Central diabetes insipidus (CDI) is a rare disorder in children. The aetiology of CDI in childhood is heterogeneous. The aim of this study is to illustrate the importance of a careful clinical and neuro-radiological follow-up of the pituitary and hypothalamus region in order to identify the aetiology and the development of associated hormonal deficiencies.
MethodsClinical and auxological variables of 15 children diagnosed with CDI were retrospectively analysed in a paediatric hospital. Evaluations of adenohypophyseal function and cranial MRI were performed periodically.
ResultsThe mean age at diagnosis of CDI was 9.6 years (range: 1.32–15.9). The aetiological diagnosis could be established initially in 9 of the 15 patients, as 7 with a germinoma and 2 with a histiocytosis. After a mean follow-up of 5.5 years (range: 1.6–11.8), the number of idiopathic cases was reduced by half. At the end of the follow-up, the aetiological diagnoses were: 9 germinoma (60%), 3 histiocytosis (20%), and 3 idiopathic CDI (20%). There is a statistically significant association between stalk thickening and tumour aetiology. At least one adenohypophyseal hormonal deficiency was found in 67% of cases, with the majority developing in the first two years of follow-up. Growth hormone deficiency (60%) was the most prevalent.
ConclusionThe follow-up of CDI should include hormone evaluation with special attention, due to its frequency, to GH deficiency. In addition, a biannual MRI in an idiopathic CDI should be performed, at least during the first 2–3 years after diagnosis, as 50% of them were diagnosed with a germinoma or histiocytosis during this period.
La diabetes insípida central (DIC) es una entidad poco frecuente en la edad pediátrica, siendo su etiología heterogénea. El objetivo de nuestro trabajo es demostrar que el seguimiento clínico y neurorradiológico de la región hipotálamo-hipofisaria, puede ayudar a establecer el diagnóstico etiológico de DIC y la presencia de otros déficits hormonales.
MétodosSe revisaron de forma retrospectiva 15 pacientes diagnosticados de DIC en un hospital pediátrico. Se analizaron las características clínicas y auxológicas; así como la valoración de la función adenohipofisaria junto con RM craneal de manera periódica.
ResultadosLa mediana de edad al diagnóstico fue de 9,6 años (rango: 1,3-15,9). El diagnóstico etiológico pudo establecerse en 9 de los 15 pacientes (germinomas: 7 e histiocitosis: 2). Tras una mediana de seguimiento de 5,5 años (rango: 1,6-11,8), los casos idiopáticos se redujeron a la mitad. Finalmente, los diagnósticos etiológicos fueron: germinoma 9 (60%), histiocitosis 3 (20%) y DIC idiopática 3 (20%). Existe una asociación estadísticamente significativa entre el engrosamiento del tallo y la etiología tumoral. El 67% desarrolló, al menos, una deficiencia hormonal adenohipofisaria, la mayoría en los dos primeros años de seguimiento. El déficit más prevalente fue el de hormona de crecimiento (60%).
ConclusionesEn todos los pacientes con DIC se deberá realizar un control auxológico y hormonal, con especial atención, por su frecuencia, a la deficiencia de GH, y en aquellos con DIC idiopática se debería incluir una RM semestral, al menos durante los 2-3 primeros años después del diagnóstico, pues en nuestro estudio el 50% fueron diagnosticados de germinomas o histiocitosis en este periodo.
Central diabetes insipidus (CDI) is a rare entity in the paediatric age group, with an annual incidence of 4 patients per 100000 individuals.1,2 It is due to the deficient secretion or release of antidiuretic hormone (ADH), also known as vasopressin, and polyuria and polydipsia are the most frequent presenting symptoms at the time of diagnosis.3 It needs to be differentiated from nephrogenic diabetes insipidus, due to resistance of the kidneys to ADH, and from primary polyuria due to excessive fluid intake.
