Paediatric inflammatory bowel disease is not very common in Spain. Its onset can be silent and an early diagnosis reduces complications and sequelae related to the disease, and can improve the prognosis. It is advisable to define the different intervals into which the time until the diagnosis is divided, as well as the peculiarities and conditions in order to be able to act on them and, to avoid, as far as possible, the diagnostic delay. The aim of this review is to provide tools to reduce the time to diagnosis.
La enfermedad inflamatoria intestinal pediátrica continúa siendo poco prevalente en nuestro medio. El comienzo de los síntomas puede ser insidioso y el diagnóstico precoz permite disminuir las complicaciones y las secuelas de la enfermedad, así como mejorar el pronóstico. Conviene definir los diferentes intervalos que componen el tiempo hasta el diagnóstico, así como sus peculiaridades y condicionantes para poder actuar sobre ellos y evitar, en la medida de lo posible, que se demore el diagnóstico, lo que repercutirá directamente sobre la salud de nuestros pacientes. Esta revisión pretende proporcionar herramientas para que cada vez sea más precoz el diagnóstico de la enfermedad.
Although the incidence and prevalence of inflammatory bowel disease (IBD) have increased in recent years,1–3 it continues to be an infrequent disease at the primary care (PC) level. In addition, primary care paediatricians (PCPs) encounter and manage diseases that are much more prevalent and with features similar to those of IBD on a daily basis, such as functional abdominal pain disorders, which include functional dyspepsia, irritable bowel syndrome, abdominal migraine and functional abdominal pain not otherwise specified, with a prevalence of 10%–20% in the paediatric population.4 Another critical aspect is the heavy caseloads that PCPs are forced to manage, of about 1002 individuals with an official public health card (PHC) per PCP (source: Statistics Webpage of the Ministry of Health, Social Services and Equality of Spain). These 3 factors are among the most important reasons for the low level of suspicion and therefore the delayed diagnosis of these diseases. We ought to mention that efforts must also be made at the hospital level, including emergency care departments, inpatient services and outpatient clinics, to reduce the time to diagnosis (TD) of IBD. Since delays in diagnosis have deleterious consequences in the short, medium and long term, it is important that we identify and understand the causes of delays in order to establish adequate strategies to reduce the time elapsed between the onset of symptoms and the confirmation of diagnosis by a paediatric gastroenterologist.
Consequences of delayed diagnosis of paediatric inflammatory bowel diseaseThe TD of IBD is particularly relevant in children. Days of missed school due to disease, the undue prolongation of the diagnostic process and social isolation are some of the potential consequences of delayed diagnosis. Untreated symptoms, such as pain or diarrhoea, can have a direct impact on the physical and psychosocial development of children and adolescents with IBD. Delayed diagnosis is associated with an increased risk of complications,5,6 growth failure and delayed puberty,7 more extensive disease,8–10 a poorer response to medical treatment,8 an increased need of surgery5 and decreased health-related quality of life.11 However, it does not seem to increase the risk of colectomy in patients with ulcerative colitis (UC).12 The onset of paediatric IBD (PIBD) usually takes place in critical periods of life, characterised by physical changes (such as a high growth velocity), and psychosocial development, when the patient is developing a sense of self, potentially leading to role confusion. Growth failure, disorders of bone metabolism, delayed puberty, malnutrition and micronutrient or vitamin deficiencies are frequently associated with PIBD. Growth failure, which is less common in UC compared to Crohn’s disease (CD), may be present at the time of diagnosis or occur during the follow-up. It is identified in 40%–50% of children with CD, persists in adulthood in 15%–20% of patients, and results in a final height below the target height in 20%. The TD of CD is one of the determinants of such growth failure.13,14 Thus, a large case series revealed a prevalence of growth failure of 9.4% in patients diagnosed within 3 months of onset, 15.7% in those diagnosed 3–6 months after onset and 22.3% in those diagnosed more than 6 months from onset.15 Delayed puberty generates feelings of isolation or rejection, more frequent than bullying, with a negative impact on relationships with peers. These patients have poor body image, decreased self-esteem, a higher risk of depression and anxiety about the future and, in the long term, missed opportunities in social and personal development.
