Thrombotic microangiopathies (TMA) are rare diseases usually presenting with renal, haematological, neurologic and cardiovascular involvement and nonspecific but severe symptoms. A registry of TMA cases managed in Spanish paediatric intensive care units (the MATUCIP Registry) was established with the aim of gaining knowledge on their clinical characteristics, diagnosis and acute-phase treatment.
MethodsWe conducted a prospective multicentre observational study in 20 paediatric intensive care units (PICUs) in Spain from January 2017 to December 2021 in children aged more than 1 month with TMAs, who were followed up through the discharge from the PICU.
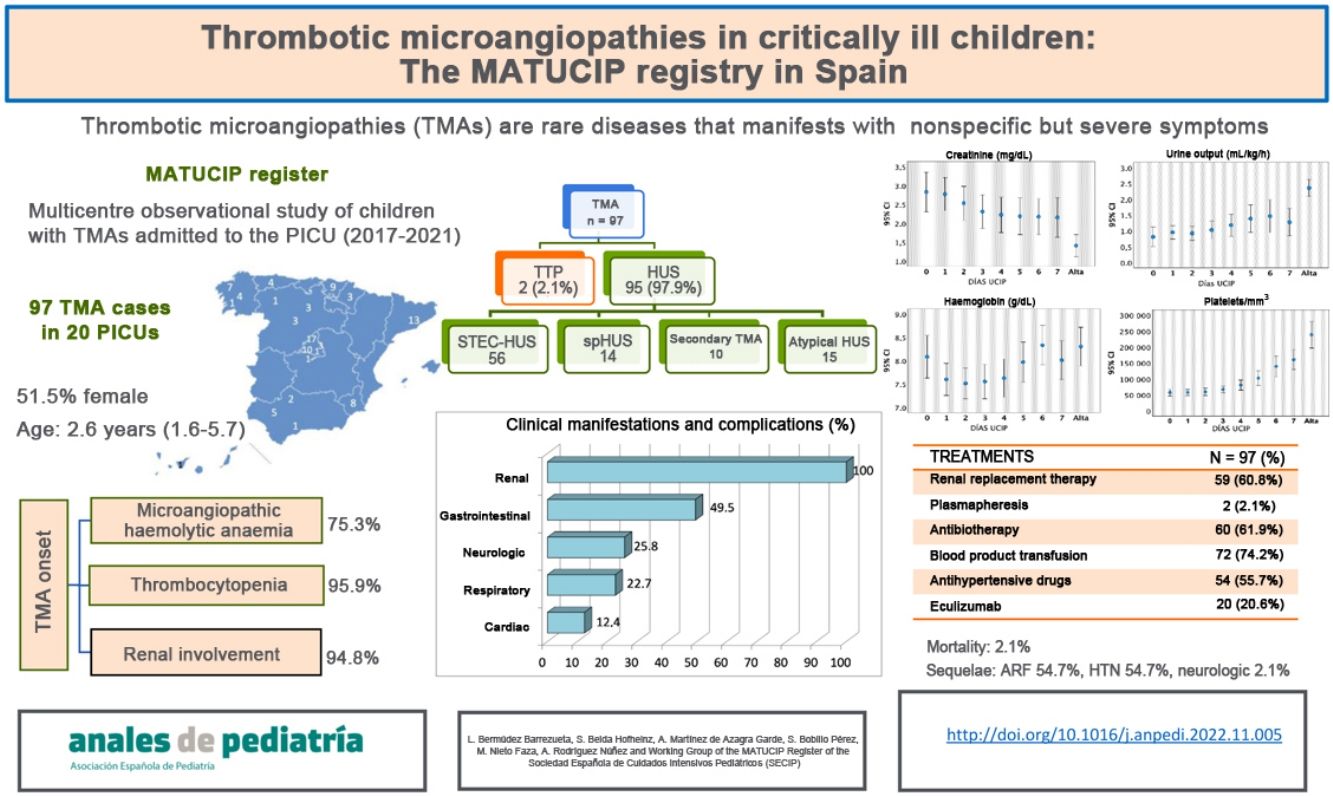
ResultsThe sample included 97 patients (51.5% female) with a median age of 2.6 years (interquartile range [IQR], 1.6–5.7). The initial manifestations were gastrointestinal (74.2%), respiratory (14.4%), fever (5.2%), neurologic (3.1%) and other (3.1%). At admission, 75.3% of patients had microangiopathic haemolytic anaemia, 95.9% thrombocytopenia and 94.8% acute kidney injury. Of the total sample, 57.7% of patients received a diagnosis of Shiga toxin-associated haemolytic uraemic syndrome (HUS), 14.4% of Streptococcus pneumoniae-associated HUS, 15.6% of atypical HUS, 10.3% of secondary TMA and 2.1% of thrombotic thrombocytopenic purpura. Eighty-seven patients (89.7%) developed arterial hypertension, and 49.5% gastrointestinal, 22.7% respiratory, 25.8% neurologic and 12.4% cardiac manifestations. Also, 60.8% required renal replacement therapy and 2.1% plasma exchange. Twenty patients received eculizumab. The median PICU stay was 8.5 days (IQR, 5–16.5). Two children died.
ConclusionsThe MATUCIP registry demonstrates the clinical variability of TMA cases requiring admission to the PICU. Knowledge of the presentation and outcomes of TMAs can facilitate early aetiological diagnosis. This registry can help improve our understanding of the clinical spectrum of these diseases, for which there is a dearth of published data.