The aetiology of CDI in the paediatric population is heterogeneous and includes forms secondary to central nervous system tumours (germinoma or craniopharyngioma, among others), cranial malformations, neurosurgery, head trauma, autoimmune hypophysitis or diseases that cause infiltration in the hypothalamic-pituitary region (neoplasia, sarcoidosis or histiocytosis). Genetic aetiologies are infrequent (genetic defects in ADH synthesis—mutation in the AVP gene—that may have an autosomal recessive, autosomal dominant or X-linked pattern of inheritance). Nephrogenic diabetes insipidus is a disorder with X-linked inheritance caused by mutations in the arginine vasopressin receptor 2 gene (AVPR2) or with autosomal or recessive inheritance cause by mutations in the aquaporin 2 gene (AQP2).1
All patients given a diagnosis of CDI should undergo a magnetic resonance imaging (MRI) examination to confirm the diagnosis (absence of the bright spot corresponding to the posterior pituitary in T1-weighted images) and perhaps even determine its aetiology.4 The presence of a thickened pituitary stalk at diagnosis of diabetes insipidus or during the follow-up is suggestive, although no pathognomonic, of pituitary infiltration associated with a tumour. At any rate, a variable percentage of CDI cases, which depending on the case series varies between 20% and 50%, with or without pituitary stalk thickening, are considered idiopathic (ICDI).5,6
The aim of our study was to analyse the clinical characteristics and outcomes of a group of patients given a diagnosis of CDI during childhood or adolescence, and to evaluate the association between pituitary stalk thickening and a neoplastic aetiology and the presence of other hormone deficiencies.
Sample , Clinical and auxological evalutation etc.• Sample. We performed a retrospective review of the cases of 15 patients given a diagnosis of CDI between 2003 and 2016 managed at a children's hospital. The diagnosis of CDI was based on a positive personal history of polyuria and polydipsia and the results of the water deprivation test.7 To determine the central aetiology of the diabetes, patients underwent a desmopressin test (DDAVP; 0.1IU/kg delivered intravenously), and CDI was considered confirmed in case of a 50% increase in urine osmolality relative to baseline. In patients what presented with a plasma osmolality greater than 295mOsm/kg and a plasma sodium level greater than 145mEq/L, the water deprivation test was not performed and was replaced by direct administration of vasopressin.
• Clinical and auxological evaluation. We retrieved data on the symptoms, anthropometric measurements and Tanner stage of pubertal development recorded at diagnosis and every 6 months during the follow-up.8,9
• Pituitary function. Every year, serum levels of insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3) were measured as indirect markers of growth hormone (GH) deficiency. Also, serum levels of thyroid-stimulating hormone (TSH), free T4 and cortisol and the serum testosterone/estradiol ratio were measured every 6 months. When considered necessary to diagnose a potential deficiency, patients underwent a growth hormone stimulation test (clonidine stimulation test and insulin-induced hypoglycaemia test), gonadotropin stimulation test (luteinising hormone-releasing hormone [LHRH] test) and adrenocorticotropin (ACTH) stimulation test.
- Thyroid-stimulating hormone deficiency was defined as a decreased free T4 level (<0.65ng/dL) accompanied by decreased levels of TSH or a normal TSH value inconsistent with the level of free T4.
- Adrenocorticotropin deficiency was diagnosed in patients with basal serum cortisol levels (8 am) of less than 8μg/dL and a peak cortisol level of less than 18pg/mL in the ACTH stimulation test.
- Gonadotropin deficiency was suspected in male patients with a testicular volume of less than 4mL at age 14 years and female patients that had not exhibited thelarche by age 13 years. When serum levels of sex steroids were in the prepubertal range in these patients, a LHRH stimulation test was performed to confirm the diagnosis of gonadotropin deficiency due to an absent or insufficient response of luteinising hormone (LH) and follicle-stimulating hormone (FSH).
• Neuroimaging. The neuroimaging evaluation consisted of a cranial MRI scan following the routine protocol (T1-weighted sagittal view, FLAIR axial view, T2-weighted coronal view and diffusion-weighted imaging) and a specific study of the pituitary region (T1- and T2-weighted sagittal views, T1-weighted coronal view and, following administration of intravenous paramagnetic contrast, post-contrast T1-weighted sagittal and coronal views). The same protocol was applied when MRI was required during the follow-up. Two neuroradiologists measured the thickness of the proximal pituitary stalk independently, with diameters of less than 3mm considered normal. Pituitary stalk thickening was classified as mild (3–3.9mm), moderate (4–6.5mm) or severe (>6.5mm).2 In patients in whom it had not been possible to establish the aetiology of the diabetes in the initial evaluation, the follow-up included performance of a cranial MRI scan every 6–12 months. Patients that received a diagnosis of neoplasm or histiocytosis underwent follow-up MRI examinations in adherence to the oncology protocol applicable to each case.
Other tests- In patients with suspected germinoma, measurement of levels of α-fetoprotein (AFP) and the ß fraction of human chorionic gonadotropin (β-HCG) in plasma and cerebrospinal fluid (CSF).
