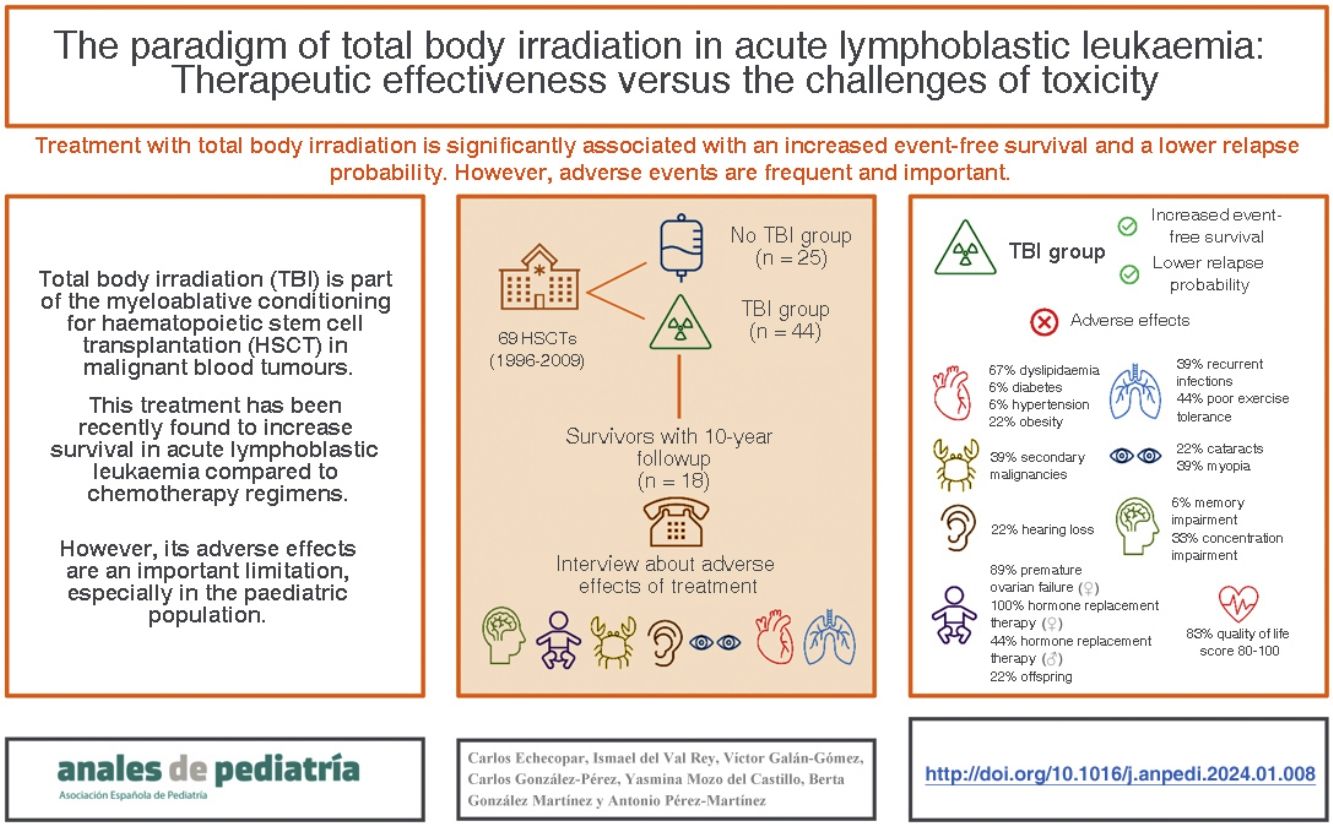
Total body irradiation (TBI) is part of the myeloablative conditioning for hematopoietic stem cell transplantation (HSCT) in malignant hematologic disorders. This therapy has recently shown improved survival in acute lymphoblastic leukemia (ALL) compared to chemotherapy-based regimens. However, side effects are a significant limitation, especially in the pediatric population.
Patients and methodsWe retrospectively analyzed the survival of patients with ALL who underwent an HSCT at a tertiary hospital between 1996 and 2009 (N = 69 HSCT in 57 patients). We differentiated a cohort that received TBI (N = 44) from another that did not (N = 25). Subsequently, we interviewed the survivors from the TBI group with a minimum of 10 years of follow-up (N = 18), asking about the presence of side effects.