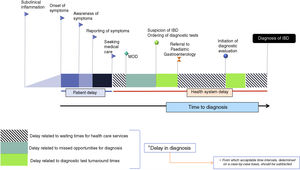
Time to diagnosis, delay in diagnosis and missed opportunities for diagnosis: concepts and intervalsDelay in diagnosis (DD) has been defined as the time elapsed from the onset of symptoms to diagnosis. In our opinion, this is an unclear term, since for instance, in the hypothetical case that a patient with UC were diagnosed in 2 weeks, this time would be referred to as a “delay”, incorrectly in our opinion. To avoid confusion, it may be better to use the term TD to refer to the time elapsed between onset of symptoms and diagnosis (Fig. 1) and DD as wasted time in diagnosis that is not justifiable including missed opportunities for diagnosis during medical care episodes. The latter includes patient-related delays and health care system-related delays. We defined missed opportunities for diagnosis as any contacts with the health care system in which the diagnostic evaluation was not initiated despite the presence of one or more signs or symptoms suggestive of IBD being reported during the history taking. We defined medical care episodes as any physician-patient interaction in primary care, emergency care or hospital care settings. Determining what constitutes a reasonable TD is challenging, as this time will depend on each patient and clinical condition. The TD includes the care system response time, defined as those periods in which the patient is waiting to undergo diagnostic tests or to be seen by a specialist.
The TD comprises different intervals that deserve individual attention (Table 1).
Time to diagnosis and intervals of diagnostic delay in PIBD.
| Author | Patient-related delay | Health care system-related delay | Time to diagnosis | |
|---|---|---|---|---|
| Interval 1 | Interval 2 | Interval 3 | ||
| Schoepfer et al. (2019)16 | 1 m (IQR, 1–4) | 1 m (IQR, 1–7) | 3 m (IQR, 1–9) | |
| El Mouzan et al. (2019)17 | 6 m (range, 1–24)a | 0.6 m (IQR, 0.4–3.4)a,c | 8 m (range, 4–24)a | |
| 2.4 m (range, 0.3–7)b | 0.8 m (0.5–3.9)b,c | 5 m (range, 2.1–8.8)b | ||
| Ricciuto et al. (2018)10 | 2.96 m (IQR, 1.6–8.3)d | 15 d (IQR, 7-45) | 4.5 m (IQR, 2.1–8.8) | |
| 6.9 m (IQR, 2.9–12.6)a | ||||
| 2.4 m (IQR, 1.3–5.3)b | ||||
| Jimenez et al. (2017)18 | 15 d (IQR, 12-30) | 2.5 m (IQR, 0.7–6.8) | 12 d (IQR, 7.7–44) | 4.2 m (IQR, 2.3–11.7) |
| 21 d (IQR, 12–57)a | 4.2 m (IQR, 1.2–11)a | 14 d (IQR, 6.5–43.7)a | 9.4 m (IQR, 3–14)a | |
| 12 d (IQR, 12–27)b | 1.1 m (IQR, 0.5–2.6)b | 17 d (IQR, 12.5–36.9)b | 3.2 m (IQR, 1.4–5.0)b | |
| Schoepfer et al. (2017)9 | 1 m (IQR, 0–3) | 3 m (IQR, 1–9)c | 4 m (IQR, 2–8)a | |
| 0 m (IQR, 0–3)b | 2 m (IQR, 1–4)b,c | 2 m (IQR, 1–7)b | ||
| Arcos-Machancoses et al. (2014)19 | 2 w (IQR, 1–3)a | 0.5 w (IQR, 0–1.25)a | 2 w (IQR, 1–4)a | 12 w (IQR, 3–51)a |
| 1 w (IQR, 1–2.5)b | 2 w (IQR, 0–4)b | 5 w (IQR, 1.5–34)b | 12 w (IQR, 4–38)b | |
| Timmer et al. (2011)15 | 4 m (IQR, 2–8) | |||
| 5 m (IQR, 2–10)a | ||||
| 3 m (IQR, 1–6)b | ||||
| Sawczenko et al. (2003)20 | 5 m (IQR, 1–108) | |||
| 6 m (IQR, 3.6–12)a | ||||
| 4.8 m (IQR, 2.4–8.4)b | ||||
| Spray et al. (2001)21 | 11.7 m (range, 1–84)a | |||
| 5 m (range, 0.5–36)b | ||||
| Heikenen et al. (1999)22 | 7.1 m (range, 0.25–36)a | |||
| 6.7 m (range, 0.1–36)b | ||||
d, days; IQR, interquartile range; m, months; w, weeks.