- Histopathological examination of skin lesions and transsphenoidal biopsies of pituitary masses.
Statistical analysis. We performed the analyses with the software SPSS version 20. We compared categorical variables by means of the Fisher exact test and evaluated the linear association between pairs of ordinal quantitative variables by means of the Spearman correlation coefficient. We defined statistical significance as a P-value of less than .05.
ResultsPatientsThe study included 15 patients with a mean age at diagnosis of 9.6 years (range, 1.3–15.9 years). The sex distribution was 66.7% female and 33.3% male. All patients presented with polyuria and polydipsia, and the mean time elapsed from onset of symptoms to the diagnosis of CDI was 3 months (range, 7–32 months). Furthermore, 53% presented associated symptoms: headache with amblyopia in 26.6% (cases 10, 12, 14 and 15; Table 1) and a decreased growth velocity in 26.6% (cases 7, 9, 11 and 13). All of them received a diagnosis of germinoma, and the ophthalmologic manifestations were due to refractive errors, with normal findings in the visual field test in all cases.
Radiological characteristics of the pituitary stalk of the patients during the follow-up.
| Patient | Age at diagnosis (years) | Stalk in MRI (thickening)a | Initial diagnosis | Time (years) | Maximum stalk thickening | Diagnosis at the end of follow-up | Order of development of hormone deficiencies |
|---|---|---|---|---|---|---|---|
| 1 | 6.2 | Mild (3mm) | Histiocytosis | – | Mild (3mm) | Histiocytosis | ADH |
| 2 | 10.6 | Mild (3mm) | Histiocytosis | 2.7* | Moderate (5mm) | Histiocytosis | ADH |
| 3 | 6.6 | No thickening | Idiopathic | 1b | Mild (3mm) | Idiopathic | ADH, GH |
| 4 | 5.7 | No thickening | Idiopathic | 1.9b | Mild (3mm) | Idiopathic | ADH |
| 5 | 7 | No thickening | Idiopathic | – | No thickening | Idiopathic | ADH |
| 6 | 1.3 | No thickening | Idiopathic | 1.2b | Moderate (6mm) | Histiocytosis | ADH, TSH, ACTH, GHc |
| 7 | 5.9 | No thickening | Idiopathic | 2.2b | Moderate (5mm) | Germinoma | ADH, GH, TSH |
| 8 | 9.9 | No thickening | Idiopathic | 2b | Severe (10mm) | Germinoma | ADH, ACTH, TSH |
| 9 | 15.9 | Moderate (5mm) | Germinoma | – | Moderate (5mm) | Germinoma | ADH, GH, TSH, FSH-LH |
| 10 | 11.6 | Severe (12mm) | Germinoma | – | Severe (12mm) | Germinoma | ADH |
| 11 | 8.5 | Severe (30mm) | Germinoma | – | Severe (30mm) | Germinoma | ADH, TSH, ACTH, GHd |
| 12 | 15.3 | Severe (26mm) | Germinoma | – | Severe (26mm) | Germinoma | ADH, TSH, ACTH, GH, LH-FSH |
| 13 | 9.6 | Moderate (4mm) | Germinoma | 2.6* | Severe (8mm) | Germinoma | ADH, GH |
| 14 | 15.6 | Severe (9mm) | Germinoma | – | Severe (9mm) | Germinoma | ADH, TSH, GH, LH-FSH, ACTH |
| 15 | 11.9 | Severe (26mm) | Germinoma | – | Severe (26mm) | Germinoma | ADH, TSH, ACTH, LH-FSH, GH |
MRI scan performed at diagnosis. When present, pituitary stalk thickening was classified as mild (3–3.9mm), moderate (4–6.5mm) or severe (>6.5mm).
Time elapsed from the initial MRI to detection of stalk thickening in patients that did not have thickening at diagnosis.*Time elapsed from the inicial MRI to the increase of stalk thickening in patients had thickening at diagnosis,
The deficiencies that developed after chemotherapy (vinblastine)c, surgery, chemotherapy per protocol and cranial irradiation (54Gy)d are presented in boldface.
ACTH, adrenocorticotropin; ADH, antidiuretic hormone; GH, growth hormone; LH-FSH, luteinising hormone/follicle stimulating hormone; MRI, magnetic resonance imaging; TSH, thyroid stimulating hormone.