ResultsThe overall survival (OS) at 2 and 5 years was 79.1% and 65.2% respectively for the TBI group and 66.2% and 55.8% for the non-TBI group, although this difference was not significant (P=.31). The event-free survival (EFS) at 2 and 5 years was 77.3% and 63.6% respectively for the TBI group and 56% and 32% for the non-TBI group (P=.02). The probability of relapse (PR) at 2 years for those who received TBI was 10% compared to 28.6% for those who did not receive TBI (P=.005). Survivors who received TBI developed secondary neoplasms (39%), dyslipidemia (67%), cognitive impairments affecting memory (44%), recurrent respiratory infections (39%), thyroid abnormalities (45%), premature ovarian failure (89%), cataracts (22%), and psychological problems (44%). However, the quality of life, as self-assessed by the patients, was considered good for 83% of the participants..
ConclusionsPatients who received TBI had significantly higher EFS and lower PR. However, adverse effects are frequent and significant, although they do not subjectively affect quality of life.
La irradiación corporal total (ICT) forma parte del acondicionamiento mieloablativo del trasplante de progenitores hematopoyéticos (TPH) en hemopatías malignas. Esta terapia ha demostrado recientemente mayor supervivencia en leucemia linfoblástica aguda (LLA) frente a regímenes basados en quimioterapia. Sin embargo, los efectos secundarios son una limitación importante, especialmente en la población pediátrica.
Pacientes y métodosAnalizamos retrospectivamente la supervivencia de pacientes con LLA que recibieron un TPH en un hospital terciario entre 1996 a 2009 (N = 69 TPH en 57 pacientes). Diferenciamos una cohorte que había recibido ICT (N = 44) y otra que no (N = 25). Posteriormente entrevistamos a los supervivientes del grupo ICT con un mínimo de 10 años de seguimiento (N = 18), preguntando acerca de la presencia de efectos secundarios.
ResultadosLa supervivencia global (SG) a los 2 y 5 años fue del 79.1% y 65.2% respectivamente para el grupo ICT y del 66.2% y 55.8% para el grupo no ICT, aunque esta diferencia no fue significativa (p = 0.31). La supervivencia libre de evento (SLE), a los 2 y 5 años fue del 77.3% y 63.6% respectivamente para el grupo ICT y del 56% y 32% para el grupo no ICT (p = 0.02). La probabilidad de recidiva (PR) a los 2 años habiendo recibido ICT fue del 10% y sin haber recibido ICT del 28.6% (p = 0.005). Los supervivientes que recibieron ICT desarrollaron neoplasias secundarias (39%), dislipemia (67%), alteraciones cognitivas (44%), infecciones respiratorias de repetición (39%), alteraciones tiroideas (45%), insuficiencia ovárica precoz (89%), cataratas (22%) y problemas psicológicos (44%), aunque la calidad de vida, valorada por ellos mismos, fue considerada como buena para el 83% de los encuestados.
ConclusionesLos pacientes que recibieron ICT tuvieron significativamente mayor SLE y menor PR. Sin embargo, los efectos adversos son frecuentes e importantes, aunque no afectan subjetivamente a la calidad de vida.
Haematopoietic stem cell transplantation (HSCT) is a consolidation treatment for high-risk acute lymphoblastic leukaemia (ALL). This treatment requires previous conditioning, whose purpose is to create room in the bone marrow of the recipient for the donor haematopoietic stem cells, achieve a sufficient level of immunosuppression to keep the immune system of the patient from rejecting the graft and eliminate as much residual disease as possible.1 Traditionally, myeloablative regimens were recommended in malignant blood tumours due to their strong antitumour effect. However, their toxicity is their main limitation in paediatric care.