Las microangiopatías trombóticas (MAT) son entidades infrecuentes que suelen causar afectación renal, hematológica, neurológica y cardiovascular, con síntomas inespecíficos pero graves. Con la finalidad de mejorar el conocimiento de sus características clínicas, proceso diagnóstico y tratamiento en la fase aguda, se ha creado el registro de MAT en las Unidades de Cuidados Intensivos Pediátricos (UCIP) de España (Registro MATUCIP).
Pacientes y métodosEstudio observacional, multicéntrico realizado en 20 UCIP españolas desde enero 2017 hasta diciembre de 2021 que incluyó niños mayores de 1 mes con diagnóstico de MAT y seguimiento hasta el alta de UCIP.
ResultadosSe incluyeron 97 pacientes (51,5% mujeres), con mediana de edad de 2,6 años (RIQ 1,6–5,7). La clínica inicial fue de tipo gastrointestinal (74,2%), respiratoria (14,4%), cuadro febril (5,2%), neurológica (3,1%) y otras (3,1%). Al ingreso, 75,3% presentaban anemia hemolítica microangiopática, 95,9% trombocitopenia y 94,8% daño renal agudo. Fueron diagnosticados de Síndrome Hemolítico Urémico (SHU) asociado a Escherichia coli productora de toxina Shiga 57,7%, SHU por Streptococcus pneumoniae 14,4%, SHU atípico 15,6%, MAT secundaria 10,3% y púrpura trombocitopénica trombótica 2,1%. Desarrollaron hipertensión arterial 89,7%, manifestaciones digestivas 49,5%, respiratorias 22,7%, neurológicas 25,8% y cardiacas 12,4%. El 60,8% requirieron depuración extrarrenal y 2,1% plasmaféresis. Recibieron eculizumab 20 pacientes. La mediana de estancia en UCIP fue 8,5 días (RIQ 5–16,5). Dos niños fallecieron.
ConclusionesEl registro MATUCIP muestra la variabilidad clínica de las MAT que ingresan en UCIP. Conocer la forma de presentación y evolución de las MAT puede facilitar el diagnóstico etiológico precoz. Este registro permite conocer mejor el espectro clínico de estas entidades donde los datos publicados son escasos.
Thrombotic microangiopathies (TMAs) are rare diseases in children (3 cases /1000000 inhabitants/year) that cause acute severe episodes of disease with renal, haematological, neurologic and cardiovascular involvement.1–4 Haemolytic uraemic syndrome (HUS) is the most important TMA on account of its relative frequency and associated morbidity and mortality.5,6
From a histopathological point of view, TMA results from endothelial lesions with activation of the complement and/or coagulation system.7,8 Platelet aggregation and thro formation of thrombi in small-calibre vessels cause ischaemia in the kidney and other organs.9–11
Haemolytic uraemic syndrome is one of the leading causes of renal failure in children. Clinically, it is characterised by microangiopathic haemolytic anaemia (MAHA), thrombocytopenia and kidney injury.6,12,13 Most cases occur following gastrointestinal infection by Shiga toxin-producing Escherichia coli (STEC-HUS, or typical HUS),3,14 with fewer cases associated with Streptococcus pneumoniae, genetic or acquired changes in the alternative pathway of the complement system, which cause atypical HUS (aHUS), inborn errors of metabolism, infection, medication and underlying conditions such tumours, transplants or autoimmune diseases (secondary TMA).6,13,15–18
Children with TMA can present with multiple nonspecific symptoms, so its diagnosis is complex.19,20 The most severe cases require intensive care and supportive care due to dysfunction of the kidneys and other organs.4,18
On account of its rarity and with the purpose of improving our understanding of its clinical characteristics, the diagnostic process and treatment of acute disease, a register of TMA cases was created in paediatric intensive care units (PICUs) in Spain (the MATUCIP register), and this article presents its general findings.
Material and methodsWe conducted a multicentre observational cohort study over a period of 5 years (retrospective from January 2017 to June 2018, and prospective from June 2018 to January 2022), with participation of 20 Spanish hospitals. The sample was obtained by the consecutive inclusion of children aged more than 1 month admitted to the PICU with a primary diagnosis of TMA, with follow-up of the patient until discharge from the PICU.
The diagnosis was suspected based on the presence of haemolytic anaemia (haemoglobin < 10g/dL and/or schistocytes > 1% in the peripheral blood smear and/or elevation of lactate dehydrogenase [LDH] and/or decreased haptoglobin), thrombocytopenia (platelets < 150000/mm3 or decrease of > 25% relative to baseline) and acute kidney injury (AKI) according to the KDIGO 2012 criteria,21 arterial hypertension22 or abnormal urine sediment (proteinuria and/or haematuria). We also documented cases of suspected TMA that did not fulfil the classical triad at admission.
We classified cases by subtype of TMA as: (a) STEC-HUS: gastrointestinal manifestations and microbiological confirmation (Shiga toxin-producing E. coli); (b) Probable STEC-HUS: clinical presentation and course compatible with STEC-HUS without a verifiable cause and in the absence of complement abnormalities; c) spHUS: HUS associated with confirmed infection by S. pneumoniae; d) TMA secondary to previous conditions or infections with microbiological confirmation; e) thrombotic thrombocytopenic purpura (TTP): deficiency of the ADAMTS-13 metalloprotease (von Willebrand factor-cleaving protease < 10%), and f) aHUS, a diagnosis of exclusion after ruling out other causes, with or without genetic confirmation of complement dysregulation.
