On 31 December 2019, the Wuhan Municipal Committee of Health and Healthcare (Hubei Province, China) reported that there were 27 cases of pneumonia of unknown origin with symptoms starting on the 8 December. There were 7 serious cases with common exposure in market with shellfish, fish, and live animals, in the city of Wuhan. On 7 January 2020, the Chinese authorities identified that the agent causing the outbreak was a new type of virus of the Coronaviridae family, temporarily called «new coronavirus», 2019-nCoV. On January 30th, 2020, the World Health Organisation (WHO) declared the outbreak an International Emergency. On 11 February 2020 the WHO assigned it the name of SARS-CoV2 and COVID-19 (SARS-CoV2 and COVID-19).
The Ministry of Health summoned the Specialties Societies to prepare a clinical protocol for the management of COVID-19. The Spanish Paediatric Association appointed a Working Group of the Societies of Paediatric Infectious Diseases and Paediatric Intensive Care to prepare the present recommendations with the evidence available at the time of preparing them.
El 31 de diciembre de 2019, la Comisión Municipal de Salud y Sanidad de Wuhan (provincia de Hubei, China) informó sobre la existencia de 27 casos de neumonía de etiología desconocida con inicio de síntomas el 8 de diciembre, incluyendo siete casos graves, con exposición común a un mercado de marisco, pescado y animales vivos en la ciudad de Wuhan. El 7 de enero de 2020, las autoridades chinas identificaron como agente causante del brote un nuevo tipo de virus de la familia Coronaviridae, denominado temporalmente “nuevo coronavirus”, 2019-nCoV. El 30 de enero de 2020 la Organización Mundial de la Salud declara el brote un Emeregencia Internacional. El día 11 de febrero la OMS le asigna el nombre de COVID-19 (Coronavirus Infectious Disease). El Ministerio de Sanidad convoca a las Sociedades de Especialidades para la elaboración de un protocolo clínico de manejo de la infección por COVID -19. La Asociación Española de Pediatría nombra un grupo de trabajo de las Sociedades de Infectologia Pediátrica y Cuidados Intensivos Pediátricos que se encargan de elaborar las presentes recomendaciones con la evidencia disponible en el momento de su realización.
As stated by the Ministry of Health, on December 31, 2019, the Wuhan Municipal Health Committee (Hubei province, China) notified the identification of 27 cases of pneumonia of unknown aetiology with onset on December 8, including 7 severe cases, with a shared history of possible exposure in a market selling shellfish, seafood and live animals in Wuhan City, but no identified source of the outbreak. The market was shut down on January 1, 2020. On January 7, 2020, the Chinese authorities identified a new virus in the Coronaviridae family as the causative agent of the outbreak, temporarily named “new coronavirus” (2019-nCoV). The Chinese authorities shared the gene sequence of the virus on January 12, 2020.1
On January 30, the World Health Organization (WHO) declared the 2019-nCoV outbreak in China a global public health emergency.2
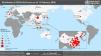
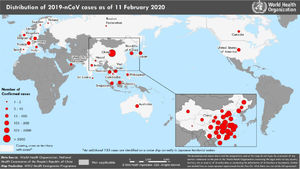
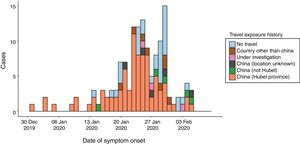
By February 12, more than 45 000 cases had been diagnosed in China, the majority clustered in the province of Hubei (33 000), while more than 450 cases had been notified outside China (https://www.worldometers.info/coronavirus/), usually in association with cases imported from China (Figs. 1 and 2). The reported fatality rates in confirmed cases has ranged from 2% to 3%, although these data must be interpreted with caution given how quickly the outbreak is evolving.1
The age distribution reflects a low incidence in the paediatric age group (0.9%) associated with a milder course of disease.3,4 Based on data of inpatient case series published to date, a high proportion of hospitalised adults and nearly all individuals that died had underlying disease.5,6
On January 31, a case of infection by 2019-nCoV was confirmed in La Gomera (Canary Islands, Spain) a German national that was a close contact of another confirmed case in Germany, and on February 9, another positive case was detected in Majorca, corresponding to a citizen of the United Kingdom that was a close contact of a case in France. Both of these cases diagnosed in Spain were in adults and had a mild course.
