There are no paediatric formulations of anti-tuberculous drugs in Spain, with the only exception being rifampicin. Some paediatricians often prescribe composite formulations (CF), while others prefer to give crushed tablets. Nevertheless, there is no consensus in this regard, or any pharmacokinetic studies validating these procedures. In this situation, the Spanish Network for the Study of Paediatric Tuberculosis (pTBred) has launched the Magistral Project, which aims at its first phase to analyse the desirability of developing child-friendly pharmaceutical formulations and other aspects regarding the anti-tuberculous drug prescription in children.
Material and methodsA cross-sectional, multicentre, nationwide study was conducted, based on an online questionnaire sent to members of pTBred between February and March 2015.
ResultsFifty-four responses from 67 consulted institutions were received. Most of the respondents reported prescribing crushed tablets. A significant number of those surveyed, although being fewer, prescribe CF, for which availability varies widely among institutions. Eighty-three percent replied that it would be essential to have fixed dose combinations of anti-tuberculous drugs, specifically adapted to paediatric doses and administered by CF or tablets. Among the surveyed institutions, differences were found in the management of latent tuberculosis infection, in the use of directly observed therapy, and in the monitoring of adverse events.
ConclusionsOur survey reveals great diversity in anti-tuberculous drug prescription in children, due to the lack of suitable infant formulations, which could have an impact on treatment adherence and outcomes. pTBred intends to develop a pioneering and useful consensus document on the management of anti-tuberculous medication in children.
En España no existen presentaciones pediátricas de fármacos antituberculosos, salvo para rifampicina. Algunos pediatras prescriben fórmulas magistrales (FM), mientras que otros administran comprimidos triturados. No existe consenso al respecto, ni estudios de farmacocinética que avalen estos procedimientos. Ante esta situación, la Red Española de Estudio de la Tuberculosis Pediátrica (pTBred) desarrolla el Proyecto Magistral, con el objetivo de analizar, en su primera fase, la conveniencia de desarrollar formas farmacéuticas específicas para niños, así como estudiar otros aspectos relacionados con la administración de antituberculosos en niños.
Material y métodosEstudio transversal, multicéntrico y de ámbito nacional, mediante encuesta on-line enviada por correo electrónico a los instituciones pertenecientes a pTBred entre febrero y marzo del 2015.
ResultadosSe recibieron 54 respuestas de 67 instituciones consultadas. La mayoría de los centros trituran los comprimidos. Un porcentaje elevado, aunque menor, administra FM, cuya disponibilidad es variable entre las instituciones. El 83% responde que sería ideal disponer de combinaciones fijas de antituberculosos, adaptadas a las dosis pediátricas y administradas mediante FM o en un comprimido. Entre las instituciones encuestadas existen diferencias en el tratamiento de la infección tuberculosa latente, el uso de la terapia directamente observada y la monitorización de efectos adversos.
ConclusionesNuestra encuesta revela gran heterogeneidad en la prescripción de antituberculosos en niños debido a la falta de formulaciones específicas para esta edad, que podría tener implicaciones en la adherencia al tratamiento y evolución. pTBred propone elaborar un pionero y útil documento de consenso sobre la administración de medicación antituberculosa en niños.
Between 2003 and 2010, the Working Group on Tuberculosis and Infection by Other Mycobacteria of the Sociedad Española de Infectología Pediátrica (Spanish Society of Paediatric Infectology [SEIP]) published the consensus documents1–6 on the diagnosis and management of tuberculosis in children, collaborating with the Sociedad Española de Neumología Pediátrica (Spanish Society of Paediatric Pulmonology [SENP])6 in the most recent one. These documents highlighted the lack of paediatric formulations prepared as solutions or suspensions for most antituberculosis agents, especially for those currently used as first-line treatment, which is one of the barriers to adherence.5,6 As of today, the challenge most frequently faced by paediatric specialists in respiratory or infectious diseases in their everyday practice still remains: how to administer antituberculosis agents to children that have yet to develop the ability to swallow solid dosage forms.