We collected data on demographic characteristics, personal and family history, baseline chronic treatment, the clinical manifestations at onset, diagnostic tests, treatments, outcomes and complications during the PICU stay. We compared baseline characteristics and outcomes in different TMA subtypes. Diagnosis and treatment were carried out independently from the register.
The study was approved by the Research Ethics Committee of Galicia (file 2018/228).
We conducted the statistical analysis with the software IBM SPSS 24.0 for Windows® (SPSS Inc, Chicago, IL, USA). We have expressed categorical as absolute frequencies and percentages. We summarised quantitative variables with the mean and standard deviation (SD) or the median and interquartile range (IQR) based on the distribution of the data (Kolmogorov–Smirnov test, P>0.05). We analysed the characteristics of TMA subtypes with the Kruskal-Wallis in the case of continuous variables, and the Fisher exact test or χ2 test for categorical variables. We considered values of P of less than 0.05 statistically significant.
ResultsCases in 99 children were recorded (1–17 patients per PICU; median, 4). We excluded 2 cases that did not meet the criteria for TMA, so the final sample comprised 97 patients.
The median age was 2.6 years (IQR, 1.6–5.7), and 51.5% of the patients were female. The symptoms at onset were gastrointestinal in 74.2% of cases, respiratory in 14.4%, fever in 5.2%, neurologic in 3.1%, and nonspecific, such as asthenia or anorexia, in 3.1%. Table 1 presents the laboratory data at the time TMA was first suspected.
Laboratory test results at the time of suspicion of TMA.
| Mean±SD | Median [IQR] | |
|---|---|---|
| Acid-base balance | ||
| pH | 7.32±0.09 | 7.31 [7.27−7.38] |
| Bicarbonate (mmol/L) | 17.5±4.3 | 17.0 [14.3−20.1] |
| BE (mmol/L) | –8±5.2 | –8.2 [11.3 a –5] |
| Lactate (mmol/L) | 1.8±2 | 1.2 [0.8−1.7] |
| Electrolytes | ||
| Sodium (mEq/L) | 133.8±5.7 | 134 [130−138] |
| Chloride (mEq/L) | 101.8±8.8 | 103 [98−108] |
| Potassium (mEq/L) | 4.8±1.1 | 4.5 [4−5.3] |
| Calcium (mg/dL) | 8.4±0.8 | 8.5 [8−8.9] |
| Haematology | ||
| Haemoglobin (g/dL) | 8.3±2.3 | 8.4 [6.8−9.9] |
| Haematocrit (%) | 24.1±6.8 | 24.3 [19.5−29.1] |
| Platelets (109/L) | 57.91±56 | 47 [27−70] |
| Reticulocytes (%); n=59 | 5.5±6.9 | 2.7 [1.8−6.8] |
| Haptoglobin (mg/dL); n = 82 | 14.2±24.9 | 5 [5−10.7] |
| Coagulation | ||
| PT (s); n=55 | 12.4±4.9 | 12 [11.1−13] |
| APTT (s) | 28.8±15 | 27 [24–29] |
| INR | 1.08±0.2 | 1.06 [0.97−1.11] |
| D dimer (ng/mL); n=34 | 5847±6421 | 3625 [1400−6867] |
| Fibrinogen (mg/dL); n=85 | 527±713 | 417 [319−506] |
| Blood chemistry | ||
| Glucose (mg/dL) | 99±24.9 | 95 [82−109] |
| Urea (mg/dL) | 154.5±105 | 126 [77−203] |
| Creatinine (mg/dL) | 2.86±2.64 | 1.93 [0.84−4.035] |
| LDH (U/L) | 3298±3348 | 2596 [1809−3900] |
| Bilirubin (mg/dL) | 1.67±1.7 | 1.2 [0.8−1.94] |
| AST (U/L) | 172.6±170 | 120 [76−210] |
| GGT (U/L) | 49.8±61.4 | 21 [11−67] |
| ALT (U/L) | 69.8±116 | 31 [16−65] |
| CK (U/L); n=40 | 200±305 | 113 [66.5−188] |
| Troponin μg/mL; n=40 | 17.8±49.8 | 5.85 [1.3−10.4] |
| Amylase; n=62 | 80.7±71.3 | 60.5 [40−97] |
| Lipase (U/L); n=36 | 122.8±185 | 41 [21−174] |
| Albumin (mg/dL); n=20 | 3±0.5 | 3 [2.7−3.22] |
| Cystatin C (mg/L); n=29 | 2.7±1.6 | 2.22 [1.34−4.4] |
ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BE, base excess; CK, creatine kinase; GGT, gamma-glutamyl transferase; INR, international normalized ratio; IQR, interquartile range; LDH, lactate dehydrogenase; PT, prothrombin time; SD, standard deviation.
In 91 cases, the presence of schistocytes was assessed in a blood smear, with positive results in 83 (91.2%). A Coombs test was ordered in 81 patients and turned out positive in 10 (12.3%).
A complement C3 test was performed in 91 cases, evincing low levels (<80mg/dL) in 39.6%. A complement C4 test was performed in 90, evincing low levels (<12mg/dL) in 22.2%. Table 2 presents the results of ADAMTS-13 and complement tests.
