence of clinical features suggestive of tuberculosis, efforts should be made to collect specimens for microbiological testing before initiating treatment. The pharmacotherapy regimen should be selected based on current clinical guidelines and the clinical response of the patient. Follow-up by specifically trained paediatricians in outpatient TB clinics in the community allows more adequate management of paediatric TB, while links to referral hospitals are important to allow further investigation.
Please cite this article as: Silva JB, Santos JC, Barbosa L, Carvalho I. Tuberculosis en la edad pediátrica: una reflexión sobre la transmisión. An Pediatr (Barc). 2021;94:403–411.