This interval depends on the patient and comprehends the time elapsed (usually unknown) between the onset of disease and the patient becoming aware of the symptoms and letting his or her parents know, or the parents becoming aware of there being a problem, plus the additional time that elapses before medical care is sought. The patient-related interval usually last days to weeks, and delays are usually due parents/patients lacking knowledge about relevant signs and symptoms (such as growth failure, asthenia, mild abdominal pain, etc.); the embarrassment elicited by gastrointestinal symptoms (diarrhoea, perianal complaints etc.) or anxiety regarding the possibility of receiving bad news (Table 2). Other factors that may be at play in this interval are the social environment of the patient, the geographical distance and accessibility of health care facilities, self-medication, believing that the symptoms are temporary, spiritual or religious beliefs and the use of alternative therapies to manage/alleviate symptoms. One aspect worth noting is that there are no differences in the duration of this interval between patients with relatives with IBD and patients with no family history of IBD.9,18
Factors that influence each interval.
| Factor | |
|---|---|
| Interval 1 | Lack of awareness of relevance of signs/symptoms of parents/patients |
| Nonspecific symptoms: growth failure, asthenia, mild abdominal pain, etc. | |
| Embarrassment caused by gastrointestinal symptoms (diarrhoea, perianal complaints, etc) | |
| Anxiety at the possibility of receiving bad news | |
| Social environment of patient | |
| Geographical distance | |
| Accessibility of health care facilities | |
| Diagnostic error and self-medication | |
| Belief that the problem is temporary | |
| Spiritual or religious beliefs | |
| Use of alternative therapies to manage/alleviate symptoms | |
| Interval 2 | Age of patient |
| Type of IBD | |
| Disease extension and location | |
| Severity of episode | |
| Presenting symptoms | |
| Lack of awareness regarding symptoms of the physician | |
| Believing the symptoms are caused by more frequent diseases | |
| Interval 3 | Organizational barriers |
| Prioritization of more severe or complicated cases | |
| Performance of superfluous diagnostic tests that do not result in a conclusive diagnosis |
This interval usually lasts months and is particularly relevant, as there is previous evidence9,10,18 that it is the main contributor to the TD. It depends strongly on the age of the patient, the type of IBD, the extension of disease, the disease activity and the presenting symptoms. This interval is mainly due to the physician not recognising the symptoms and attributing them to more frequent diseases, such as infections.