Complement and ADAMTS-1 levels.
| N=97 (%) | Mean±SD | Median | Range | IQR | |
|---|---|---|---|---|---|
| C3 (mg/dL) | 91 (93.8%) | 86.2±27.2 | 87.5 | 1−153 | 66−105.7 |
| C4 (mg/dL) | 90 (92.8%) | 17.4±8.4 | 16 | 2.8−53.2 | 12.6−21 |
| CH50 | 33 (34%) | 111.4±154 | 68 | 0−810 | 43−104 |
| ADAMTS-13 (%) | 68 (70%) | 64.8±26.4 | 68 | 0−112 | 50−85 |
ADAMTS-13, A disintegrin and metalloproteinase with a thrombospondin type-1 motif, member 13; CH50, 50% haemolytic complement; IQR, interquartile range; SD, standard deviation.
An infectious aetiology was confirmed in 86.6% of patients. Eighty underwent testing for detection of enterohaemorrhagic Shiga-toxin producing E. coli, with positive results in 53 patients (Table 3). Some other culture was positive in 32/97 cases (13 blood cultures, 6 stool cultures, 8 urine cultures, 2 pleural fluid cultures and 3 tracheal aspirate cultures). Tests for detection of respiratory viruses were conducted in 48 patients, with detection of at least 1 virus in 16, most frequently rhino/enterovirus infections (11 cases).
Microbiological methods used to test for enterohaemorrhagic Shiga-toxin producing E. coli.
| Analysed samples | Positive result | Diagnostic yield | |
|---|---|---|---|
| n=80 | n=53 | ||
| ELISA | 25 | 14 | 56% |
| Stool culture in sorbitol-MacConkey | 23 | 15 | 65.2% |
| PCR for Shiga 1 and 2 toxin genes | 32 | 24 | 75% |
ELISA, enzyme immunoassay; PCR, polymerase chain reaction. Diagnostic yield: total positive samples/total tested samples × 100%.
Fifty-three children (54.6%) received a diagnosis of confirmed STEC-HUS, 3 (3.1%) of probable STEC-HUS, on account of a clinical presentation and course compatible with STEC-HUS but negative stool culture results, 14 (14.4%) of spHUS, 9 (9.3%) of TMA secondary to other infections, 1 (1%) of TMA secondary systemic lupus erythematosus, 15 (15.6%) of aHUS and 2 (2.1%) of TTP. We found differences between TMA subtypes in both baseline characteristics and in the clinical and laboratory characteristics and outcomes in the PICU, which are summarised in Table 4.
Differential diagnosis of thrombotic microangiopathies in patients included in the MATUCIP Register.
| STEC-HUS (n=56) | spHUS (n=14) | Secondary TMA (n=10) | Atypical HUS (n=15) | TTP (n=2) | P | |
|---|---|---|---|---|---|---|
| Age, years | 2.7 [2−5.2] | 1.4 [1.1−1.8] | 4.8 [2.3−10] | 3.2 [1.6−7.9] | 8.3 [7.6−9] | 0.002 |
| Female sex | 26 (46.4%) | 6 (42.9%) | 6 (60%) | 10 (66.7%) | 1 (50%) | 0.622 |
| Weight (kg) | 14.3 [12.3−19.5] | 10.4 [10−11.7] | 21.9 [13.5−26.7] | 15.4 [11.5−24.5] | 35 [31–39] | 0.001 |
| Initial presentation | <0.001 | |||||
| Gastrointestinal | 55 (98.2%) | 1 (7.1%) | 5 (50%) | 10 (66.7%) | 1 (50%) | |
| Respiratoria | 0 | 11 (78.6%) | 2 (20%) | 1 (6.7%) | 0 | |
| Neurologic | 1 (1.8%) | 0 | 0 | 2 (13.3%) | 0 | |
| Other symptoms | 0 | 2 (14.3%) | 3 (30%) | 2 (13.3%) | 1 (50%) | |
| Classical triad at onseta | 40 (71.4%) | 14 (100%) | 5 (50%) | 7 (46.7%) | 0 | 0.003 |
| Onset with: | ||||||
| Anaemia (Hb<10g/dL) | 41 (73.2%) | 14 (100%) | 6 (60%) | 10 (66.7%) | 2 (100%) | 0.124 |
| Haemoglobin (g/dL) | 8.3 [6.9−10.2] | 6.2 [5.1−8.4] | 9.3 [7−10.7] | 9 [7.2−10.7] | 7.6 [6.8−8.4] | 0.051 |
| Thrombocytopenia | 55 (98.2%) | 14 (100%) | 10 (100%) | 12 (80%) | 2 (100%) | 0.022 |
| Platelets (103/μl) | 46.5 [28−66.5] | 19 [13–29] | 70 [51−101] | 70 [38−95] | 19 [10–37] | <0.001 |
| Creatinine (mg/dL) | 2.1 [1.1−5.2] | 1.2 [0.7−1.3] | 1.8 [0.7−3.5] | 2.5 [0.9−4.6] | 0.6 [0.5−0.6] | 0.038 |