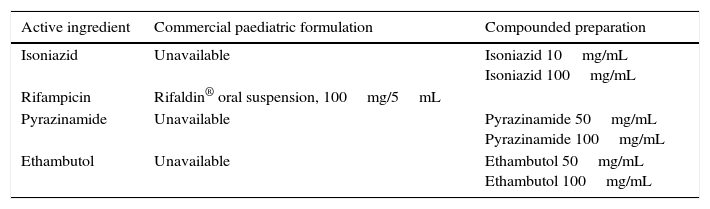
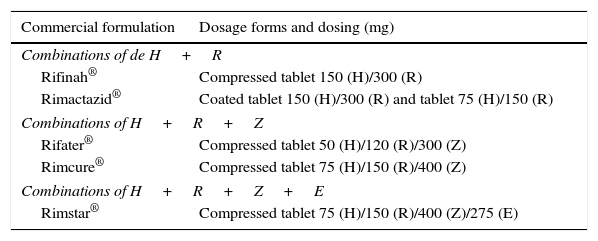
In Spain, the only first-line antituberculosis agent that is commercially available as a liquid dosage form is rifampicin7 (Table 1). Under these circumstances, some paediatricians prescribe compounded preparations (CPs), while others prescribe tablets to be crushed and subsequently diluted in various fluids for their administration. There is no consensus on these practices, nor any pharmacokinetic studies to support them. There is also wide variability in the use of CPs, with variations in stability between different dosage forms and the excipients used in their formulation and in the conditions surrounding their preparation. The published literature on the stability of CPs is also scarce.8,9 There is also few data on the use of fixed dose combinations (FDCs) of antituberculosis drugs (Table 2), and furthermore their use is not approved in children aged less than 8 years.10 In 2009, the World Health Organization established guidelines for the use of FDCs in children based on body weight, and warned that none of the existing combinations was ideal for use in children.11
Antituberculosis agents available as oral solution or suspension in Spain.
| Active ingredient | Commercial paediatric formulation | Compounded preparation |
|---|---|---|
| Isoniazid | Unavailable | Isoniazid 10mg/mL Isoniazid 100mg/mL |
| Rifampicin | Rifaldin® oral suspension, 100mg/5mL | |
| Pyrazinamide | Unavailable | Pyrazinamide 50mg/mL Pyrazinamide 100mg/mL |
| Ethambutol | Unavailable | Ethambutol 50mg/mL Ethambutol 100mg/mL |
Fixed-dose combinations available in Spain. As of July 2015, the marketing authorisation for Rimactazid® and Rimcure® was suspended, based on data from the Centro de Información online de Medicamentos de la Agencia Española de Medicamentos y Productos Sanitarios (Online Medicines Information Centre of the Spanish Agency of Medicines and Medical Devices [CIMA-AEMPS]).
| Commercial formulation | Dosage forms and dosing (mg) |
|---|---|
| Combinations of de H+R | |
| Rifinah® | Compressed tablet 150 (H)/300 (R) |
| Rimactazid® | Coated tablet 150 (H)/300 (R) and tablet 75 (H)/150 (R) |
| Combinations of H+R+Z | |
| Rifater® | Compressed tablet 50 (H)/120 (R)/300 (Z) |
| Rimcure® | Compressed tablet 75 (H)/150 (R)/400 (Z) |
| Combinations of H+R+Z+E | |
| Rimstar® | Compressed tablet 75 (H)/150 (R)/400 (Z)/275 (E) |
E, ethambutol; H, isoniazid; R, rifampicin; Z, pyrazinamide.
The Red Española de Estudio de Tuberculosis Pediátrica (Spanish Network for the Study of Paediatric Tuberculosis [pTBred]) was created in 2013.12 As of February 2015, the network comprises more than 120 researchers from 67 institutions and has gathered information on more than 200 children with tuberculosis in Spain.
