The onset of obesity at young ages is strongly associated with the early development of type 2 diabetes (T2D). The shape of the curves of glucose and insulin curves during an oral glucose tolerance test (OGTT) could predict the risk of developing T2D.
ObjectiveTo analyse the morphology of the OGTT and determine T2D risk factors in a mainly Caucasian population of children and adolescents.
MethodsObservational retrospective study including 588 patients (309 males, 279 females) with a mean age of 11.1±2.8 years, and of whom 90.3% were Caucasian. Risk factors for T2D were compared in patients with a monophasic or biphasic pattern during the performance of an OGTT, as well as anthropometric and biochemical variables, insulin resistance, and beta-cell function.
ResultsThe shape of the glucose curve was monophasic in 50.2% of patients (50.8% male), biphasic in 48.5% (47.6% males), and indeterminate in 1.3%. The monophasic pattern showed lower insulin-sensitivity and worse beta-cell function. Patients with a biphasic pattern had a higher BMI, waist circumference, and blood pressure, although the results were not significant. Latin-American patients had significantly lower serum glucose levels with higher insulin levels during the OGTT.
ConclusionsThe pattern of response to an OGTT reflects different metabolic phenotypes. Paediatric patients with a biphasic pattern have lower risk-profiling for T2D. OGTT would be useful to implement early intervention strategies in children and adolescents with obesity, in order to prevent the development of pre-diabetes or T2D.
El aumento en la prevalencia de obesidad en la edad pediátrica se asocia a mayor incidencia de diabetes mellitus tipo2 (DM2). El tipo de respuesta de la glucemia y de la insulina a la sobrecarga oral de glucosa (SOG) podría predecir el riesgo de DM2 en pacientes con obesidad.
ObjetivoValorar la respuesta a la SOG y relacionar con factores de riesgo de DM2 en niños y adolescentes obesos.
MétodosEstudio observacional retrospectivo sobre 588 pacientes (309 varones, 279 mujeres); 90,3% caucásicos; edad media 11,1 ± 2,8 años. Según el tipo de respuesta en la SOG se establecieron dos grupos: monofásico y bifásico. Se analizaron parámetros antropométricos, bioquímicos e índices relacionados con sensibilidad a la insulina y la función de la célulaβ.
ResultadosEl 50,2% de los pacientes tuvieron un patrón de glucosa monofásico (50,8% varones), el 48,5% bifásico (47,6% varones) y el 1,3% indeterminado. La respuesta monofásica mostró menor sensibilidad a la insulina y peor función de la célula β; los pacientes con patrón bifásico presentaron mayor índice de masa corporal, perímetro de cintura y presión arterial, sin ser estos resultados estadísticamente significativos. Los pacientes latinos tuvieron glucemias significativamente menores en la SOG a expensas de una mayor insulinemia.
ConclusionesEl patrón de respuesta de la SOG refleja fenotipos metabólicos diferentes. Los pacientes pediátricos con un patrón bifásico tienen un perfil con menor riesgo de desarrollar DM2. Una SOG en niños y adolescentes obesos podría ser útil para implementar estrategias de intervención precoz y prevenir la aparición de prediabetes o DM2 en esta población.
The incidence of type 2 diabetes (T2D) in adolescents has increased in recent decades in association with an increase in the prevalence of obesity in the paediatric age group.1 Some studies have reported larger increases in specific groups of adolescents aged 15–19 years, with an incidence of T2D of 17.0–49.4 per 100,000 individuals per year,2 even outnumbering new cases of type 1 diabetes in some ethnic groups.3 In adults, the progression from prediabetes to T2D takes approximately 5–10 years; in paediatric patients, the progression is more rapid, which is probably related to the transient physiologic insulin resistance that occurs in puberty.4
Research conducted in the United States has reported a higher incidence of T2D in paediatric patients with obesity and biochemical markers of impaired carbohydrate tolerance,5 but has been unable to establish clinical variables or specific serum levels that can accurately predict the risk of diabetes in these patients.6 The assessment of the response of glucose and insulin levels to an oral glucose tolerance test (OGTT) provides important information to establish the individual risk of each patient.7
There is evidence that the risk of T2D is associated not only with baseline and 2-hour glucose concentrations in the OGTT, but also with the morphological characteristics of the glucose response curve of individual patients, which can reflect early metabolic changes that help predict the risk of future T2D.8 Monophasic responses show a continuous and gradual increase in serum glucose levels, while biphasic responses exhibit an initial increase in glucose, followed by a decline and then a further increase at the end. In adults, the monophasic pattern has been associated with insulin resistance, impaired β-cell function and increased risk of developing T2D, while levels in individuals with normal glucose tolerance usually exhibit a biphasic pattern.7,9 These responses reflect changes in insulin sensitivity and/or secretion, both of which play a role in T2D.9 A study of Latino adolescents with obesity reported results similar to those described in adults, suggesting that the OGTT can contribute to the early detection of risk of T2D in adolescents.5
The aim of this study was to analyse the shape of the response to the OGTT in a predominantly Caucasian paediatric cohort, and assess whether there was an association between different response patterns and possible risk factors for T2D.