The median duration of follow-up of the patients was 5.5 years (range, 1.6–11.8 years). As of this writing, 6 patients (40%) remain in follow-up, with a mean age of 17.6±0.78 years.
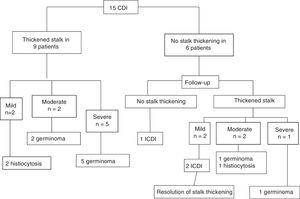
NeuroimagingIn the initial MRI evaluation, 9 of the 15 patients presented with a thickened pituitary stalk with diameters ranging from 3 to 30mm (mild in 2, moderate in 2 and severe in 5). The final diagnosis in these 9 patients was germinoma in 7 and histiocytosis in 2 (Fig. 1). The bright spot corresponding to the posterior pituitary gland was missing in all patients in the initial MRI evaluation. Patients 1 and 2 had received a Langerhans cell histiocytosis (LCH) diagnosis based on the histopathological examination of samples of skin lesions that were the initial presenting symptom in both cases at ages 2.2 and 1.6 years. After 4 and 9 years of follow-up, respectively, they developed symptoms compatible with CDI. The MRI evaluation of 4 and the 7 patients with a diagnosis of germinoma revealed a pineal gland cyst.
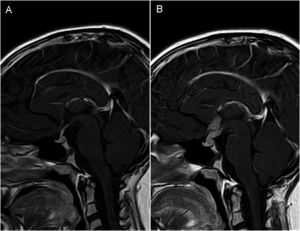
In 5 of the 6 patients that did not have a thickened pituitary stalk at diagnosis, follow-up MRI examinations revealed thickening. The final diagnoses in these patients were germinoma (n=2), histiocytosis (n=1) and ICDI (n=2), with a mean delay of 1.6±0.5 years from the initial MRI examination. Patient 6 received a diagnosis of ICDI at age 1.3 years, with normal MRI findings at diagnosis. After 1.2 years of follow-up, imaging revealed thickening of the pituitary stalk coinciding the development of skin lesions; the histopathological examination of biopsy specimens of these lesions led to the diagnosis of histiocytosis. Fig. 2 shows the progressive thickening of the pituitary stalk in one of the patients that had received an initial diagnosis of ICDI.
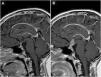
(A and B) MRI imaging in patient with central diabetes insipidus (Patient 8, Table 1). Post-contrast T1-weighted sagittal view, with 24 months elapsed between scans. (A) Initial MRI scan at diagnosis: no abnormal findings, with normal pituitary stalk. (B) Marked thickening of pituitary stalk with nodular enhancement suggestive of neoplastic disease (germinoma eventually confirmed by biopsy). Absence of hyperintense spot corresponding to posterior pituitary in T1-weighted images.
The thickening developed by patients 3 and 4 (Table 1), who received a diagnosis of ICDI, was mild and resolved spontaneously in approximately 1 year. Patient 5 (Table 1), with a diagnosis of ICDI, remained in follow-up for 6.8 years until age 17 years, with no evidence of pituitary stalk thickening at any point.
At the end of follow-up, the aetiological diagnoses were the following: germinoma in 9 patients (60%), LCH in 3 (20%) and ICDI in 3 (20%) (Fig. 1). We found a statistically significant association between pituitary stalk thickening and confirmation of a neoplastic aetiology by the end of follow-up (P=.02).
Histopathological examinationA biopsy specimen was obtained from the 8 patients in whom the MRI examination revealed the presence of a suprasellar mass, and the histological features were compatible with germinoma in all. The remaining patient with a diagnosis of germinoma had been referred from their local hospital with a suspected diagnosis of craniopharyngioma with compatible MRI findings. In our hospital, they underwent a lumbar puncture, and the examination of the specimen revealed atypical cells compatible with a germline tumour, so, foregoing a biopsy, treatment was initiated following the established protocol.
Patients 1, 2 and 6 received a diagnosis of histiocytosis based on the findings of the biopsy of their skin lesions.
A histopathological evaluation was not performed in the rest of the patients, as they did not present with significant thickening of the pituitary stalk.