Total body irradiation (TBI) is a treatment included in some myeloablative conditioning regimens, with delivery of radiation to the whole body at a dose of about 12 Gy.2 Conditioning with TBI is the standard of care for HSCT since more than 70 years ago, with clinical outcomes that have yet to be surpassed by chemotherapy-based conditioning regimens. The results of the FORUM study, an international, multicentre phase III trial comparing TBI and chemotherapy, were published in 2021, showing an increased 2-year overall survival and decreased relapse risk with TBI.3
However, the FORUM study did not assess long-term outcomes of TBI in the paediatric population. Total body irradiation is a treatment associated with multiple adverse events in the medium and long term, especially in paediatric patients.4–6 Its adverse effects increase with decreasing patient age, and TBI is not recommended in children aged less than 4 years.7,8
In recent years, variables known as patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs) have been analysed to help measure the impact of a given intervention from the perspective of the patient,9 and their study is particularly important in patients with a high risk of morbidity, such as HSCT recipients.10
The aim of our study was to make a retrospective analysis of survival in patients with ALL treated with HSCT, comparing those who had undergone TBI as part of their conditioning regimen and those who had not. A secondary objective was to describe the cumulative morbidity of patients who underwent TBI as part of their conditioning regimen, taking into account PREMs to obtain more comprehensive information about the adverse effects associated with TBI based on the personal experience of the patients.
Sample and methodsWe conducted a retrospective study analysing the HSCT performed for treatment of ALL in the department of paediatric haematology and oncology of a tertiary care hospital between 1996 and 2009. During this time, 69 HSCTs were performed in 57 patients. We recorded clinical data, grouping patients based on whether they had received TBI as part of the conditioning regimen and those who only received chemotherapy (Table 1). We collected clinical data from the health records and expressed quantitative variables as mean and standard deviation.
Baseline clinical characteristics of patients by conditioning regimen.
| Total | TBI | No TBI | P | |
|---|---|---|---|---|
| Frequency of cases, n (%) | 69 | 44 (64%) | 25 (36%) | ― |
| Age (years), mean ± SD | 14.9 ± 7.6 | 17.9 ± 7.2 | 9.6 ± 5.2 | .000 |
| Age at diagnosis (years), mean ± SD | 6.3 ± 3.9 | 7.5 ± 3.4 | 4.1 ± 3.7 | .000 |
| Age at HSCT (years), mean ± SD | 8.5 ± 4.3 | 9.8 ± 3.4 | 6.3 ± 4.7 | .001 |
| Sex, n (%) | .941 | |||
| Male | 41 (59%) | 26 (59%) | 15 (60%) | |
| Female | 28 (41%) | 18 (41%) | 10 (40%) | |
| ALL subtype, n (%) | .075 | |||
| B-cell ALL | 58 (84%) | 39 (89%) | 19 (76%) | |
| Precursor B-cell ALL | 3 (4%) | ― | 3 (12%) | |
| Philadelphia + ALL | 5 (8%) | 3 (7%) | 2 (8%) | |
| T-cell ALL | 2 (3%) | 2 (4%) | ― | |
| Biphenotypic ALL | 1 (1%) | ― | 1 (4%) | |
| Number of HSCT, n (%) | .014 | |||
| 1 HSCT | 60 (87%) | 42 (96%) | 18 (72%) | |
| 2 HSCT | 7 (10%) | 1 (2%) | 6 (24%) | |
| 3 HSCT | 2 (3%) | 1 (2%) | 1 (4%) | |
| Type of donor, n (%) | .015 | |||
| Unrelated | 17 (25%) | 8 (18%) | 9 (36%) | |
| Haploidentical | 29 (42%) | 16 (36%) | 13 (52%) | |
| Autologous | 23 (33%) | 20 (46%) | 3 (12%) | |
| Source of stem cells, n (%) | .005 | |||
| Peripheral blood | 34 (49%) | 26 (59%) | 8 (32%) | |
| Bone marrow | 25 (36%) | 16 (36%) | 9 (36%) | |
| Umbilical cord blood | 10 (15%) | 2 (5%) | 8 (32%) | |
| Disease at time of HSCT, n (%) | .656 | |||
| Complete remission | 62 (90%) | 39 (89%) | 23 (92%) | |
| Partial remission | 7 (10%) | 5 (11%) | 2 (8%) | |
ALL, acute lymphoblastic leukaemia; HSCT, haematopoietic stem cell transplantation; SD, standard deviation; TBI, total body irradiation.
Statistically significant P values are presented in boldface.
We used the Kaplan-Meier method for the survival analysis. The outcomes we analysed were the overall survival (OS), the event-free survival (EFS), with event defined as relapse or death, the relapse probability (RP) and transplant-related mortality (TRM). We compared these outcomes using the log-rank test.