| Urea at admission (mg/dL | 145 [92−224] | 94 [77−140] | 132 [75−166] | 128 [72−189] | 42.5 [38−47] | 0.070 |
| LDH (U/L) | 2813 [1972−4155] | 3815 [1963−4751] | 1622 [840−2995] | 2438 [1713−2987] | 1990 [1540−2441] | 0.089 |
| Haptoglobin (mg/dL) | 5 [3.9−12.1] | 5.5 [5−6.8] | 5.7 [5−44.3] | 5 [4.8−10.3] | 5 [12.5–20] | 0.858 |
| AST (U/L) | 105 [70.5−186] | 197 [158−317] | 92 [41−153] | 118 [69−229] | 86.7 [72−101] | 0.007 |
| Complement | ||||||
| C3 (mg/dL) | 93.3 [78−109] | 66.7 [50−90] | 76.7 [59−93] | 84 [59.5−102] | 76.3 [60−93] | 0.029 |
| C4 (mg/dL) | 16 [12.4−20.8] | 13 [7.6−15] | 17.3 [11.8−32] | 18.2 [17–23] | 16 [12.8−19] | 0.069 |
| Low C3 levels | 14/50 (28%) | 9/14 (64.3%) | 6/10 (60%) | 6/15 (40%) | 1/2 (50%) | 0.084 |
| Low C4 levels | 11/49 (22.4%) | 6/14 (42.9%) | 3/10 (30%) | 0 /15 (0%) | 0/2 (0%) | 0.070 |
| ADAMTS-13% activity | 68 [52–85] | 50.3 [44−66] | 58 [42−107] | 76 [60−86] | 0 | 0.060 |
| Positive Coombs (n=81) | 1/47 (2.1%) | 8/9 (88.9%) | 1/9 (11%) | 0 | 0 | <0.001 |
| Acute kidney injury | 55 (98.2%) | 14 (100%) | 9 (90%) | 14 (93.3%) | 0 | <0.001 |
| Hypertension | 49 (87.5%) | 14 (100%) | 10 (100%) | 13 (86.7%) | 1 (50%) | 0.158 |
| Proteinuria | 45 (80.3%) | 10 (71.4%) | 8 (80%) | 14 (93.3%) | 2 (100%) | 0.776 |
| Haematuria | 42 (75%) | 9 (64.3%) | 8 (80%) | 13 (86.7%) | 2 (100%) | 0.952 |
| Clinical manifestations | ||||||
| Gastrointestinal | 33 (58.9%) | 4 (28.6%) | 6 (60%) | 4 (26.7%) | 0 | 0.041 |
| Neurologic | 11 (19.6%) | 5 (35.7%) | 5 (50%) | 4 (26.7%) | 0 | 0.232 |
| Cardiac | 3 (5.4%) | 5 (35.7%) | 3 (30%) | 1 (6.7%) | 0 | 0.004 |
| Respiratory | 5 (8.9%) | 14 (100%) | 3 (30%) | 0 | 0 | <0.001 |
| CRRT | 35 (62.5%) | 11 (78.6%) | 5 (50%) | 8 (53.3%) | 0 | 0.209 |
| Days of CRRT (n=59) | 6 [4–11] | 8.5 [5−11.5] | 11 [10–15] | 5 [4–10] | – | 0.421 |
| Blood products | 42 (75%) | 12 (85.7%) | 9 (90%) | 10 (66.7%) | 2 (100%) | 0.524 |
| Eculizumab | 3 (5.4%) | 1 (7.1%) | 2 (20%) | 14 (93.3%) | 0 | <0.001 |
| PICU stay, days | 6.5 [4.5−12.5] | 16.5 [9–22] | 13.5 [7–23] | 9 [4.5−14.5] | 6 [4–8] | 0.026 |
| Sequelae at discharge from PICU | ||||||
| Acute renal disease | 27 (48.2%) | 4 (28.6%) | 6 (60%) | 8 (53.3%) | 0 | 0.389 |
| Hypertension | 27 (48.2%) | 7 (50%) | 8 (80%) | 10 (66.6%) | 0 | 0.250 |
| Neurologic | 0 | 0 | 0 | 2 (13.3%) | 0 | – |
ADAMTS-13: A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; AST, aspartate aminotransferase; HUS, haemolytic uraemic syndrome; LDH, lactate dehydrogenase; CRRT, continuous renal replacement therapy; PICU, paediatric intensive care unit; spHUS, S. pneumoniae-associated haemolytic uraemic syndrome; STEC, Shiga toxin-producing E. coli; TTP, thrombotic thrombocytopenic purpura.
Categorial variables expressed as n (%), and quantitative variables as median [interquartile range].
At the time of admission to the PICU, only 66 patients (68%) presented with the classical triad (MAHA, thrombocytopenia and AKI). Also, 24.7% did not present with anaemia, schistocytes were not observed in 8.8%, 4.1% did not present with thrombocytopenia and 5.2% did not meet the diagnostic criteria for AKI.
When it came to the course of disease, patients developed different clinical manifestations and complications. All had some form of renal involvement: AKI in 92 (94.8%), arterial hypertension in 87 (89.7%) and abnormalities in urine sediment in 83 (85.6%). Gastrointestinal involvement was present in 48 patients (49.5%), respiratory in 22 (22.7%), neurologic in 25 (25.8%) and cardiac in 12 (12.4%) (Table 5).