Spain has no airports with direct flights to Wuhan. The cancellation of flights from Wuhan or to and from China decreases the probability of arrival of infected individuals. Nevertheless, it is possible that cases imported from the high-risk area may still arise in Spain. Under the current circumstances, it is estimated that the risk of an imported case emerging in Spain is moderate.1
The recommendations presented in the current document are based on and adapted from the guidance for the clinical management of severe acute respiratory infection by 2019-nCoV published by the WHO on January 28,7 which in turn were adapted from the recommendations given for management of severe acute respiratory infection with suspected involvement of MERS-CoV, and the recently published recommendations of the National Clinical Research Center for Child Health de China8 and expert’s consensus statement.9 These guidelines may change as the outbreak evolves, including the case definition, which could extend to a larger geographical area, and the recommendations for prevention, isolation, protection or treatment, based on emerging evidence. These clinical practice guidelines do not include recommendations on the management of close contacts.
On February 11, 2020 the WHO changed the interim name of 2019-nCoV to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus infectious disease (COVID-19).
Human coronavirus (HCoV)Since human coronaviruses (HCoV) were first identified in the 1960s, 6 viruses, including HCoV 229E, HCoV OC43, HCoV NL63, HCoV HKU1, severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) have been recognised as causative agents of a broad range of respiratory infections.
HCoV NL63 and HCoV HKU1 were first described in 2004 and 2005, respectively, and combined with HCoV 229E and HCoV OC43 are responsible for 35% of upper respiratory tract infections, usually in the context of outbreaks. Human coronavirus OC43 is the most prevalent, with the majority of cases occurring in children under 5 years. They are often detected in cases of coinfection by other respiratory viruses, which makes it difficult to establish their particular role in disease. These viruses are associated with a greater severity of disease requiring hospital admission, usually due to bronchospasm, especially in children with underlying diseases. There have been fatal cases of HCoV NL63 infection in immunocompromised patients.10–12
The SARS-CoV was described in 2003 in relation to an outbreak originating in China that caused more than 700 deaths, with 20%–30% of cases requiring mechanical ventilation and a 10% case fatality rate and a very high mortality in patients with underlying disease. The MERS-CoV virus was first identified in 2012 in association with a similar clinical picture, but with a higher fatality rate (36%). This virus has not been fully eradicated and sporadic cases continue to emerge. Both are zoonotic diseases transmitted to humans through animal vectors, bats in the case of SARS and dromedary camels in the case of MERS, although cases of human-to-human transmission have been reported, mainly in health care settings, with a low rate of transmission.13
SARS-CoV-2 and COVID-19Like other human coronaviruses, SARS-CoV2 is an enveloped single-stranded RNA virus. It has a diameter of 60–140 nm and a round or oval shape, and exhibits some pleomorphism.14 There is evidence of a high sequence identity (percent identity of 86.9%–89%) with the genome of a SARS-like coronavirus found in bats (bat-SL-CoVZC45).14,15 The sequence of the main protein in the viral envelope of SARS-CoV-2 also exhibits high identity with the sequences found in bat-SL-CoVZC45 (84%) and SARS-CoV (78%).
The physical and chemical properties of the virus responsible for COVID-19 have not been fully established, but it is believed that it is sensitive to ultraviolet radiation and heating. For instance, based on previous research on SARS-CoV and MERS-CoV, the virus could be inactivated by heating it to a temperature of 56 °C for 30 min and using solvents effective on lipids such as 70% ethanol, disinfectants containing chlorine, peroxyacetic acid and chloroform, but not with chlorhexidine.8
EpidemiologyThe main source of infection are individuals infected by SARS-CoV-2. There have been reports of transmission during the incubation period in asymptomatic individuals.16 The virus is transmitted in airborne droplets (>5 microns) when diseased individuals cough, speak or sneeze. Close contact is another potential source of transmission (such as touching the mouth, nose or the ocular conjunctiva with a contaminated hand). There are no documented cases of vertical transmission17, but there has been a case of infection in the newborn of an infected mother, who tested positive at 30 h post birth. It is not known whether the virus can be transmitted through breastfeeding. The paediatric cases documented to date have been less frequent and milder compared to adults, although it appears that the full spectrum of severity is possible in the paediatric population. There have been no reports of fatal cases in children. Familial clusters have been described, a salient one including the case of 2 children, one with mild disease and another asymptomatic with abnormal imaging findings.15
Definitions and criteriaAt the time of this writing (11/2/2020), cases are identified applying the criteria presented in Table 1.