Abnormalities in the hypothalamic-pituitary axisAll patients in the sample had an initial diagnosis of CDI and 67% developed at least one pituitary hormone deficiency, in most cases in the first 2 years of follow-up. The most frequent deficiency was GH deficiency (60%) followed by TSH deficiency (53%), ACTH deficiency (40%) and LH/FSH deficiency (33%). Only 5 patients (patients 1, 2, 4, 5 and 10) had isolated ADH deficiency (Tables 1 and 2). All patients that had more than 2 pituitary hormone deficiencies developed them before receiving cancer treatment, except 2: patient 6 developed ACTH and GH deficiency after treatment with vinblastine, and patient 11 developed TSH, ACTH and GH deficiency after undergoing surgery, chemotherapy per protocol and cranial irradiation (54Gy).
Association between degree of thickening and type/number of associated hormone deficiencies.
| Hormone deficiencya | Maximum thickening of pituitary stalk | Total | |||
|---|---|---|---|---|---|
| Type | Absent | Mild | Moderate | Severe | |
| ADH | 1 | 3 | 4 | 7 | 15 |
| GH | 0 | 1 | 3 | 5 | 9 (60%) |
| TSH | 0 | 0 | 3 | 5 | 8 (53%) |
| ACTH | 0 | 0 | 1 | 5 | 6 (40%) |
| LH/FSH* | 0 | 0 | 1 | 3 | 4 (28%) |
| Number of deficienciesb | Absent | Mild | Moderate | Severe | |
|---|---|---|---|---|---|
| Isolated ADH deficiency | 1 | 2 | 1 | 1 | 5 (33.3%) |
| 2 | 0 | 1 | 0 | 1 | 2 (13.3%) |
| 3 | 0 | 0 | 1 | 1 | 2 (13.3%) |
| 4 | 0 | 0 | 2 | 1 | 3 (20%) |
| 5 | 0 | 0 | 0 | 3 | 3 (20%) |
| Total | 1 (6.7%) | 3 (20%) | 4 (26.7%) | 7 (46.7%) | 15 |
ACTH, adrenocorticotropin; ADH, antidiuretic hormone; GH, growth hormone; LH-FSH, luteinising hormone/follicle stimulating hormone; TSH, thyroid stimulating hormone.
The mean time elapsed from diagnosis of CDI to the development of additional hormone deficiencies was 0.43 years (range, 0–2.3) for TSH (8 patients); 0.46 years (range, 0–7.6) for GH (9 patients) and 0.92 years (range, 0–2.3) for ACTH (6 patients). Four out of 14 patients (28.5%, 2 female and 2 male) developed gonadotropin deficiency (LH/FSH). In patient 7, it was not possible to assess a probable LH/FSH deficiency because the patient was in the prepubertal stage at the time of the study. Table 1 presents the order in which hormone deficiencies developed in each patient, and Table 2 the association between the type of thickening and the type and number of deficiencies. We found a positive linear correlation (r=0.64) between the number of hormone deficiencies and pituitary stalk thickening at the end of follow-up, which was statistically significant (P=.009). However, we did not find a statistically significant association between the presence of multiple hormone deficiencies (CDI plus two or more pituitary hormone deficiencies) and a neoplastic aetiology.
Other testsAll patients given a diagnosis of germinoma (n=9) exhibited elevation of AFP (>0.4ng/mL) and ß-HCG (>5mIU/mL) in CSF. On the other hand, increased serum levels of AFP (>15ng/mL) were only found in 1/8 patients (12.5%), and elevation of serum β-HCG (>5mIU/mL) in only 2/7 patients (28.6%).
DiscussionWe made a retrospective review of the characteristics and clinical outcomes of the 15 patients given a diagnosis of CDI in the past 13 years in our hospital. The study was particularly focused on the presence at diagnosis or subsequent development of a thickened pituitary stalk and its association with the presence or development of other pituitary hormone deficiencies.
In our sample, the time elapsed from onset of symptoms and the aetiological diagnosis of CDI was typically long, of more than 2 years, especially in patients with mild or no abnormal imaging findings at the time of diagnosis of CDI, which can be explained by the challenges in diagnosis a disease with a slow course and localised in a hard-to-access region that has multiple functions, such as the hypothalamic-pituitary region.