A nationwide register, SPIDER, that included 149 patients with a recent diagnosis of PIBD in Spain revealed that 73.5% visited more than 1 physician, 29.5% 3 or more physicians and up to 32.7% were evaluated more than 4 times by the same physician before being referred to a paediatrician specialised in paediatric gastroenterology. Of all patients, 52% had received a diagnosis of acute gastroenteritis; other initial diagnoses included lactose intolerance, irritable bowel syndrome, gluten intolerance, haemorrhoids, anal fissure, perianal abscess, recurrent abdominal pain, food allergy, short stature and gastritis.18
The 3 factors at play in this interval are the level of suspicion of IBD, the lines of communication with the reference unit that the patient needs to be referred to and previous experience with the disease. It is important that PCPs be aware of the possibility of IBD, as the prevalence and incidence of this disease are low. According to the Instituto Nacional de Estadística (National Institute of Statistics), the population aged less than 14 years in Spain as of December 31, 2018 was of 6 438 614 inhabitants, and the PHC/PCP ratio in 2018 was of 1002 children (source: Statistics Webpage of the Ministry of Health, Social Services and Equality of Spain), so given that the incidence of PIBD in Spain is of 2.8 cases per 105 inhabitants,1 each paediatrician would encounter 0.18 cases of PIBD per year. A PCP working for 35 years is likely to manage a total of 6.3 patients with IBD over the course of his or her professional career. These data evince the need for PCPs to maintain a high level of suspicion, since compared with abdominal pain, which accounts for 8%–18% of all paediatric PC visits, very few patients in this setting will have IBD.
On the other hand, we need to take into account the waiting times and the pathways connecting PC settings to the reference unit that these patients need to be referred to, factors that vary between autonomous communities. In some cases, and depending on the clinical condition of the patient, the waiting times may be excessive, so it is essential that alternative pathways for referral to paediatric gastroenterology services are established in order to shorten this interval.
This interval is significantly lower in patients with UC or IBD unclassified (IBDU), aged more than 11 years, with colonic (B2)23 or ileocolonic (B3) forms of CD compared to CD with exclusive involvement of the distal 1/3 ileum (L1) and patients with any of the following signs or symptoms: diarrhoea, rectal bleeding, bloody diarrhoea, fever or weight loss.10,15 Abdominal pain was not a symptom that affected DD, but growth velocity was.10 This is particularly relevant, as growth failure in and of itself is not recognised as a sign of IBD and therefore the latter is not included in some of the algorithms for evaluation of short stature published in the literature,24 even though the prevalence of growth failure is of 10%–56% in patients with CD and of 0%–10% in patients with UC, and that up to 46% will exhibit a decreased height velocity before the onset of any other symptom related to IBD. Furthermore, only 12% of children with IBD exhibit a normal growth velocity at the time of diagnosis.13–15
Interval 3: from referral to paediatric gastroenterology to diagnosisThis interval usually lasts days, and delays are mainly due to organizational problems and the prioritization of more severe or complicated cases.10,18,19 In such cases, the presence of rectal bleeding contributes to a quicker diagnosis.10
One factor to take into account in this interval is that certain pathogens may produce mucosal lesions found that are similar to those associated with IBD on endoscopic examination (Table 3), which highlights the importance of a rigorous and standardised diagnosis in these patients. Thus, it is recommended that patients be evaluated in facilities with sufficient experience in the management of these cases,31 applying the Porto criteria32 and correctly identifying the IBD subtype, thus reducing the number of cases diagnosed as IBDU.33
Main infectious agents that cause lesions detected by endoscopy similar to those found in IBD.
| Microorganism | Potential ileal involvement | Appearance similar to CD | Appearance similar to UC |
|---|---|---|---|
| Plesiomonas shigelloides | No | + | +++ |
| Entamoeba histolytica | No | + | +++ |
| Aeromonas spp. | No | + | ++ |
| Clostridium difficile | No | + | + |
| Escherichia coli | No | + | + |
| Klebsiella oxytoca | No | + | + |
| Vibrio parahaemolyticus | No | + | + |
| Mycobacterium tuberculosisb | Yes | +++ | + |
| Yersinia enterocoliticaa | Yes | +++ | + |
| Campylobacter spp.a | Yes | ++ | + |
| Salmonella enteritidisa | Yes | + | ++ |
| Shigella dysenteriae | Yes | + | +++ |
| Cytomegalovirus | Yes | + | +++ |
Neisseria gonorrhoeae, Chlamydia trachomatis, herpes simplex virus, cytomegalovirus, Treponema pallidum, Giardia lamblia, Haemophilus ducreyi and Entamoeba histolytica may cause proctitis in sexually active adolescents, consider possibility of sexually-transmitted disease.