Epidemiological, clinical and laboratory criteria for assessment and identification of infection by novel coronavirus.
| Epidemiological and clinical criteria: | |
| A | Any individual with clinical manifestations compatible with acute respiratory infection of any severity presenting with fever and any of the following symptoms: shortness of breath, cough or malaise |
| AND | |
| History of travel to Hubei province, China, in the 14 days preceding onset | |
| B | Any individual with fever of respiratory manifestations such as shortness of breath or cough |
| AND | |
| History of close contact with a probable or confirmed case in the 14 days preceding onset, with close contact defined as follows: | |
| - Caring for an individual with probable or confirmed case while the case was symptomatic (health care workers that did not use appropriate protective measures, family members or other individuals with similar physical contact with the case). | |
| - Having been in the same space as a probable or confirmed case, at a distance of less than 2 metres, while the case was symptomatic (such as household members or visits). | |
| - In an airplane, close contact is defined as being a passenger seated within a 2-seat radius from a probable or confirmed case while the case was symptomatic, or a member of the crew coming in contact with a case (see Appendix A). | |
| C | Any individual requiring hospital admission for fever and clinical features of severe acute respiratory illness |
| AND | |
| History of travel to mainland China in the 14 days preceding onset. | |
| Laboratory criteria: | |
| Positive result of PCR screening and positive result in the confirmatory PCR analysis of an alternative gene | |
- •
Person under investigation (PUI): meeting at least 1 of the 3 criteria given in Table 1.
- •
Laboratory-confirmed case: case that meets the laboratory criteria given in Table 1.
- •
Probable case: case under investigation in which the laboratory results are inconclusive or only 1 generic coronavirus polymerase chain reaction (PCR) test is positive.
- •
Ruled out case: case under investigation in which laboratory tests do not detect infection by SARS-CoV-2.
The diagnosis is made by reverse transcription PCR (RT-PCR) for detection of coronavirus RNA or by sequencing of the viral genome. Testing can be performed on pharyngeal swab, nasopharyngeal swab (more appropriate in children), sputum, stool or blood specimens. The virus can be isolated from airway epithelial cell cultures, but this can only be performed in referral laboratories.18
We recommend using a respiratory viral panel to rule out coinfection with other viruses, especially influenza viruses.
Although several laboratories in the different autonomous communities of Spain can perform the microbiological diagnosis, samples for all positive cases must be submitted to the Centro Nacional de Microbiología (National Microbiology Centre, CNM) for definitive confirmation. Samples must be stored and transported at 4 °C and handled as a category B infectious substance.
Clinical picture of respiratory infection by SARS-CoV-2Infection by SARS-CoV-2 may present as mild, moderate or severe disease, including severe pneumonia, acute respiratory distress syndrome (ARDS), sepsis and septic shock.
To date, few paediatric cases have been described in the literature,8,9 and they appear to be milder19. The incubation period lasts between 2–14 days (median, 3–7 days). In all cases, patients have recovered in 1–2 weeks. No paediatric deaths have been reported to date.
Early identification of patients with severe disease (Table 2) allows prompt delivery of optimised supportive care and quick and safe admission (or referral to) an intensive care unit in accordance with regional or national criteria and protocols. Table 3 presents the most frequent laboratory and imaging abnormalities found in these patients. Overall, the findings are compatible with viral respiratory infection, similar to those of other such viral illnesses, such as influenza.