In the second part of the study, we identified a total of 27 survivors of HSCT performed for treatment of ALL that had been followed up for a minimum of 10 years, of who 21 agreed to be interviewed. Of this total, 18 patients (86%) received TBI as part of the conditioning regimen and 3 patients (14%) did not undergo TBI. Due to the small number of patients in the no TBI group (Appendix A, supplemental material 1), it was not possible to compare the two groups, so we decided to conduct a descriptive analysis of the adverse events in the TBI group.
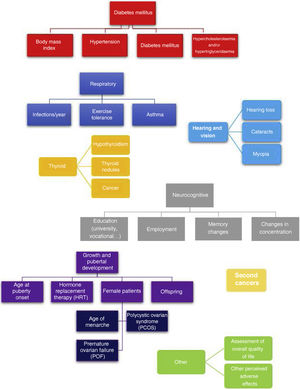
A single researcher conducted all of the telephone interviews, administering a questionnaire developed in house consisting of close-ended questions that is shown in Fig. 1. This questionnaire assessed for the most frequent adverse events experienced after HSCT: (a) pulmonary toxicity,11 (b) metabolic syndrome,12 (c) hypothyroidism and thyroid cancer,13 (d) development of cataracts,13 (e) tinnitus and hearing loss,14 (f) neurocognitive deficits,15,16 (g) pubertal and gonadal development17 and (h) secondary malignancies.18 To complete the interview, we added 2 open-ended questions to allow patients to describe any other relevant adverse effects not covered before and to report their perceived current quality of life on a scale from 0-100.
The study was approved by the Clinical Research Ethics Committee of the hospital on May 28, 2022.
ResultsEpidemiological dataTable 1 presents the clinical characteristics of the patients included in the study. The mean age of the patients who underwent HSCT was 14.9 years (SD, 7.6) with a mean age at diagnosis of 6.3 years (SD, 3.9) and a mean age at the time of HSCT of 8.5 years (SD, 4.3). Patients who received TBI were significantly older at diagnosis, the time of HSCT and at the time of followup compared to patients who did not receive TBI (P < .001). The number of transplants was significantly greater in patients who did not undergo TBI and there were also significant differences between the groups in the type of donor and source of the stem cells. There were no differences based on the sex of the patient, the type of leukaemia or the disease status prior to HSCT.
Survival analysis comparing total body irradiation vs no total body irradiationThe mean duration of followup in the overall sample was 8.4 years (SD, 7) and 12.4 years (SD, 6.6) in survivors. Table 2 summarises the results of the analysis of survival.
Survival analysis based on conditioning regimen.
| Total | TBI | No TBI | P | |
|---|---|---|---|---|
| 2-year OS (95% CI) | 74.6 (69.3-79.9) | 79.1 (72.9-85.3) | 66.2 (56.4-76) | .31 |
| 2-year EFS (95% CI) | 70 (64.5-75.5) | 77.3 (71-83.6) | 60 (50-70) | .02 |
| 2-year RP (95% CI) | 16.5 (11.7-21.3) | 10 (5.2-14.8) | 18.3 (12-24.6) | .005 |
| Death, n (%) | 29 (42%) | 18 (41%) | 11 (44%) | .803 |
| TRM, n (%) | 16 (23%) | 10 (23%) | 6 (24%) | .968 |
CI, confidence interval; EFS, event-free survival; OS: overall survival; RP, relapse probability; TBI, total body irradiation; TRM, transplant-related mortality.
Statistically significant P values are presented in boldface.
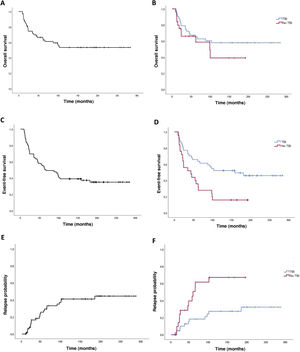
The 2-years overall survival (OS) was 74.6% (95% CI, 69.3-79.9) and the 5-year OS was 64.8% (95% CI, 70.7-58.9) (Fig. 2). Comparing the TBI vs no TBI groups, we found a 2-year OS of 79.1% in the TBI group (95% CI, 72.9-85.3) compared to 66.2% in the no TBI group (95% CI, 56.4-76). The 5-year OS was 65.2% in the TBI group (95% CI, 57.9-72.5) compared to 58.8% for the no TBI group (95% CI, 47.6-70), and the differences were not statistically significant (Fig. 2).