In light of the difficulty and lack of consensus that exists in the administration of antituberculosis agents to children, a group of researchers of the pTBred developed the Proyecto Magistral (Compounding Project, pTBred#3) in January and February 2015 with the main objective of assessing, in an initial phase, the suitability of developing specific dosage forms for children based on body weight of first-line oral antituberculosis drugs. The study also sought to determine the pattern of antituberculosis drug prescription in the member institutions of the pTBred, the availability of CPs in these centres, the regimens commonly prescribed for the management of latent tuberculosis infection (LTBI), the percentage of institutions that order directly observed therapy (DOT) and how adverse effects to first-line drugs are monitored.
Materials and methodsWe conducted a cross-sectional, multicentre, descriptive, multidisciplinary nationwide study. The data for the study was collected by means of a survey to be completed on a voluntary basis, which was sent to the member institutions of the pTBred by electronic mail and created using Google Drive®. The survey was approved by the Scientific Committee of the pTBred and conducted between February 15, and March 1, 2015.
The survey (Appendix A) included two parts. The first one was designed to determine the characteristics of the surveyed institutions, and the second comprised 21 questions arranged into three sections by subject: 7 questions on concepts related to the approach to the management of tuberculosis in children, 8 questions pertaining to the use of the most common antituberculosis agents, and lastly 6 questions on the availability of CPs in the different hospitals.
The number of institutions that were members of the pTBred as of February 1, 2015 was 67. We estimated that we needed to receive responses from at least 51 institutions to achieve a large enough sample to be able to generalise the results to all the pTBred member institutions with a margin of error of less than 3%, a level of heterogeneity of 95% and a 95% confidence interval (CI). The sole inclusion criterion was to be a member of the pTBred, and we only accepted one response per institution.