Clinical presentations associated with COVID-19.
| Uncomplicated infection | Patients with uncomplicated upper respiratory tract infection may develop nonspecific symptoms, such as fever, cough, sore throat, nasal congestion, headache, muscle aches or malaise. No signs of dehydration, sepsis or respiratory distress. |
| Mild lower respiratory tract infectiona | Cough, respiratory distress and rapid breathing (in breaths per minute): <2 months, |
| ≥60 bpm; 2–11 months, ≥50 bpm; 1–5 years, ≥40 bpm in absence of features of severe pneumonia. | |
| Ambient oxygen >92%. May or may not have fever. | |
| Severe lower respiratory tract infectionb | Cough or respiratory distress and at least 1 of the following: central cyanosis or SatO2 <92% (<90% in preterm infants); severe respiratory distress (such as grunting, severe chest retractions); inability to feed or difficulty feeding, lethargy or loss of consciousness or convulsions. May present with other features, such as chest retractions, rapid breathing (in breaths per minute): age <1 year, ≥70 bpm; age ≥1 year, ≥50 bpm. Blood gases: PaO2 < 60 mmHg, PaCO2 > 50 mmHg. This is a clinical diagnosis, chest imaging can rule out complications (atelectasis, infiltration, effusion). |
| Other manifestations associated with severe illness | Coagulation disorders (prolonged prothrombin time and D-dimer elevation), myocardial damage (elevation of cardiac enzymes, ST-T wave changes on ECG, cardiomegaly and heart failure), intestinal failure, elevation of liver enzymes and rhabdomyolysis. |
| Acute respiratory distress syndrome20 | Onset: onset with ARDS or worsening of respiratory symptoms in the past 10 days. |
| Chest X-ray, CT or US: new infiltrate(s) compatible with acute involvement of lung parenchyma. | |
| Origin of pulmonary oedema: respiratory failure in absence of other possible causes such as heart failure or volume overload. | |
| Oxygenation (OI and OSI calculated using the SpO2): | |
| • Bilevel NIV or CPAP ≥ 5 cmH2O using full face mask: PaO2 / FiO2 ≤ 300 mmHg o SpO2/ FiO2 ≤ 264 | |
| • Mild ARDS (invasive ventilation): 4 ≤ OI <8 or 5 ≤ OSI <7.5 | |
| • Moderate ARDS (invasive ventilation):8 ≤ OI < 16 or 7.5 ≤ OSI <12.3 | |
| • Severe ARDS (invasive ventilation): OI ≥ 16 or OSI ≥ 12.3 | |
| Sepsis21 | Suspected or confirmed infection with ≥ 2 criteria for SIRS, which should include either abnormal body temperature or abnormal white blood cell count. |
| Septic shock22 | Any degree of hypotension (SBP <5th percentile or more than 2 SD below normal for age) or 2–3 of the following: altered mental status; tachycardia o bradycardia (HR < 90 bpm or >160 bpm in infants and <70 bpm or >150 bpm in children); prolonged capillary refill time (>2 s) or warm shock with normal pulses; tachypnoea; mottled skin or petechial or purpuric rash; lactate elevation, oliguria, hyperthermia or hypothermia. |
ARDS, acute respiratory distress syndrome; CPAP, continuous positive airway pressure; CT, computed tomography; ECG, electrocardiogram; HR, heart rate; NIV, non-invasive ventilation; OI, oxygenation index; OSI; oxygen saturation index; SBP, systolic blood pressure; SD, standard deviation; SIRS, systemic inflammatory response syndrome; SpO2, peripheral capillary oxygen saturation; US, ultrasound.