Forty percent of the patients in the sample (6–15) received an initial diagnosis of idiopathic CDI; a percentage that dropped in a relatively short period of time (between 1 and 2.2 years) by 50% to only 20% of the patients. This reduction over time in the proportion of cases of CDI considered idiopathic was greater compared to similar studies, which have reported decreases of approximately 20% (18%–22%), with a median duration of follow-up of 2.3–10 years.6,10,11
The final proportion of ICDI (20% in our sample) varies widely among recently published case series,11,12 ranging between the 5.7% reported by Hunter et al.13 and the 52% reported by Maghnie et al.14 This variability is mostly due to the heterogeneity of the follow-up protocols applied to these patients, especially in relation to the interval at which follow-up MRI examinations are performed, as shorter time intervals, especially in the first few years after diagnosis (in our hospital, ever 6–12 months for at least the first 2–3 years) allow the early detection of pituitary stalk thickening, thus manifesting the presence of an underlying disease, frequently of a neoplastic nature and potentially severe.15,16 However, the detection of pituitary stalk thickening, especially if mild, does not necessarily correspond to the presence of a tumour. Two of our patients with suspected ICDI developed mild thickening of the stalk during the follow-up that eventually resolved spontaneously. These transient thickening suggests the possibility of hypophysitis of an inflammatory and/or autoimmune aetiology.17 There have been occasional reports of the detection of antibodies against vasopressin and other antibodies in this context whose measurement could be useful in the diagnosis of some patients, but which was not performed in our sample. This clinical picture is associated with a low risk of developing histiocytosis or germinoma in the future.18
In our sample, the most frequent aetiology was neoplastic, identified in 60% at the time of diagnosis and in 80% by the end of follow-up.19 Early detection of thickening is essential, as our case series demonstrates that the presence of a thickened pituitary stalk is associated with an increased risk of underlying tumours. In the case series presented by Werny et al.,6 the presence of a thickened pituitary stalk at diagnosis was significantly associated with a neoplastic aetiology.
Germinomas amount to approximately 5% of all central nervous system tumours.20 In our study, it was the most frequent cause of CDI, accounting for 60% of cases, although other studies with larger samples have reported lower percentages (10%-38%).21,22 Thickening of part or all of the pituitary stalk may be the sole presenting feature of small germinomas.10
When it came to the patients with LCH, two ouf of three had a thickened pituitary stalk at the time of diagnosis of CDI; Grois et al.23 found this feature in the initial evaluation in 71% of cases of CDI secondary to LCH. This difference could be due to the small number of patients given an initial diagnosis of histiocytosis. We did not find any cases of familial neurogenic diabetes insipidus or CDI of a genetic aetiology, which was consistent with previous studies11,13; in larger studies, monogenic forms account for less than 10% of the total cases of CDI.6,10,14
In our study, the hyperintense signal characteristic of the posterior pituitary was absent in the initial MRI scan of 100% patients with CDI, but this was a higher percentage compared to studies with larger samples (81%6–94%14). Thus, the presence of this bright spot does not rule out CDI.23
The hormone evaluation revealed more than 1 deficiency in 67% of our patients, which was consistent with previous studies that have reported percentages of patients with more than 1 hormone deficiency ranging from 53.8% (Liu et al.)12 to 81.4% (Di Iorgi et al.).10 The most frequent deficiency concerned GH, which was also consistent with the literature.
Another test that should be performed in patients with CDI of suspected neoplastic aetiology is the measurement of AFP and β-HCG levels in plasma and CSF, which are tumour markers.24 As observed in other studies, most patients with germinoma had normal serum levels of AFP and β-HCG with elevation in CSF. The measurement of these markers in plasma show low sensivity sensitive as in CSH. Thus, they should be measured routinely in CSF, especially considering that their elevation is highly suggestive of a germ cell tumour.25
Based on a detailed analysis of the data in our study and a review of the literature, we may conclude the following:
- •
All patients with CDI require monitoring of auxologic parameters and hormone levels at least every 6 months to identify pituitary deficiencies that can develop gradually over time, especially in the first 2 years following diagnosis of CDI, with particular emphasis on GH deficiency on account of its high frequency.
- •
The follow-up of patients with ICDI should include a MRI scan every 6–12 months based on the detected hormonal abnormalities, at least for the first 2–3 years after diagnosis, as 50% of the patients in our sample received a diagnosis of germinoma and histiocytosis in this time interval.
- •
The presence and degree of pituitary stalk thickening in patients with CDI could be directly correlated to the presence or future development of other hormone deficiencies.
The authors have no conflicts of interest to declare.
Please cite this article as: Corredor Andrés B, Muñoz Calvo MT, López Pino MA, Márquez Rivera M, Travieso Suárez L, Pozo Román J, et al. Engrosamiento del tallo hipofisario en niños y adolescentes con diabetes insípida central: causas y consecuencias. An Pediatr (Barc). 2019;90:293–300.