We performed a descriptive analysis of qualitative variables by calculating their relative frequencies using the statistical software SPSS v20.0. We did not include any patient data, as the survey only asked about aspects of clinical practice, and thus did not deem it necessary to submit the survey for evaluation by ethics committees. Only researchers that participated in the study had access to the data of the survey, which were collected solely for the purpose of statistical analysis.
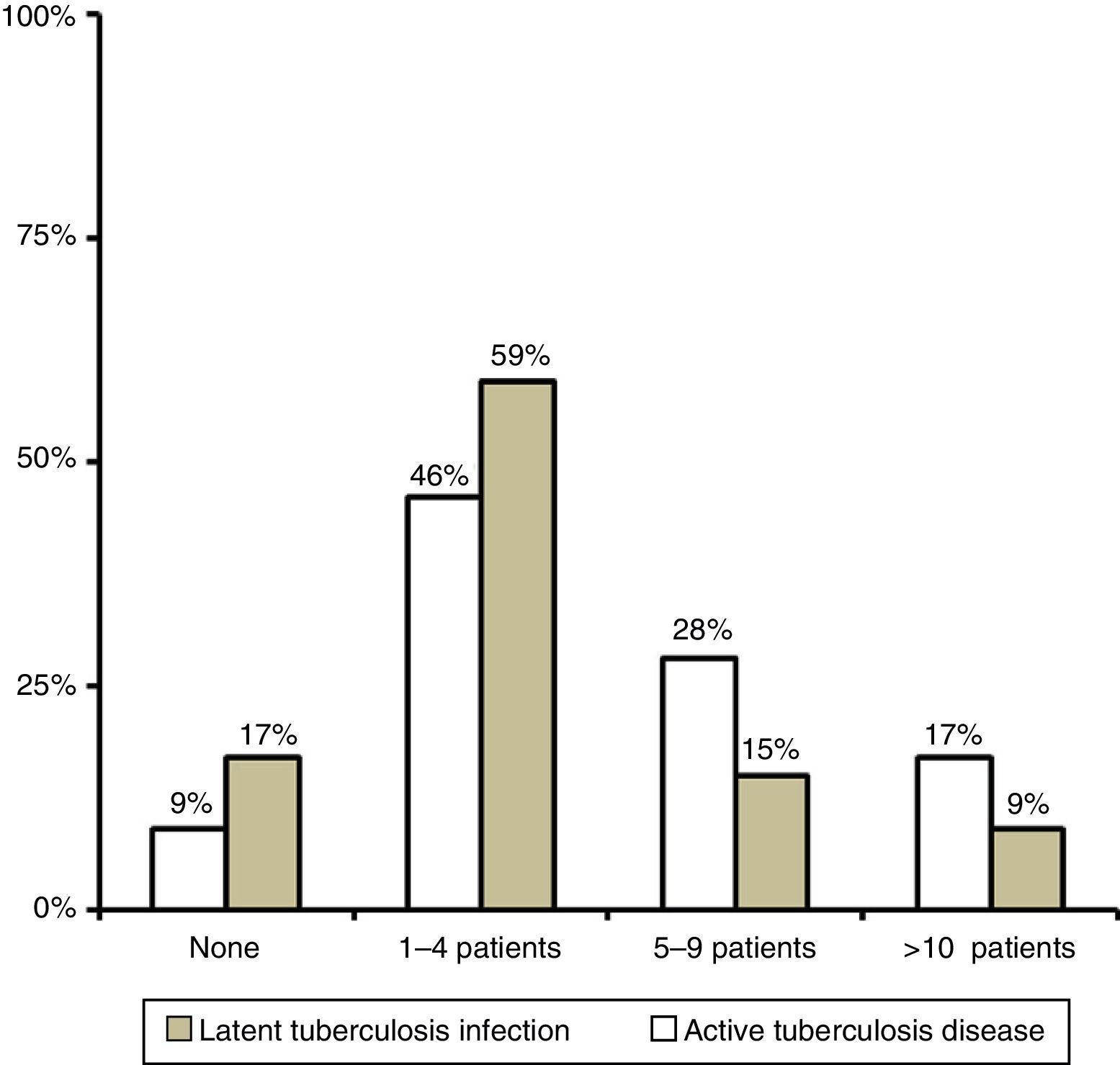
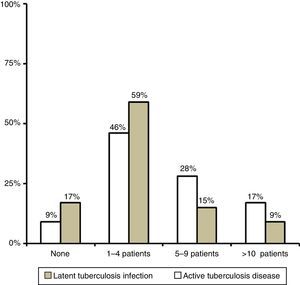
ResultsWe received 54 responses: 24 from institutions in the Autonomous Community of Madrid, 10 from Catalonia, 4 from Andalusia and 16 from other autonomous communities, all of which were represented except for Aragón, Murcia and Castilla-León. In 2014, most of the surveyed centres had served one to four children with LTBI and one to four children with active tuberculosis disease (Fig. 1).
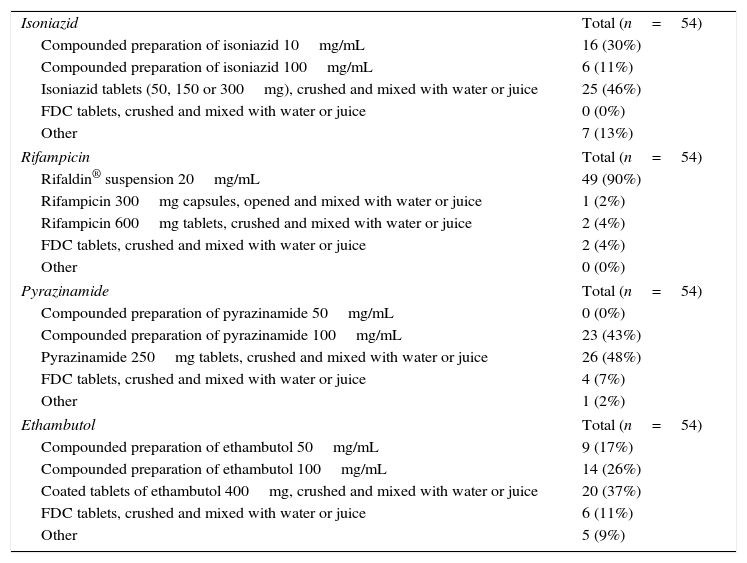
The most frequently used method to administer antituberculosis agents in children unable to swallow solid dosage forms was crushing compressed tablets, except in the case of rifampicin, which is available as a solution (Table 3). The second most frequent method was the administration of drugs in CPs, although the preparation and availability was highly variable between members of the pTBred (Table 4). When it came to the use of FDCs, more than half of the institutions reported using them regularly, although the questionnaire did not specifically ask whether they were only used in older children and adolescents or also in children aged less than 8 years, and since this may lead to these results being biased, we decided not to include them in the discussion of the study.