Possible clinical manifestations and laboratory and laboratory changes in COVID-19 in children.
| Mild | Severe | |
|---|---|---|
| Clinical features | Fever (not always present), cough, nasal congestion, rhinorrhoea, coughing with phlegm, diarrhoea, headache. | Malaise, irritability, food refusal, hypoactivity 1 week from onset. |
| In some cases, rapid progression (1–3 days) with respiratory failure refractory to supplemental oxygen, septic shock, metabolic acidosis, coagulation disorder or haemorrhage. | ||
| Complete blood count | Normal white blood cells or mild leukopenia and lymphopenia | Progressive lymphopenia |
| C-reactive protein | Normal | Normal or elevated (suggestive of bacterial superinfection) |
| Procalcitonin | Normal | PCT > 0.5 ng/mL (suggestive of bacterial superinfection). |
| Blood chemistry | Normal | Elevation of transaminases, muscle enzymes, myoglobin, D-dimers |
| Chest X-ray | Normal or peripheral interstitial infiltrates | Bilateral ground glass opacity and consolidation in several locations. Pleural effusion is infrequent |
| Chest CT scan | Ground glass opacities and infiltrates are more discernible in CT compared to X-ray. | Possible multilobar consolidation |
In patients with mild disease, hospital admission may not be necessary unless there is concern for rapid deterioration. All discharged patients must be instructed to return to hospital if their symptoms worsen. For the time being, we recommend that all patients that test positive be hospitalised.
Recommendations for the management of confirmed casesUncomplicated infection- 1
Adhere to general preventive measures. Have the patient (if possible) and family members wear surgical masks. Ideally, maintain the patient at least 2 m apart (minimum of 1 m) from all other patients at all times.
- 2
The staff caring for the patient must adhere to recommended preventive measures (contact and airborne precautions), wearing a FFP2 mask, gloves, a fluid-resistant gown and protective goggles to perform physical examinations, during history taking or during sample collection procedures.
- 3
Administration of commonly used antipyretic agents as needed (paracetamol or ibuprofen).
- 4
If the patient has fever, we recommend a chest x-ray, blood tests (complete blood count [CBC], C-reactive protein [CPR], procalcitonin [PCT], blood culture, liver enzymes, electrolytes and coagulation) to rule out bacterial superinfection.
- 5
Depending on the epidemiological context and household conditions, choose between hospitalization or home care provision, in the latter case providing clear instructions on how to proceed in case the condition of the patient worsens. The WHO has published recommendations for outpatient follow-up.23
- 1
The same general measures applied in case of uncomplicated infection.
- 2
We recommend hospitalization in isolation and monitoring for all patients.
- 3
Non-invasive vital sign monitoring, including measurement of oxygen saturation (SatO2) by means of pulse oximetry.
- 4
Performance of chest radiograph, blood tests (CBC, CPR, PCT, blood culture, blood chemistry including liver enzymes, electrolytes, coagulation and blood gas analysis) and placement of a peripheral catheter.
- 5
Bronchodilators may be used when indicated based on the findings of physical examination (wheezing), preferably delivered with a metered-dose inhaler (MDI) and spacer.
- 6
Commonly used analgesics (paracetamol or ibuprofen).
The option of admitting one of the parents along with the patient may be considered. Preferably a single individual, always the same one, would stay with the child, always adhering to the indicated precautions (mask, gown, gloves and goggles). There may also be cases in which both the parents and the child are infected, and all can be admitted together.
Severe lower respiratory tract infection- 1
In addition to all the interventions mentioned in the previous sections:
- 2
Conservative fluid management, as aggressive fluid resuscitation may worsen oxygenation (routine fluid therapy with administration of 2/3 of baseline requirements). We recommend against using hypotonic saline solutions (or hydroxyethyl starches or gelatines for fluid resuscitation).
- 3
Supplemental oxygen therapy with a target saturation of >92%.
- 4
In case of suspected bacterial superinfection (leucocytosis and elevation of CPR or PCT), start antibiotherapy with intravenous (IV) amoxicillin-clavulanic acid.
Admission to the PICU should be considered if the patient has lower respiratory tract infection that meets criteria for definition of severe disease or with extrapulmonary manifestations associated with severe illness (Table 2) and/or exhibiting progressive worsening. We recommend hospitalization in an isolation room with verified negative pressure. If performance of aerosol-generated procedures (Table 4) is necessary to provide appropriate care, the level of protection should be heightened due to the increased risk of contamination. The staff that gets exposed should be kept to the minimum necessary and should wear FFP3 masks, in addition to long-sleeve fluid-resistant gowns, fitted goggles or face shields and gloves. Given the limited information that is currently available and until the mechanisms of transmission and associated risks are well understood, when this additional level of risk occurs in the PICU it would be reasonable to add the use of a coverall or long impermeable gown with a hood to completely avoid exposure to the generated droplets.