MethodologyWe conducted a retrospective observational study. We included 588 patients, 309 male (52.5%) and 279 female (47.5%), aged 3–17 years (mean±standard deviation [SD], 11.1±2.8), that received care at the obesity clinic of a tertiary hospital. We collected data on anthropometric measurements—weight (kg), height (cm), body mass index (BMI, kg/m2), waist circumference (cm)—and blood pressure (mmHg). Blood pressure (BP) was measured in the right arm of the patient by means of a digital sphygmomanometer with the patient in a seated position; the cuff used depended on the size of the arm. If the BP was greater than 90mmHg, it was measured again using a manual sphygmomanometer. We defined pubertal status using the Tanner scale.10 We defined obesity as a BMI at or above 2 SDs from the mean (Hernández growth charts).11 During the initial visit, tests were performed to measure plasma concentrations of glycated haemoglobin (HbA1c) (Menarini high-performance liquid chromatography analyser; normal range, 5.31±0.31), total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglycerides. To conduct the OGTT, patients ingested a dose of 1.75g/kg of glucose to a maximum of 75g following a 10-h fast. Blood samples were collected at 0, 30, 60, 90 and 120min to measure plasma glucose and insulin concentrations using a standardised method.12 We defined impaired fasting glucose (IFG) as a concentration of 100mg/dL or greater, impaired glucose tolerance (IGT) as a glucose concentration of 140mg/dL or greater at 120min in the OGTT, and diabetes (DM) as a fasting glucose concentration of 126mg/dL or greater, or a 2-h glucose concentration of 200mg/dL in the OGTT. When patients exhibited an abnormal response to the OGTT, the test was repeated at least once to confirm the results.
We classified the phenotype of the response to the OGTT as monophasic, biphasic or undetermined following the criteria described in previous studies.4,13 The glucose change threshold of 4.5mg/dL was established to minimise the bias produced by fluctuations in glucose concentrations that could be caused by the method of glucose analysis. We defined monophasic response as a response exhibiting an initial gradual rise in plasma glucose concentrations followed by a subsequent decrease during the 120min of the test. A biphasic response was characterised by a gradual rise in glucose, followed by a fall of at least 4.5mg/dL, with a second rise of glucose of at least 4.5mg/dL. Participants who exhibited a gradual increase in plasma glucose without a corresponding fall were classified as “undetermined” and excluded from the study.
The variables we used to assess insulin resistance and β-cell functioning were the homeostatic model assessment insulin resistance (HOMA-IR) and β-cell (HOMA-βC) indices,14,15 and we assessed insulin sensitivity by means of the Matsuda,14 quantitative insulin sensitivity check index (QUICKI)16 and insulin sensitivity14 indices.
The study was approved by the ethics committee of our hospital, and we received the consent of both the patients and their parents or guardians for the participation of the former in the study.
We analysed the data with SPSS® version 15 and GraphPad Prism® version 6. We assessed differences between groups using the Student's t, Mann–Whitney U and χ2 tests.
ResultsThe study included 588 patients (309 male and 279 female) with a mean age of 11.1±2.8 years and a mean BMI of 27.4±3.7kg/m2 (BMI z-score, 3.8±1.3). In our sample, 90.3% of the patients were Caucasian and 9.7% Latino. We found a monophasic response in 50.2% of the patients (50.8% male) and a biphasic response in 48.5% (47.6% male). Eight patients exhibited an undetermined pattern and were excluded from the analysis. We found no differences in age or sex between the two groups, so they were comparable. We consider the fact that we did not have access to data regarding the family history of diabetes mellitus or gestational diabetes for patients in our sample one of the limitations of our study. Table 1 summarises the general characteristics of the patients included in the study.