Most frequent methods used to administer antituberculosis agents to children that are not able to swallow solid dosage forms. Multiple choice, single answer questions.
| Isoniazid | Total (n=54) |
| Compounded preparation of isoniazid 10mg/mL | 16 (30%) |
| Compounded preparation of isoniazid 100mg/mL | 6 (11%) |
| Isoniazid tablets (50, 150 or 300mg), crushed and mixed with water or juice | 25 (46%) |
| FDC tablets, crushed and mixed with water or juice | 0 (0%) |
| Other | 7 (13%) |
| Rifampicin | Total (n=54) |
| Rifaldin® suspension 20mg/mL | 49 (90%) |
| Rifampicin 300mg capsules, opened and mixed with water or juice | 1 (2%) |
| Rifampicin 600mg tablets, crushed and mixed with water or juice | 2 (4%) |
| FDC tablets, crushed and mixed with water or juice | 2 (4%) |
| Other | 0 (0%) |
| Pyrazinamide | Total (n=54) |
| Compounded preparation of pyrazinamide 50mg/mL | 0 (0%) |
| Compounded preparation of pyrazinamide 100mg/mL | 23 (43%) |
| Pyrazinamide 250mg tablets, crushed and mixed with water or juice | 26 (48%) |
| FDC tablets, crushed and mixed with water or juice | 4 (7%) |
| Other | 1 (2%) |
| Ethambutol | Total (n=54) |
| Compounded preparation of ethambutol 50mg/mL | 9 (17%) |
| Compounded preparation of ethambutol 100mg/mL | 14 (26%) |
| Coated tablets of ethambutol 400mg, crushed and mixed with water or juice | 20 (37%) |
| FDC tablets, crushed and mixed with water or juice | 6 (11%) |
| Other | 5 (9%) |
Availability of compounded preparations in the hospital.
| Availability of compounded preparations in the hospital (n=54) | Isoniazid | Pyrazinamide | Ethambutol |
|---|---|---|---|
| Unknown | 6 (11%) | 6 (11%) | 5 (9%) |
| Never | 6 (11%) | 6 (11%) | 9 (17%) |
| Rarely | 1 (2%) | 3 (6%) | 3 (6%) |
| Sometimes | 5 (9%) | 6 (11%) | 8 (15%) |
| Almost always | 9 (17%) | 10 (19%) | 7 (13%) |
| Always | 27 (50%) | 23 (42%) | 22 (40%) |
Compounded preparations: multiple choice, single answer question.
Of the respondents that reported using crushed tablets, 58% dissolved them in water, 19% in juice and 4% in milk. Thirteen percent reported not having a preference in terms of the fluid used for dissolving them, and six percent reported never using crushed tablets. Of the 50 institutions that reported using crushed tablets, 76% did not recommend a specific type of pill splitter or crusher. Eighty-nine percent of respondents administered all the drugs under fasting conditions, while the rest recommended taking them with food in case the patient had poor tolerance.
When it came to the question “Do you think that a compounded preparation or FDC with the following combination of antituberculosis agents: 150mg H, 200mg R, 350mg Z±200mg E (or else 75mg H, 100mg R, 175mg Z±100mg E) would be useful in the treatment of tuberculosis in children?” (we calculated these doses based on the recommendations of the SEIP6 and the Committee on Medications of the AEP13 [CM-AEP], which in turn are based on the recommendations of the WHO14 and recent pharmacokinetics studies10,15), 83% answered that this would be ideal, 4% that the currently available FDCs suffice, while 13% considered that the doses proposed for pyrazinamide and ethambutol are suboptimal or that it would be better to administer each drug separately.