Potential aerosol-generating procedures and strategies recommended to reduce their risk if they are strictly necessarya.
| Procedure | Strategyb,c |
|---|---|
| Aspiration of respiratory secretions | Only perform when strictly necessary. |
| Closed suction in case of MV | |
| Aerosol therapy | Use MDI with spacer. |
| Collection of respiratory samples | Only perform when strictly necessary. |
| Bronchoalveolar lavage | Avoid if possible. |
| High flow oxygen therapy | Avoid. |
| Non-invasive ventilation (NIV) | Avoid if possible. |
| If necessary, ensure correct seal of the interface. | |
| Use a double-limb configuration. | |
| Manual ventilation with bag-valve-mask | If possible, avoid ventilation with a bag-valve-mask system and, if absolutely necessary, use a system with a high efficiency filter between the self-inflating bag and the mask to prevent viral contamination, avoiding hyperventilation and leaks. |
| Intubation | Use cuffed endotracheal tubes to prevent leaks with cuff pressures <25 cmH2O. |
| If required, deliver preoxygenation with a non-rebreather mask and carry out rapid sequence intubation. Intubation should be performed by experienced staff to minimise the completion time and the number of intubation attempts. | |
| Mechanical ventilation | Use of high-efficiency filters preventing viral contamination in both the inspiratory and expiratory limbs. |
| Use closed suction systems. | |
| Use a heat-moisture exchanger fitted with a high-efficacy filter preventing viral contamination instead of active heated humidifiers. | |
| Prevent accidental disconnection. | |
| Cardiopulmonary resuscitation |
MDI, metered-dose inhaler; MV, mechanical ventilation; NIV, non-invasive ventilation.
The entire care team and support staff and the person accompanying the patient in the PICU should use the recommended personal protective equipment (PPE), following the established protocol for putting it on and taking it off, under supervision, and having received previous training on the PPE and related procedures.
Transport routes to and from the PICU should be predetermined, and necessary precautions and infection control measures should be implemented during transport to prevent transmission during the transfer process.
During the hospital stay, transport of the patient should be kept to a minimum, performing the necessary diagnostic examinations (such as radiographs or ultrasound scans) with portable equipment in the isolation box whenever possible. If diagnostic equipment cannot be exclusively dedicated to these patients, it should be disinfected following preventive medicine guidelines. Disposable supplies should be used whenever possible and supplies that are not disposable should be disinfected following the established protocol. Medical waste from the care of these patients is considered biohazardous and should be handled and disposed of following the corresponding protocol.
Health care staff will plan their tasks in advance and stay in the room the minimum time required to perform them. If possible, the staff should remain at least 2 m away from the patient. A record should be kept of every staff member that comes into contact with the patient.
Based on current knowledge, supportive care delivered at the PICU for severe patients presenting with ARDS, sepsis or organ failure does not significantly differ from the international guidelines that already exist for management of these conditions.24–29
If patients with COVID-19 require respiratory support, non-invasive ventilation (NIV) poses a higher risk of contamination due to the generation of aerosols. In case of deterioration of respiratory function, use of invasive mechanical ventilation should be considered, following the lung-protective strategies recommended at the Pediatric Acute Lung Injury Consensus Conference (PALICC)24,26 for management of ARDS in the paediatric population (low tidal volumes (4−8 mL/kg), optimised positive end-expiratory pressure, inspiratory plateau pressure of up to 28–32 cmH2O, driving pressure of less than 15 cmH2O, permissive hypercapnia, etc.), prone positioning and, if necessary, neuromuscular blockade.
Fluids must be managed appropriately, avoiding fluid overload and preventing a positive balance, both of which are associated with poorer respiratory outcomes and increased morbidity and mortality. Patients with sepsis, in addition to cautious volume expansion during the initial resuscitation, may require vasoactive drugs, to be delivered in adherence with current international paediatric guidelines.28,29 These patients may also require continuous renal replacement therapy.