Descriptive characteristics of the sample by phenotype of glucose response to OGTT.
| Parameter | Total | Monophasic | Biphasic | P |
|---|---|---|---|---|
| BMI (kg/m2) | 27.37±3.71 | 27.0±3.9 | 28.4±3.9 | 0.21 |
| BMI (z) | 3.80±1.28 | 3.75±0.06 | 3.83±0.08 | 0.99 |
| Waist circumference (cm) | 88.42±11.06 | 86.03±1.92 | 90.45±1.79 | 0.16 |
| Systolic BP (mmHg) | 113.56±10.9 | 114.0±0.7 | 112.9±0.7 | 0.38 |
| Diastolic (mmHg) | 59.63±8.48 | 87.9±0.5 | 88.5±0.5 | 0.91 |
| HbA1c (%) | 4.64±2.82 | 5.3±0.04 | 5.4±0.2 | 0.32 |
| Total cholesterol (mg/dL) | 159.27±31.02 | 160.4±1.9 | 159.4±1.9 | 0.92 |
| HDL cholesterol (mg/dL) | 43.94±14.28 | 44.2±0.9 | 45.1±0.8 | 0.66 |
| LDL cholesterol (mg/dL) | 91.69±33.21 | 92.1±1.9 | 91.7±1.9 | 0.87 |
| Triglycerides (mg/dL) | 80.43±46.78 | 78.5±2.8 | 83.3±2.7 | 0.07 |
BMI, body mass index; HbA1c, glycated haemoglobin.
P-values are for tests comparing monophasic and biphasic patients.
The BMI and systolic and diastolic BP values were similar in the monophasic and the biphasic response groups (Table 1). We found a small difference in waist circumference that was not statistically significant (p=0.09), with smaller values in patients with a monophasic pattern. We also found no statistically significant differences in the lipid profile between the two groups. Although the differences were not statistically significant, the HOMA-IR and HOMA-βC indices were higher in patients with a monophasic response, which may reflect a greater insulin resistance compared to patients with a biphasic response. These results were consistent with the results of the QUICKI, which was higher in the group of patients with a biphasic response, a difference that was statistically significant (0.026+0.0018 vs 00211±0.0014; p<0.001), as well as with a higher insulin sensitivity index and a lower Matsuda index in the biphasic group compared to the monophasic group (Table 2).
Assessment of glucose and insulin homeostasis and β-cell function.
| Parameter | Total | Monophasic | Biphasic | P |
|---|---|---|---|---|
| HOMA-βC | 2.62±2.14 | 2.64±0.15 | 2.61±0.09 | 0.20 |
| HOMA-IR | 3.44±3.41 | 3.36±0.20 | 3.29±0.13 | 0.18 |
| Insulin sensitivity index | 175.86±139.61 | 174.9±8.9 | 178.9±7.4 | 0.28 |
| QUICKI | 0.024±0.028 | 0.026±0.001 | 0.0211±0.001 | <0.001 |
| Matsuda index | 4.72±2.91 | 4.81±0.18 | 4.68±0.17 | 0.54 |
| Insulin sensitivity index | 1.79±1.91 | 1.63±0.13 | 1.97±0.9 | 0.96 |
P-values are for tests comparing the given variable in monophasic and biphasic patients.
Beta-cell function, estimated by means of the insulin sensitivity index, was superior in patients with a biphasic pattern compared to patients with a monophasic pattern (1.97+0.9 vs 1.63+0.13), although the difference was not statistically significant. In patients with a monophasic pattern, the area under the glucose curve was larger, while the area under the insulin curve was smaller (Table 2).
We found statistically significant differences along the OGTT glucose response curve at minutes 30, 60, 90 and 120 (p<0.01, p<0.01, p<0.05 and p<0.001, respectively). Baseline plasma glucose concentrations were similar in both groups. Patients with a monophasic pattern had higher glucose concentrations at 30 and 90min, with a greater secretion of insulin in the first 30min, although they had reached a lower glucose concentration by 120min, with the difference in insulin concentration between baseline and the end of the curve exceeding 6mU/L (Fig. 1).
Glucose (A) and insulin (B) response curves during OGTT in monophasic (solid line) and biphasic (dashed line) groups. Plasma glucose concentrations in patients with a monophasic pattern were significantly greater at 30 and 90mins after ingestion of glucose solution, coinciding with a rise in insulin at 30min and decreased insulin concentrations at 90min, and a greater insulin response at 120min associated with a greater reduction of plasma glucose concentrations. *p<0.05; ** p<0.001; *** p<0.0001.