The regimens used for treating LTBI varied between institutions. Thus, 50% used a 9-month isoniazid regimen, 30% a 6-month isoniazid regimen, and 20% a 3-month isoniazid and rifampicin regimen. As for ethambutol, 69% of institutions used it routinely for the treatment of tuberculosis disease, except in cases in which the strain in the patient or the index case was known to be sensitive to isoniazid, rifampicin or pyrazinamide; 4% used it in all cases, regardless of what was known about the sensitivity of the strain; and the rest chose other answer options, for example using it exclusively for specific forms of disease such as tuberculous meningitis.
As for DOT, 72% of respondents ordered it only if there seemed to be risk factors for poor adherence to treatment, 17% never ordered it, and 11% almost always ordered it, save for select patients in whom adherence to treatment was expected to be optimal. No institution reported always ordering DOT.
We also found differences in the monitoring of adverse effects. When it came to monitoring liver function, 52% of institutions ordered liver enzyme tests prior to initiating treatment, but not after, unless the patient developed manifestations compatible with liver toxicity; 28% performed serial tests after initiating treatment; 7% did not order liver function tests before or after starting treatment; and seven institutions reported having approaches other than those proposed in the answers (before and after, weekly, monthly, etc.).
Eye examinations were ordered by 33% of institutions for children aged less than 5 years treated with ethambutol, compared to 9% that never ordered them and 58% that only ordered them in specific cases, for instance, for patients taking doses of 25mg/kg/day or greater for treatment of tuberculous meningitis.
DiscussionThe results of our survey show a lack of consensus in Spain when it comes to how to administer antituberculosis drugs to children, except for rifampicin, which is widely administered in the form of oral suspension. Most paediatricians prescribe tablets that need to be crushed and dissolved, while the rest prescribe CPs. More than 20% of the respondents reported that compounding was not available in their institution or not knowing whether it was available. It is difficult to make sense of the variability found in the administration of antituberculosis agents in Spain, and also hard to accept the lack of specific paediatric dosage forms for the administration of a treatment the success of which depends directly on patient adherence.
The website of the Grupo Español de Farmacia Pediátrica (Spanish Group of Paediatric Pharmacy), which is part of the Sociedad Española de Farmacia Hospitalaria (Spanish Society of Hospital Pharmacy [SEFH]), offers instructions on how to compound various drugs in Spain.16 For antituberculosis agents, the site only features directions for isoniazid 10mg/mL and pyrazinamide 100mg/mL. A CP of isoniazid at that concentration is not too useful in children, as it produces large volumes that are poorly received by these patients. At the time of this writing, this site did not describe the steps to compound any preparation of ethambutol, although many pharmacy departments already have information on how to prepare an oral suspension with this active ingredient. As far as we know, no clinical trials have been conducted recently to assess and guarantee the safety of the administration of these drugs as CPs, and the data available is from old trials.17 However, as is the case of many other drugs in paediatric practice, their off-label use is authorised. Such use requires obtaining verbal consent from the parents or legal guardians, and while legal, it is not covered by the guarantees of drug regulatory agencies, and pharmaceutical companies would not be liable if complaints were to be filed.18
Eighty-three percent of the surveyed institutions stated that it would be ideal to have fixed dose combinations specifically made for children with doses calculated based on the latest recommendations6,13,14 of the SEIP, CM-AEP and WHO. However, 13% declared that the proposed doses for pyrazinamide and ethambutol would be suboptimal, which is up for debate, as different dose ranges based on weight have been recommended, or believed that it was preferable to administer each drug separately, an opinion that is consistent with that of some experts in relation to the use of FDCs in adults.19 Others believe that, all things considered, combination drugs are currently the best treatment option.20,21
When it came to the use of crushed tablets, there was also disagreement as to the type of fluid used to dissolve them. In fact, while 13% of the surveyed centres answered that it does not make any difference, both milk and the juice of some fruits can decrease the bioavailability of some drugs, so the recommendation of administering these medications with dairy or juices such as grapefruit juice should always be given after careful consideration.22
The results of the survey also evinced that, despite the availability of consensus documents1–6 and that members of the pTBred12 are expected to have a homogeneous approach, there is considerable variability between these institutions.