Clinical manifestations and complications in patients included in the register.
| Involvement | n=97 | % |
|---|---|---|
| Renal | 97 | 100% |
| Acute kidney injury | 92 | 94.8% |
| Hypertension arterial | 85 | 87.6% |
| Proteinuria and/or haematuria | 82 | 84.5% |
| Gastrointestinal | 48 | 49.5% |
| Diarrhoea and/or vomiting | 30 | 30.9% |
| Lower gastrointestinal bleeding | 6 | 6.2% |
| Colitis | 4 | 4.1% |
| Pancreatitis | 4 | 4.1% |
| Peritonitis | 3 | 3.1% |
| Ileus/ intestinal pseudo-obstruction | 3 | 3.1% |
| Rectal prolapse | 1 | 1% |
| Cholestatic hepatitis | 1 | 1% |
| Respiratory | 22 | 22.7% |
| Pneumonia±pleural effusion | 14 | 14.4% |
| Respiratory failure and mechanical ventilation | 10 | 10.3% |
| Isolated pleural effusion | 4 | 4.1% |
| Acute respiratory distress syndrome | 2 | 2.1% |
| Acute pulmonary oedema | 1 | 1% |
| Neurologic | 25 | 25.8% |
| Altered level of consciousness | 15 | 15.5% |
| Seizures | 7 | 7.2% |
| Stroke | 3 | 3.1% |
| Encephalitis | 2 | 2.1% |
| Irritability | 3 | 3.1% |
| Hemiparesis | 2 | 2.1% |
| Coma | 1 | 1.6% |
| Brain death | 1 | 1.6% |
| Cardiac | 12 | 12.4% |
| Shock/haemodynamic instability | 6 | 6.5% |
| Heart failure | 3 | 4.8% |
| Pericardial effusion | 2 | 3.2% |
| Ventricular hypertrophy | 1 | 1% |
As concerns renal function, the urine output on day 1 varied, with anuria in 28.9% of patients and oliguria (<1mL/kg/h) in 32%. At the time of admission, creatinine levels ranged 0.1 and 13.3mg/dL (median, 1.93mg/dL) and 25% had levels below 4mg/dL. Urea levels ranged from 25 to 532mg/dL (median, 126mg/dL) and 25% of patients had values under 200mg/dL. Of the 92 patients that met the criteria for AKI, 17 had stage 1 disease, 10 stage 2 and 65 stage 3 according to the KDIGO 2012 classification. Fig. 1 presents the changes in urine output and creatinine and urea levels.
At admission, 73 patients (75.3%) had anaemia, and 100% developed MAHA in the first week, while 72 (74.2%) required at least one transfusion of packed red blood cells. Also, 93 patients (95.9%) had thrombocytopenia at admission and 2 experienced a decrease in the platelet count (< 150000/mm3) in the days that followed; 41 (42.3%) required platelet transfusions. Fig. 2 shows the changes in haemoglobin levels and platelet counts.
When it came to treatment, during the acute phase, 50.5% of patients received at least 1 bolus of crystalloid fluids and 24.7% at least 1 albumin bolus. Continuous renal replacement therapy (CRRT) was used in 59 patients (60.8%) for a median duration of 7 days (IQR, 4–12). In 38 cases, the modality used was continuous veno-venous haemofiltration (CVVHF); in 16, peritoneal dialysis, and in 5 cases, both. The median age of patients that started with peritoneal dialysis was 2.5 years (IQR, 1.7–3.9) compared to 2.4 years (IQR, 1.6–5.9) in patients in which the initial approach was CVVHF, without significant differences in the type of CRRT used based on age (P=0.97).
Twenty children received eculizumab (20.6%), of who 14 had a diagnosis of aHUS and 6 other diagnoses (2 of secondary TMA, 1 of spHUS and 3 of STEC-HUS with severe neurologic manifestations or persistent renal failure). The median time elapsed from diagnosis of TMA to initiation of eculizumab was 2.5 days (IQR, 1.5–5). Table 6 presents the treatments used.
Treatments used during the PICU stay.
| N=97 (%) | |
|---|---|
| Renal replacement therapy | 59 (60.8%) |
| Hemodiafiltration | 38 (39.2%) |
| Peritoneal dialysis | 16 (16.5%) |
| Haemodiafiltration+peritoneal dialysis | 5 (5.2%) |
| Plasmapheresis | 2 (2.1%) |
| Antibiotherapy | 60 (61.9%) |
| Blood products | |
| Packed red blood cell transfusion | 72 (74.2%) |
| Platelet transfusion | 41 (42.3%) |
| Fresh frozen plasma | 4 (4.1%) |
| Antihypertensive drugs | 54 (55.7%) |
| Eculizumab | 20 (20.6%) |
Two patients died (2.1%). One had pneumococcal meningitis associated with HUS, and progressed to brain death on day 1. The other had STEC-HUS and experienced progressive worsening with severe neurologic manifestations (status epilepticus and strokes), dying at 9 days.
The remaining 95 patients were discharged from the PICU after a median of 8.5 days (IQR, 5–16.5). At the time of discharge, 52 (54.7%) had acute renal disease: 3 stage 1, 11 stage 2 and 38 stage 3 disease, according to the KDIGO 2012 classification. At discharge, the median creatinine level was 0.9mg/dL (IQR, 0.44–2), the median urea level 78mg/dL (IQR, 38.8–119) and the median urine output 2.2mL/kg/h (IQR, 1.5–3). In addition, 52 children (54.7%) had arterial hypertension and 2 neurologic sequelae (epileptic encephalopathy and left hemiparesis).