When we compared the response patterns by sex, we observed that patients of either sex that had a monophasic response exhibited a peak in insulin release at 30min that coincided with higher glucose concentrations, and a peak of insulin at 120min that allowed them to have lower concentrations of glucose compared to patients with a biphasic pattern, who had a lower insulin peak at 30min, followed by a gradual decline through the end of the curve (Appendix A, Supplementary Fig. 1).
When we compared the glucose and insulin concentration curves based on pubertal status (male patients: 194 prepubertal and 115 pubertal; female patients, 95 prepubertal and 184 pubertal), we found that in pubertal patients, insulin and glucose concentrations were significantly higher at baseline and at 60min of the OGTT test (Appendix A, Supplementary Fig. 2). At 30min, patients that exhibited a monophasic pattern, both pubertal and prepubertal, had similar insulin concentrations (Appendix A, Supplementary Fig. 3).
Among male patients, 54.1% of prepubertal patients and 45.2% of pubertal patients exhibited a monophasic response, compared to 44.8% of prepubertal and 52.6% of pubertal patients that exhibited a biphasic response. In female patients we found a monophasic pattern in 47.4% of prepubertal girls and 48.9% of pubertal girls, and a biphasic pattern in 52.6% of prepubertal girls and 49.5% of pubertal girls.
In our series, 32 patients (12 female and 20 male; 23 pubertal) had IFG, and 50% of them exhibited a monophasic response pattern. Twenty-seven patients had IGT, of who 19% exhibited a monophasic pattern (14 male and 13 female; 18 pubertal). However, the proportion of patients with concentrations of HbA1c of 5.7% or greater, the threshold used to define prediabetes, was similar in the monophasic and the biphasic groups.
We found glucose concentrations greater than 155mg/dL at 60min of the OGTT in 8.3% (49/588) of the patients. This was not associated with any anthropometric variable or the baseline concentration of any of the blood chemistry markers, although we did find a direct correlation with the areas under the glucose and insulin curves (p<0.001) and with a glucose concentration greater than 140mg/dL at 120min (p<0.001), as well as an inverse correlation to the Matsuda index (p<0.05). We did not find a correlation with concentrations of HbA1c greater than 5.7%.
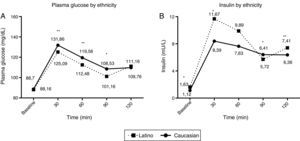
When we analysed OGTT responses by ethnicity, we found that 52% of Caucasian patients exhibited a monophasic response compared to 60% of Latino patients, although the difference was not statistically significant. However, there were clear differences in plasma glucose and insulin concentrations between the two groups, as we found higher insulin concentrations in Latino patients at minutes 30, 60 and 120 of the OGTT, followed by concentrations similar to those of Caucasian patients at 120min, which reflects a greater degree of insulin resistance (Fig. 2). We did not find any differences between the two groups in the Matsuda, HOMA-IR or insulin sensitivity indices (p<0.05).
Glucose (A) and insulin (B) response curves during OGTT in Caucasian patients (solid line) and Latino patients (dashed line). Latino patients had significantly lower plasma glucose concentrations throughout the curve, but reached similar concentrations to those of Caucasian patients by 120min. They also exhibited significantly higher plasma insulin concentrations at baseline and at 30, 60 and 120min. * p<0.05; ** p<0.001.
The identification of obese patients with an increased risk of future T2D is key for the purpose of intensifying lifestyle interventions or even initiating preventive pharmacological treatment in patients with abnormal OGTT results.17,18 Consequently, efforts have been made in recent years to develop prediction models to identify populations at risk of developing T2D.