Generally, the main factors that play a role in DD are colonic involvement in CD, which acts as a protective factor leading to a quicker diagnosis, and diarrhoea or rectal bleeding, which also act as protectors. Risk factors associated with delayed diagnosis include onset before age 5 years and CD with isolated ileal involvement. Table 4 summarises the findings regarding the factors with an impact on the TD.
Factors involved in the diagnosis of IBD.
| Factor | OR (95% CI) | Type of analysis | P | Ref. |
|---|---|---|---|---|
| Crohn’s disease | 0.62 (0.56–0.68)b | MV | NA | 15 |
| IBDU | 0.80 (0.66–0.96)b | MV | NA | 15 |
| Family history | 1.45 (1.07–1.96)b | UV | NA | 15 |
| 8.84 (0.98–76)CD | MV | .051 | 17 | |
| Age (year increase) | 1.07 (1.06–1.08)a,b | MV | NA | 15 |
| Age <5 years | 0.11 (0.018–0.66) | UV | .015 | 19 |
| Age >10 years | 15.625 (1.23–200)UC | MV | .034 | 17 |
| L2 or L2 + L4 | 1.52 (1.25–1.85)b | MV | NA | 15 |
| L1 | 0.35 (0.13–0.95) | MV | .040 | 17 |
| B3 | 0.83 (0.73–0.97)b | UV | NA | 15 |
| G1 | 0.66 (0.53–0.72)b | UV | NA | 15 |
| MEI | 0.89 (0.77–1.01)b | UV | NA | 15 |
| Severity of episode | 1.53 (1.00–2.38) | UV | .051 | 10 |
| Diarrhoea | 3.44 (1.35–9.0) | UV | .010 | 10 |
| Rectal bleeding | 3.03 (1.17–7.69) | UV | .021 | 10 |
| Bloody diarrhoea | 3.57 (1.19–10) | MV | .024 | 10 |
| Fever | 4.34 (0.93–20) | UV | .061 | 10 |
| Weight loss | 2.63 (1.01–6.6) | UV | .047 | 10 |
| Delayed growth | 0.48 (0.31–0.75) | MV | .001 | 10 |
| Abdominal pain | 2.94 (1.06–8.33) | UV | .037 | 10 |
| Hypoalbuminaemia | 1.26 (1.11–1.42) | MV | .001 | 10 |
| Anaemia | 1.05 (1.02–1.09) | UV | .001 | 10 |
| Elevated CRP | 1.02 (1.0–1.03) | UV | .065 | 10 |
| Elevated ESR | 1.03 (1.01–1.05) | UV | .015 | 10 |
Dependent variable: early diagnosis.
B3, Crohn’s disease with stricturing behaviour; CD, Crohn’s disease; CRP, C-reactive protein; EIM, extraintestinal manifestations; ESR, erythrocyte sedimentation rate; G1, growth failure; IBDU, inflammatory bowel disease unclassified; L, location of Crohn’s disease (L1: distal 1/3 ileum; L2: colon; L3: ileocolon; L4: upper gastrointestinal); MV, multivariate; NA, not available; Ref, reference; UC, ulcerative colitis; UV, univariate.
During interval 2, patients frequently receive one or several diagnoses as clinicians attempt to identify a plausible explanation for the clinical presentation out of a collection of diseases that are more prevalent than IBD. It is important that the duration of symptoms is established and that a diagnostic evaluation for IBD be initiated if they persist, that is, once they become an alarm sign/symptom. In other cases, the alarm signs are abnormal laboratory findings (Table 5).