DiscussionOur register is the first one to focus on TMA cases in the PICU setting and contributes relevant information on the clinical spectrum and heterogeneity of TMAs in addition to their management and short-term outcomes.
The classification of TMAs has been updated as knowledge increased on its pathophysiological mechanisms, aetiology and diseases involved in their development.4,8,15 In our register, 57.7% of children had STEC-HUS and 15.6% had aHUS. These results diverge from “classical” case series according to which 90% of cases corresponded to STEC-HUS and less than 10% to aHUS.6,10,23 Although there could be biases at play in the lower proportion of STEC-HUS and the higher proportion of aHUS in the register due to the study setting (children in the PICU), our findings suggest that the relative frequency of TMA subtypes may be shifting due to improvements in the identification of these diseases.15,24
Despite the different causes and triggers, TMAs share some common pathophysiological aspects, characterised by an endothelial proinflammatory state with thrombi formation in capillaries and arterioles that results in the triad of MAHA, thrombocytopenia and organ damage.6 However, these three manifestations are not always found together, as observed in our register, in which only 68% of patients presented all 3 at admission to the PICU. Similarly, previous studies have described cases of TMA without thrombocytopenia or schistocytes, with renal involvement being the most consistent manifestation at diagnosis.10,19,23,25,26 Fremeaux-Bacchi et al.26 studied a cohort of 89 children with aHUS and observed that the initial presentation did not include thrombocytopenia in 15% or anaemia in 6%. The lack of a complete triad hinders clinical suspicion and early diagnosis, which are essential in these diseases.
Although the Coombs test and the peripheral blood smear are basic tests used in the diagnosis of MAHA, they were not performed in all patients. Nevertheless, the diagnosis was supported by other criteria, such as elevation of LDH and/or decreased levels of haptoglobin, and MAHA was identified in all cases in the first week in the PICU.
We found clinical differences between TMA subtypes. Thus, patients with spHUS were younger and the classical triad was present at diagnosis in only 46.7% of cases of aHUS compared to 100% of cases of spHUS and none of the cases of TTP. Anaemia and thrombocytopenia were more severe in patients with spHUS and TTP, and creatinine levels were higher in patients with aHUS and STEC-HUS. The Coombs test was positive in 89% of cases of spHUS, and was predominantly negative in all other forms of disease. We found complement deficiency in practically every TMA subtype (more marked in spHUS), with a lower frequency in patients with STEC-HUS.
Typical STEC-HUS chiefly affects children aged less than 5 years.10,27 It is estimated that 15% of children with infection by enterohaemorrhagic E. coli develop HUS.6,20 In the cohort under study, the presence of Shiga toxin-producing E. coli was assessed in the 80 patients who presented with gastrointestinal or nonspecific symptoms, with positive results in 53 (66.3%). Three were considered probable cases due to their age, presentation and course of disease, taking into account that the shedding of enterohaemorrhagic E. coli and its toxins is temporary, so that the probability of detection decreases from day 7.14,28,29 The children with STEC-HUS in the register had ages ranging from 2 to 5 years and had relatively favourable outcomes, with a shorter stay in the PICU compared to other types of HUS, although one of these patients, who had neurologic complications, died. Although the prognosis of STEC-HUS has improved in recent years, its mortality continues to be estimated at 2%–5%, and there is neurologic involvement in up to 25% of these cases.14
Atypical HUS deserves special attention, as specific treatment with eculizumab and other complement inhibitors is available for it.15,23,24,30 Its incidence is estimated to be of 3.5–7 cases per million children,20 with 70% of affected patients experiencing the first episode before age 2 years.6,24 Atypical HUS results from the genetic or acquired dysregulation of the alternative complement pathway; however, a change is not identified in any of the genes known to be associated with the disease in 30%–50% of patients.6,20,31 In the MATUCIP register, 15 children (15.6 %) received a diagnosis of aHUS, at a median age of 3.2 years, similar to the findings of Ito et al.32 These patients initially presented with nonspecific symptoms, with gastrointestinal, neurologic and respiratory manifestations, mild or absent thrombocytopenia at onset in 20% of cases, neurologic and cardiac complications and creatinine elevation, although with a short duration of CRRT and without fatalities. Ninety-three percent 93% received eculizumab. Although it has been reported that up to 30% of children with aHUS may have gastrointestinal symptoms, in our cohort they appeared in two thirds of the cases, which should be taken into account in the differential diagnosis of STEC-HUS. The diagnosis of aHUS was made excluding other causes, and while genetic tests were ordered, their results were not available during the stay in the PICU, so the decision to initiate treatment with eculizumab was based on clinical and laboratory criteria. Until this drug became available, the prognosis of aHUS was poor and the disease carried a high morbidity and mortality15; its use has allowed to reduce the progression to terminal renal failure or death from 30% to 50% in the past to currently 9% in children.3,12 The favourable outcome of cases of aHUS documented in the register with early treatment with eculizumab (median, 2.5 days) is consistent with this evidence.