Event-free survivalThe 2-year event-free survival (EFS) in the overall sample was 70% (95% CI, 64.5-75.5), and the 5-year EFS was 53.6% (95% CI, 47.6-59.6). Comparing by conditioning regimen, EFS was significantly greater in the TBI group, with a 2-year EFS of 77.3% (95% CI, 71-83.6) compared to 60% (95% CI, 50-70) in the no TBI group, and a 5-year EFS of 63.6% (95% CI, 56.3-70.9) compared to 32% (95% CI, 22.7-41.3) in the no TBI group (P =.02) (Fig. 2).
We assessed 2-year EFS based on the variables for which we had found statistically significant differences between the TBI and no TBI groups, and found no significant differences except in relation to the type of donor (Appendix A, supplemental material 2).
Probability of recurrenceIn this case series, there were 25 relapse cases (36%). In the overall sample, the mean time elapsed to relapse was 3.4 years, with a 2-year relapse probability (RP) of 16.5% (95% CI, 11.7-21.3) and a 5-year RP was 20.8% (95% CI, 12.6-29) (Fig. 2). Comparing the probability based on the conditioning regimen, the 2-year and 5-year PR in the TBI group were 10% (95% CI, 5.2-14.8) y 18.3% (95% CI, 12-24.6) compared to 28.6% (95% CI, 18.6-38.6) and 56% (95% CI, 44.6-67.4) in the no TBI group (P = .005).
Treatment-related mortalityIn this case series, 16 patients died due to the HSCT (23.2%). We did not find statistically significant differences in the treatment-related mortality (TRM) based on the conditioning regimen used.
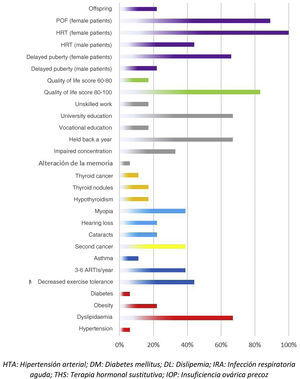
Adverse events in the total body irradiation groupFig. 3 presents the adverse events experienced by HSCT survivors who had undergone conditioning with TBI.
There were 7 cases of secondary cancer among the survivors. They corresponded to 2 cases of follicular thyroid carcinoma, 2 skin tumours, 1 granular cell tumour and basal cell carcinoma, an unspecified soft tissue tumour, a malignant neoplasm of unspecified ovary and a neurofibroma.
When it came to the respiratory system, 44% of patients reported decreased exercise tolerance compared to their peers. As for cardiovascular risk factors, 22% of the patients had obesity and 67% dyslipidaemia. Forty-five percent of the interviewees reported some form of thyroid involvement (17% thyroiditis, 17% thyroid nodules and 11% thyroid cancer). When it came to sexual development, the 9 interviewed female patients required hormone replacement therapy (HRT) and 8 of them had premature ovarian failure. In terms of other adverse events, we found that 22% developed cataracts, 39% myopia, 22% hearing problems, 33% concentration problems and 67% poor academic performance.
Among the adverse effects reported by patients themselves, we ought to highlight psychological problems related to adjustment to life after discharge and resuming everyday activities (56%). Male patients also reported premature balding (23%).
When it came to the self-reported quality of life, most patients (83%) had scores between 80 and 100 points.
DiscussionAlthough we did not find significant differences in the 2-year OS between the TBI and no TBI groups, the EFS and RP were better in patients who underwent TBI for conditioning. These findings were similar to those of the prospective FORUM study.3 This suggests that TBI has a protective effect against relapse.
In our study, there was a higher proportion of autologous HSCT in the TBI group compared to the no TBI group, in which the proportion of allogeneic HSCT was greater. Autologous HSCT does not produce graft versus tumour effects that may be present in allogeneic HSCT, in spite of which the survival outcomes were better in the group conditioned with TBI. This suggests that TBI has a significant effect against leukaemia, but it is also important to remember that autologous HSCT has not been recommended for treatment of paediatric ALL for a decade.