In case of severe respiratory failure or cardiorespiratory arrest refractory to conventional treatment, the use of extracorporeal membrane oxygenation (ECMO) may be considered based on the same indications applied for other health conditions.
Empirical antibiotherapyAntibiotherapy should be used in case of suspected bacterial superinfection and in patients with sepsis or septic shock (in the latter case, it is essential that antibiotherapy is given within one hour or as soon as possible). The regimen should be individualised, considering the previous condition and characteristics of the patient (comorbidities, healthy patient, mechanical ventilation etc.). Samples for microbiological testing should be obtained before initiation of antibiotherapy whenever possible, remembering to withdraw or taper down treatment as necessary based on the results.
In the cases described at the beginning of the outbreak,6 there was radiological evidence of infiltrates in every patient, and all received empirical antibiotherapy, but at present, close monitoring is recommended, reserving antibiotherapy for cases of suspected bacterial superinfection.
Systemic steroids and immunomodulatorsSystemic steroids are not generally recommended. Previous studies in patients with SARS, MERS and even influenza have shown that they offer no benefits and may even delay viral clearance.20,30,31 Their use can be considered in patients with ARDS, septic shock, encephalitis, hemophagocytic syndrome or with severe bronchospasm associated with wheezing. If indicated, we recommend: IV methylprednisolone (1−2 mg/kg/day) for 3–5 days, avoiding prolonged use.8,9
Intravenous immunoglobulin therapy has been used in severe cases, but its indications and efficacy need to be evaluated. The recommended doses are: 1 g/kg/day for 2 days, or 400 mg/kg/day for 5 days.
Specific antiviral treatmentThere is no specific antiviral agent proven to be effective in case of infection by HCoV, SARS or MERS. The WHO does not recommend any antiviral agents.
There are several drugs currently being tried on an experimental basis.32
- •
Oseltamivir: neuraminidase inhibitors have been used in cases of MERS-CoV infection, and oseltamivir has been used in the initial response to the COVID-19 outbreak in China. Its efficacy is not clear and it may have been used to manage influenza coinfection. Its use is not recommended at present.
- •
Inhaled interferon-alfa has been recommended in combination with antiviral therapy with lopinavir/ritonavir in adults, and a clinical trial has been set up to assess its efficacy. Interferon-alfa has broad-spectrum antiviral effects and is used to treat hepatitis B virus. Formulations of this drug for inhalation are not currently available in Spain. Chinese guidelines recommend nebulisation of interferon-α2b at a dose of 100 000–200 000 IU/kg in mild cases and 200 000–400 000 IU/kg in severe cases, given in twice a day for 5–7 days.
- •
Lopinavir is a protease inhibitor use for management of HIV in combination with ritonavir as a booster. Lopinavir and/or lopinavir/ritonavir have exhibited activity against coronavirus in vitro. In the management of the SARS outbreak in Hong Kong, there was evidence that compared to ribavirin alone, treatment with lopinavir/ritonavir plus ribavirin decreased the risk of ARDS or death.33,34
- •
Remdesivir could be the best possible option for treatment of COVID-19. It is an antiviral agent originally developed to fight Ebola in the class of nucleotide analogues. Experiments in animals infected with MERS-CoV found reduced viral titres and an improvement in lung tissue damage in animals treated with remdesivir compared to controls, and that outcomes were better in the group treated with remdesivir compared to the group treated with lopinavir/ritonavir combined with interferon-β. A phase III clinical trial of remdesivir for treatment of infection by Ebola virus has been completed, and there is ample data on its pharmacokinetics and safety. However, the efficacy and safety of remdesivir in patients with SARS-CoV-2 infection remains to be established. This drug is not available in Spain. In the United States, it has been used in at least one patient with favourable results.35,36
- •
Other drugs such as chloroquine (antimalarial) or baricitinib (Janus kinase inhibitor) have been considered as alternative treatment options, although their use has not been assessed in clinical trials.37,38
In conclusion, there currently is no evidence in support or against the use of antiviral therapy or a specific antiviral agent. Table 5 presents the dosage of different antiviral agents.