Cases of TMA secondary to other diseases are extremely rare.10,23 We identified 10 cases, chiefly associated with infection. These patients presented heterogeneous symptoms, with absence of anaemia at onset in 40% of cases, respiratory, neurologic and cardiac complications, longer duration of CRRT and prolonged stay in the PICU. Thrombotic microangiopathies are complex processes with interacting imbalances in immunity, coagulation and complement in which infection can act as a trigger in patients with genetic predisposition.33 There is evidence of complement activation in secondary TMAs, and there is a very narrow distinction between these diseases and aHUS.10,20 Since in approximately half of children primary aHUS can be triggered by intercurrent events, it is not unreasonable to consider whether secondary TMAs should be included in that label.10 Therefore, although the evidence on the subject is not definitive, some authors have proposed the use of eculizumab for treatment of secondary TMA.33
When it came to spHUS, the observed proportion (14.4%) was similar to the one reported in the most recent series published in the post-vaccine era.3,16,34 Haemolytic uraemic syndrome develops in 0.4%–0.6% of pneumococcal infections16,17,20 and the incidence of spHUS seems to be increasing on account of nonvaccine serotypes.16,34 In our register, children with spHUS were younger, had onset with clinically significant anaemia and thrombocytopenia and poorer outcomes, with neurological and cardiac complications, need of CRRT and longer PICU stays. A patient with pneumococcal meningitis and spHUS died. Similarly, other authors have reported a more severe course of disease in patients with spHUS compared to STEC-HUS.3,16,17,35,36
Thrombotic thrombocytopenic purpura is very rare in children with a high morbidity and mortality.4,23,37 In our register, 2 patients had a diagnosis of acquired TTP and were characterised by being older, presenting with clinically significant anaemia and thrombocytopenia, preserved renal function and absence of neurologic manifestations, and had good outcomes after early treatment with steroid therapy and plasmapheresis. Although classically TTP has been characterised by the pentad of haemolytic anaemia, thrombocytopenia, neurologic dysfunction, renal failure and fever, few patients present all of these symptoms, as observed in our cohort.3,38
As regards treatment, more than half the patients required some form of CRRT, and the modality used most frequently was CVVHF, which evinces the displacement of peritoneal dialysis by slow continuous therapies in critically ill children. The selection of renal replacement modality was not associated with the age of the patient, and, while we do not know the actual reasons, it probably was influenced by the experience in each PICU.39
There are limitations to our study. The MATUCIP is a nationwide voluntary register that does not include all children in Spain with a diagnosis of TMA in the period under study. Since it was an observational study, the diagnosis and treatment of TMAs varied between PICUs. In addition, the follow-up was limited to the stay in the PICU, so the study could not contribute data on long-term outcomes.
ConclusionEarly detection of TMAs and their differential diagnosis based on their aetiology poses a challenge in the intensive care setting due to their low incidence and clinical heterogeneity. In some types of TMA, early specific treatment is essential to reduce the risk of irreversible organ damage or death. The MATUCIP Register evinces the characteristic features of TMAs from the perspective of real-world paediatric intensive care practice, describing the clinical course and outcomes and the effects of treatments used in the acute phase. Our findings provide a detailed perspective of the process of diagnosis of TMAs at an early stage in the PICU setting with the aim of improving the therapeutic approach to these diseases.
FundingThis research was not funded by specific grants from any institutions in the public, private or not-for-profit sectors.
Conflicts of interestThe Working Group of the MATUCIP Register has received partial funding for holding work meetings from Alexion SA. Antonio Rodríguez Núñez, Sylvia Belda Hofheinz and Amelia Martínez de Azagra Garde have participated in educational activities funded by Alexion SA.
We thank the members of the Working Group of the MATUCIP Register of the SECIP and everyone that has collaborated with the register.
Lorena Bermúdez Barrezueta (Hospital [H.] Clínico Universitario de Valladolid), Sylvia Belda Hofheinz and Ana Arias Felipe (H. Universitario 12 de Octubre), Amelia Martínez de Azagra Garde and María I. Iglesias Bouza (H. Infantil Universitario Niño Jesús), Sara Bobillo Pérez (H. Sant Joan de Déu), Manuel Nieto Faza (H. Universitario de Cruces), Juan Francisco Collado Capar and María Miñambres Rodríguez (H. Clínico Universitario Virgen de la Arrixaca), Raquel Díaz Soto and Ángela Ferrer Barba (Complejo Hospitalario Universitario de La Coruña), Inmaculada Sánchez Ganfornina and Elena González Río (H. Universitario Virgen del Rocío), Corsino Rey Galán (H. Central de Asturias), Débora Sanz Álvarez and María José Santiago Lozano (H. General Universitario Gregorio Marañón), María Luisa Palacios Loro (H. Universitario de Navarra), Esteban Gómez Sánchez (H. Universitario de Burgos), Andrés Alcaraz Romero (H. Universitario de Getafe), Lourdes Artacho González (H. Universitario de Málaga), Raúl Montero Yéboles (H. Universitario Reina Sofía), Luis J. Ferrero de la Mano (H. Universitario de León), Raúl Borrego Domínguez (Complejo Hospitalario de Toledo), Diana Álvarez Demanuel (H. Álvaro Cunqueiro de Vigo), Victoria Guerra Martín (H. de Tenerife), Paula Santos Herráiz and Raúl Borrego (H. Universitario de Toledo).
Coordinator: Antonio Rodríguez Núñez (H. Clínico Universitario de Santiago de Compostela).
The members of the Study Group of the MATUCIP Registry of the Spanish Society of Pediatric Intensive Care (SECIP) are presented in Appendix A.
Previous presentations: preliminary results of this study were presented at the 35th National Congress of the Sociedad Española de Cuidados Intensivos Pediátricos (SECIP), May 17–21, de 2021.