Signs, symptoms and laboratory findings suggestive of inflammatory bowel disease.
| Symptoms | Asthenia or anorexia |
| Nocturnal bowel movements. Urgency. | |
| Diarrhoea lasting ≥2–4 weeksa (Bristol stool chart type 5–7) or ≥2 episodes in the past 6 months with no other apparent cause | |
| Bloody diarrhoea lasting >1 weeka | |
| Recurrent abdominal painb >14 days or ≥2 episodes of abdominal pain in the past 5 months (not meeting ROMA IV criteria for a functional disorder) | |
| First-degree relative with IBDb,c | |
| Unintended weight loss >1 kgb,c | |
| Anal bleeding in absence of constipation (based on ROMA IV criteria)c | |
| Signs | Recurrent mouth ulcers |
| Recurrent perianal diseasec (abscesses,a fistulae,a fissures or skin tags) | |
| Prolonged feverb | |
| Extraintestinal manifestations (episcleritis, scleritis, uveitis, erythema nodosum, gangrenous pyoderma, psoriasis, arthritis or digital clubbing)c | |
| Delayed linear growthb,c (at least 1 SD below the target height) | |
| Delayed puberty | |
| Laboratory findings | Positive ASCA or ANCA antibodiesb |
| Elevated faecal calprotectinb | |
| Negative stool culture/parasites | |
| Haemoglobin concentration below the normal range for age and sexc | |
| Hypertransaminasaemia | |
| Elevated CRP > 10 mg/Lb,c | |
| Platelet count > 450 × 109/mm3c | |
| Elevated ESR > 20 mm/hb,c |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SD, standard deviation.
None of the signs, symptoms or laboratory findings described above are pathognomonic for IBD, which is why efforts are being made to develop and validate algorithms that would increase the level of suspicion of physicians and PCPs and other specialists, facilitating the early referral of patients. Danese et al.38 developed the Red Flag Index, consisting of 8 items rated on a scale from 2 to 5 points. A Red Flag Index greater than 8 points can discriminate adult patients with CD from those with suggestive symptoms with a sensitivity and a specificity of 94%, a positive likelihood ratio of 5.1 and a negative likelihood ratio of 0.066. Holtman et al.35 conducted a prospective study that included 90 patients with gastrointestinal symptoms (prolonged diarrhoea or chronic/recurrent abdominal pain) referred from PC for assessment in a paediatric gastroenterology unit. The authors reported that adding faecal calprotectin results to the alarm symptoms (Table 4) significantly increased the area under the curve from 0.80 (0.67–0.92) to 0.97 (0.93–1.00), which did not occur with the inclusion of CRP. Decision curves confirmed that the combination of alarm symptoms with faecal calprotectin produced the diagnostic test strategy with the highest net benefit at reasonable threshold probabilities.
Recently, Turner et al. developed and validated the IBD-REFER criteria to guide the selection of patients requiring referral to paediatric and adult gastroenterology units due to suspected IBD. The IBD-REFER includes 3 major criteria and 8 minor criteria, which we detail in Table 5 along with other criteria for referral published by other authors. The IBD-REFER criteria have exhibited a higher sensitivity and specificity when used both in children and adults compared to the Red Flags Index of the International Organization For the Study of Inflammatory Bowel Disease.37
Proposed measures of improvementIn light of all of the above, we believe that collaboration in the framework of an interdisciplinary team is necessary to achieve the proposed objectives. The following are some of the strategies that we consider essential:
- a)
Development and dissemination of guidelines and protocols in collaboration with PC to facilitate the early identification of these patients (Fig. 2).39
- b)
Establishment of quick and effective channels of communication between PC centres, hospitals and referral centres to allow early referral of these patients.40
- c)
Develop alternative referral routes to reduce the time to diagnosis (delivery of urgent or acute care by nursing staff, online visits, phone visits, etc.).
The authors have no conflicts of interest to declare.
Please cite this article as: Martín-de-Carpi J, Jiménez-Treviño S, Pujol-Muncunill G, Martín-Masot R, Navas-López VM. Tiempo hasta el diagnóstico en la enfermedad inflamatoria intestinal pediátrica: claves para un diagnóstico precoz. An Pediatr (Barc). 2020;92:242.