At present, the glucose response to the OGTT is considered a better predictor of risk of T2D than fasting glucose concentration.19 A study conducted in adult patients in the United States showed that their risk for progression to T2D could be stratified based on the relationship between the baseline and the post-load plasma glucose concentrations in the OGTT, and that a faster return to the fasting plasma glucose concentration or to levels below it was associated to a lower risk of T2D.9 This strategy allows the identification of two groups of patients: those with abnormalities in insulin secretion, and those with impaired insulin sensitivity, both of which are pathophysiological mechanisms associated with T2D.9,20 Similar results have been reported in Caucasian adults, although patients with abnormal baseline glucose levels were at higher risk of T2D. Patients whose plasma glucose concentration falls below the baseline at 30 or 60min are at lower risk than patients whose glucose concentration does not decrease, as the latter have been shown to have greater degrees of hepatic and peripheral insulin resistance and β-cell dysfunction.9
While IFG and IGT are considered diagnostic of prediabetes, the evaluation of glucose and insulin response patterns in OGTTs can provide additional information on insulin sensitivity and the potential for developing T2D of each individual.4,20 In our study, while patients with a monophasic pattern exhibited greater insulin resistance, we did not find a greater incidence of prediabetes, which may be explained by the age of the patients. The followup of these patients will allow us to improve our prediction of the risk of future progression to T2D. Our results suggest that insulin sensitivity and β-cell function is better in patients that exhibit a biphasic response compared to those with a monophasic response, which is consistent with the findings of previous studies on adults and Latino adolescents.4,9 We ought to highlight that in our sample, the response to the OGTT showed a stronger association with insulin sensitivity than with clinical parameters like BMI, BP and waist circumference, so that changes in the shape of oral glucose tolerance curves could be considered more sensitive predictors than those other parameters.
Evidence from 3-h OGTTs in patients with suspected gestational diabetes shows that a greater number of phases in the patients’ responses is associated with a healthier metabolic profile, with better insulin sensitivity and β-cell function and a lower incidence of prediabetes or T2D, suggesting that a biphasic response is associated with a decreased risk of diabetes in this subset of the population.7
The authors of a recent multivariate analysis proposed that 60-min or 90-min plasma glucose concentrations are the most sensitive markers for predicting the risk of T2D in adults,21 assigning OGTTs an important role in T2D prediction models. In our sample, we found a positive correlation between 60-min plasma glucose concentrations of more than 155mg/dL and concentrations above 140mg/dL at 120min, which suggests that shorter OGTTs could have a significant predictive value if the cut-off points were modified. It is worth noting that some authors have stated that 60-min and 90-min glucose levels are better predictors of diabetes than the glucose concentration at 120min.12 We must take into account that generally speaking, the prognostic yield of all models decreases as the duration of followup increases, especially in those that include OGTT.21
Some authors consider that the usefulness of the OGTT in obese adolescents is limited, and argue that fasting indices of insulin sensitivity correlate better to insulin resistance than OGTT-derived surrogates independently of glucose tolerance status, with the HOMA and QUICKI indices performing similarly as predictors of insulin resistance.22 However, the comparison performed in this study only took into account the concentration values at each time point of the OGTT, and not the shape of the curve. In our patients, the shape of the curve suggested early changes in the pattern of the response to hyperglycaemia, providing information that supplemented that of the fasting indices. This interpretation is supported by the finding that the QUICKI and the insulin sensitivity index were higher in the group of patients with a biphasic response compared to patients with a monophasic response, while the Matsuda index was lower.
The natural history of T2D in the paediatric age group is less well understood compared to adults, and impaired β-cell function is the hypothesis that is most supported at present from a pathophysiological perspective.4 Research conducted in adolescents in the United States showed that the OGTT response curve could be used to differentiate the risk of T2D independent of obesity. Participants with a biphasic response had significantly better β-cell function, higher insulin sensitivity and secretion as well as a lower glucose area under the curve and HbA1c concentration.4 However, no prospective studies have been conducted to confirm these findings.
To date, there was no study that compared the metabolic response to the OGTT of Caucasian and Latino patients, and we found significant differences in plasma glucose and insulin concentrations between the two groups. However, there were few Latino patients in our study compared to Caucasian patients, which limits the validity of our analysis.
Consistent with the results previously described in adults and adolescents, our results show that monophasic responses in the OGTT suggest a pattern of insulin resistance that carries an increased risk of future T2D. The analysis of the response pattern exhibited by patients during the OGTT could be used as an early marker of dysregulation in carbohydrate metabolism that would allow early intensification of lifestyle interventions in these patients.
Conflict of interestThe authors have no conflict of interests to declare.
Please cite this article as: Herrera-Martínez AD, Enes P, Martín-Frías M, Roldán B, Yelmo R, Barrio R. La respuesta monofásica a la sobrecarga oral de glucosa como factor predictivo del riesgo de diabetes tipo 2 en pacientes pediátricos con obesidad. An Pediatr (Barc). 2017;87:211–217.