For example, 30% of the surveyed centres use the 6-month isoniazid regimen to treat LTBI, when our guidelines recommend the 9-month regimen.5,6 When it comes to DOT, 16% of the responding institutions never order it, and nearly 75% only order it when there are risk factors for poor adherence to treatment. We must take into account the legal framework for the role of health professionals and the health authorities in ensuring adherence to treatment with the purpose of preventing the spread of tuberculosis in the community.5
We also found inconsistencies in relation to patient safety and the monitoring of drug adverse effects. Different hospitals performed different numbers of liver function tests. The SEIP does not recommend routinely monitoring liver enzyme levels if patients have no risk factors for developing hepatitis.5,6 However, more than 90% of respondents reported performing at least one transaminase test, usually prior to initiating treatment. There also seems to be no agreement in relation to the performance of eye examinations when children are treated with ethambutol. We know that toxicity is low and reversible in most cases by discontinuing treatment, that it is dose-dependent, and that the prevalence of this adverse effect may be underestimated.23,24 In Spain, most providers perform some type of examination, but only for doses of 25mg/kg/day or higher.
The main limitation of this study is that we only sent the survey to the member institutions of the pTBred, which obviously are not the only facilities in Spain that diagnose and treat tuberculosis in children.
However, and despite this limitation, we consider that the results of the survey reflect a reality that must be reviewed, analysed and changed by paediatricians and pharmacists. There are enough clinical practice guidelines in Spain that recommend an appropriate and uniform approach to the management of tuberculosis in children, but they are useless if they are not applied or we cannot ensure adherence to treatment. We calculate the exact doses of antituberculosis agents by kilogram of body weight, but the drugs are administered with no knowledge of the final dose received by the children or their safety as it regards their pharmacokinetics. We also found significant differences in regards to the monitoring of potential side effects and the initiation of DOT.
In conclusion, our survey showed that at present, and despite the available consensus documents, there is variability in the management of tuberculosis in children and the administration of antituberculosis agents in Spain. The Red Española de Estudio de la Tuberculosis Pediátrica has proposed the analysis of the differences found in this survey and the development of a pioneering and practical consensus document on the administration of antituberculosis drugs in children, with particular emphasis on infants and young children that may be unable to swallow solid dosage forms. This proposal of the pTBred has already secured the collaboration of the SEIP and the SENP, and will request the collaboration of the SEFH, the CM-AEP and the Agencia Española de Medicamentos y Productos Sanitarios (Spanish Agency of Medicines and Medical Devices) for the joint development of this document. The goal is to create a consensus guideline for the compounding of suspensions of isoniazid, pyrazinamide and ethambutol, with an emphasis on concentrations of 100mg/mL. Along the same lines, we will study the possibility of developing paediatric dosage forms with fixed doses of two, three or four antituberculosis agents at the doses needed in the paediatric population, titrated based on kilogram of body weight. Last of all, we will review aspects in which the approach of providers across Spain are inconsistent, such as the management of LTBI, the need of DOT to ensure adherence to treatment, or the monitoring of the side effects of first-line antituberculosis agents.
FundingStudy supported by the Comité de Medicamentos de la Asociación Española de Pediatría (Committee on Medicines of the Spanish Association of Pediatrics [CM-AEP]).
The pTBred was awarded the 2013 Research Grant of the AEP. Specific funding was not awarded to this particular study.
Conflicts of interestThe authors have no conflicts of interest to declare.
Validation of the daily unit dose of isoniazid at 10mg/kg of body weight in infants aged less than 3 months. Ref. PI13/01740
Phase IIA open-label clinical trial to study the absorption of a 10mg/mL isoniazid suspension for the treatment of tuberculosis infection in patients aged less than 6 years. Ref. ICI14/00228
Asociación Española de Pediatría. Research Grant 2013.
Province: free text.
Autonomous community: free text.
Hospital: free text.