Dosage of antiviral drugs. Lopinavir/ritonavir (as specified in summary of product characteristics).
| Paediatric dosing guidelines based on body weight (age >6 months and <18 years) | ||
|---|---|---|
| Body weight (kg) | Dose (mg/kg every 12 h) | Volume of oral solution given with food every 12 h (80 mg lopinavir/20 mg ritonavir per mL solution) |
| 7 to 15 kg | 12/3 mg/kg | |
| 7 to 10 kg | 1.25 mL | |
| >10 kg to <15 kg | 1.75 mL | |
| 15–40 kg | 10/2.5 mg/kg | |
| 15−20 kg | 2.25 mL | |
| >20−25 kg | 2.75 mL | |
| >25−30 kg | 3.50 mL | |
| >30−35 kg | 4 mL | |
| >35-40 kg | 4.75 mL | |
| >40 kg | Adult dose | 400 mg/100 mg every 12 h |
| Dosage in infants aged 2 weeks to 6 months | ||
|---|---|---|
| By weight (mg/kg) | By BSA (mg/m2) | Frequency |
| 16/4 mg/kg (equivalent to 0.2 mL/kg) | 300/75 mg/m2 (equivalent to 3.75 mL/m2) | Given twice daily with food |
The volume in mL of oral solution represents the average dose for the weight range = . Weight-based dosing recommendations are based on limited data.
BSA, body surface area.
BSA can be calculated with the following equation: √ (length (cm) x weight (kg)/3600).
Use of lopinavir/ritonavir is not recommended before 15 days post birth.
The following could be considered:
- •
In mild cases requiring uncomplicated management. Consider oseltamivir in case of coinfection with influenza only at an early stage. Another possible option is lopinavir/ritonavir.
- •
In severe cases requiring hospital admission, consider initiating treatment with lopinavir/ritonavir.
- •
If remdesivir can be obtained, it may be useful in severe cases.
It is important to take into account that lopinavir/ritonavir often causes gastrointestinal adverse events at treatment initiation (diarrhoea, vomiting).
Conflicts of interestThe authors declare no conflicts of interest.
Members of the Working Group of the AEP: José Tomás Ramos1 (Infectious Disease Unit. Department of Paediatrics. Hospital Clínico San Carlos. Department of Public Health and Mother and Child Health. Madrid), Fernando Baquero-Artigao1,2,3, Maria Luisa Navarro1 (Department of Paediatrics and Infectious Diseases. Hospital Universitario Gregorio Marañón. Madrid), Carlos Rodrigo1 (Paediatrics Clinical Management, Hospital Universitario Germans Trias i Pujol, Badalona, Barcelona), Olaf Neth1 (Department of infectious Diseases, Rheumatology and Immunodeficiencies. Hospital Infantil Universitario Virgen del Rocio, Instituto de Biomedicina de Sevilla [IBIS], Seville), Victoria Fumadó1 (Infectious and Imported Disease Unit [CSUR]. Infectious Disease High Level Isolation Unit [CSUR]. Hospital Universitario Sant Joan de Deú, Barcelona), Juan José Menendez Suso4. Department of Intensive Care and High Level Isolation Unit. Hospital Universitario La Paz-Hospital Carlos III (Madrid), María Slocker Barrio4 and Amaya Bustinza Arriortua4 (Paediatric Intensive Care Unit. Hospital Materno Infantil Gregorio Marañón. Madrid), Iolanda Jordán García4 (Paediatric Intensive Care Unit. Hospital Sant Joan de Dèu. Barcelona), Javier Pilar Orive4 (Paediatric Intensive Care Unit. Hospital Universitario de Cruces. Bizkaia).
Please cite this article as: Calvo C, López-Hortelano MG, Vicente JCdC, Martínez JLV. Recomendaciones sobre el manejo clínico de la infección por el «nuevo coronavirus» SARS-CoV2. Grupo de trabajo de la Asociación Española de Pediatría (AEP). An Pediatr (Barc). 2020;92:241.
The Appendix Alists the names of the authors members of the Working Group of the Asociación Española de Pediatría (AEP).