To make a comprehensive assessment of TBI, we sought to describe the adverse events in patients who had received it as part of their conditioning regimen, including the personal perspective of the patient applying the recently established performance measures, PROMs and PREMs.9
The development of secondary malignancies is a known adverse event of HSCT, and there are studies demonstrating an increased risk of secondary malignancy when TBI is used for conditioning.6 In our series of patients treated with TBI, 39% developed secondary malignancies, most of them involving the thyroid or skin, an incidence much higher compared to the previous literature, in which the reported incidence is approximately 3% to 5%.19
According to the existing evidence, one of the main adverse effects of TBI is pulmonary toxicity, with the main adverse events including pulmonary fibrosis, decreased lung function as measured by spirometry and an increased frequency of respiratory infections.11 In our patients, we found that 44% reported poorer exercise tolerance compared to their peers and 11% a diagnosis of asthma. In addition, all had a mild to moderate level of recurrent respiratory infections, with 11 patients (61%) reporting fewer than 3 episodes a year and 7 (39%) 3 to 6 episodes a year.
Thirty-three percent of patients in the TBI group reported concentration problems, in agreement with the previous literature, in which impaired concentration is described as one of the major neurocognitive adverse events of radiation therapy, found in 50% of patients.16
Cataracts developed in 22% of patients treated with TBI at a mean age of 14 years and a latency time of approximately 2 to 2.5 years after performance of the HSCT, which was consistent with the previous literature, in which an incidence of 28% is reported following TBI.20
Up to 50% of patients who underwent TBI received a diagnosis of hypercholesterolaemia, at a mean age of 17 to 18 years, and most achieved adequate control with lifestyle and dietary measures. Diabetes and metabolic syndrome are both common adverse events in patients managed with radiation therapy, and can develop even in patients with a normal body mass index.21
When it comes to adverse effects in the area of sexual development, they are most frequent in female patients, and most of those affected (89%) develop premature ovarian failure, require lifelong hormone replacement therapy and are unable to conceive. A previous study found that female patients treated with TBI are 30% less likely to be able to have children.17 Male patients are less affected, and in our interview, many reported premature balding.
However, when asked about quality of life, most interviewed patients (83%) reported a moderate to very good quality of life, in agreement with previous evidence in which only 12% rated it as poor.19 However, we must highlight that patients also reported mental health problems in the early years after the disease, especially features of anxiety and depression, in many cases requiring professional help.22
Despite these adverse events, most of the patients in our study reported a good quality of life, with scores above 80 or 90 points, which was consistent with the previous literature, in which 57% of the patients rated their quality of life as good or excellent and only 12% as poor.23
Although the use of TBI as part of the conditioning regimen for HSCT in childhood ALL is still recommended,24 TBI has significant associated toxicities in the medium and long term. However, current chemotherapy-only conditioning regimens are clearly inferior, so randomised controlled trials should be conducted to explore the possibility of combining them with other post-transplantation strategies to intensify the anti-leukaemia effect without increasing toxicity, for instance, through the use of drugs such as tyrosine kinase inhibitors or immunotherapy with adoptive cell transfer.
Another alternative that is currently being considered is reducing the dose of TBI to 8 Gy, an approach already supported by evidence in adults with ALL but it has yet to be confirmed in the paediatric population.25
ConclusionPatients who received TBI for conditioning had a greater EFS and a lower RP compared to patients who underwent chemotherapy for conditioning. This suggests that TBI has a protective effect against relapse, in agreement with the findings of the FORUM study.
Another of the goals of our study was to assess the potential adverse events associated with TBI, taking into account the subjective perspective of patients through PREMs. We found that a high proportion of the survivors experienced respiratory complications in the form of recurrent respiratory infections and dyspnoea, metabolic syndrome with obesity, HTN, diabetes and dyslipidaemia, thyroiditis and thyroid cancer, cataracts and myopia, hearing problems, difficulty concentrating and school failure, premature ovarian failure in female patients and secondary malignancies (especially thyroid and skin cancers).
Strategies for followup, monitoring and intervention in the medium and long term need to be established for these patients on account of the high morbidity that they experience throughout the lifespan. To this end, it is important to take into account the experience of the patient and directly involve the patient in the assessment of the outcomes of treatment and the impact in their quality of life of the adverse events resulting from HSCT.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are thankful for the support of the European Regional Development Fund (ERDF) (FIS) PI18/01301 and the Fundación Cris contra el Cáncer (http://criscancer.org).