Number of children with latent TB infection and active TB disease diagnosed in your centre in 2014 (does not include exposure to TB):
None
1–5 children
5–10 children
More than 10 children
Select the combination drugs or FDCs that are widely used in your centre (multiple-choice, multiple answer question):
Combinations of 150mg isoniazid+300mg rifampicin (Rifinah®)
Combinations of 50mg isoniazid+120mg rifampicin+300mg pyrazinamide (Rifater®)
Combinations of 75mg isoniazid+150mg rifampicin+400mg pyrazinamide+275mg ethambutol (Rimstar®)
Other, note which: free text.
Which is the regimen used most commonly in your hospital for the treatment of latent tuberculosis infection?
Isoniazid for 9 months
Isoniazid for 6 months
Isoniazid+rifampicin for 3 months
Rifampicin for 4 months
Other. Which?: free text.
Use of directly observed therapy or treatment monitoring in children:
We always request it for all cases.
We almost always request it, except in select cases in which it is believed that the family will adhere optimally to the treatment.
We requested for approximately 50% of cases.
We only request it when we consider that there are risk factors for poor adherence to treatment.
We never request it.
In regards to the use of ethambutol as the fourth antituberculosis drug (multiple choice, multiple answer):
It is used in specific cases of tuberculosis disease (miliary, meningeal, etc.).
It is used when the drug sensitivity of the strain of the index case is unknown.
It is used routinely in immigrants from regions where the prevalence of isoniazid resistance is greater than 4%.
It is used in all cases, regardless of the sensitivity of resistance of the index case strain.
It is used routinely unless the strain (of the paediatric patient or the index patient) is known to be sensitive to H, R and Z.
It is never used.
Other: free text.
In regards to eye checkups in children aged less than 5 years receiving ethambutol:
We always order them, in all cases.
We almost always order them, but not in all cases.
We order them sometimes, approximately in half of the cases.
We order them only occasionally, for example when doses of 25mg/kg/day are used for treatment of tuberculous meningitis.
We never order them, as there is not enough evidence on the development of optical neuritis in children treated with ethambutol.
In regards to liver enzyme tests:
We never order them, unless the patient develops manifestations compatible with hepatic cytolysis.
We always test before initiating treatment, but not after unless the patient develops manifestations compatible with hepatic cytolysis.
We test before initiation of treatment and periodically thereon.
Other: free text.
Isoniazid
Describe the form(s) in which you usually prescribe isoniazid to children unable to swallow pills (multiple choice, multiple answer):
Compounded preparation of isoniazid 10mg/mL.
Another compounded preparation of isoniazid. At which concentration?: free text.
Isoniazid tablets (50, 150 or 300mg), crushed and mixed with water or juice.
FDC tablets, crushed and mixed with water or juice.
Describe the form in which you most frequently prescribe isoniazid to children unable to swallow pills (multiple choice, single answer):
- –
Compounded preparation of isoniazid 10mg/mL.
- –
Isoniazid compounded preparation (another). At which concentration?: free text.
- –
Isoniazid tablets (50, 150 or 300mg), crushed and mixed with water or juice.
- –
FDC tablets, crushed and mixed with water or juice.
Rifampicin
Describe the form(s) in which you usually prescribe rifampicin to children unable to swallow pills (multiple choice, multiple answer):
Rifaldin® 20mg/mL suspension.
Compounded formulation. At which concentration?: free text.
Rifampicin 300mg capsules, opened and mixed with water or juice.
Rifampicin 600mg tablets, crushed and mixed with water or juice.
FDC tablets, crushed and mixed with water or juice.
Describe the form in which you most frequently prescribe rifampicin to children unable to swallow pills (multiple choice, single answer):
- –
Rifaldin® 20mg/mL suspension.
- –
Compounded formulation. At which concentration?: free text.
- –
Rifampicin 300mg capsules, opened and mixed with water or juice.
- –
Rifampicin 600mg tablets, crushed and mixed with water or juice.
- –
FDC tablets, crushed and mixed with water or juice.
Pyrazinamide
Describe the form(s) in which you usually prescribe pyrazinamide to children unable to swallow pills (multiple choice, multiple answer):
Compounded formulation of pyrazinamide 100mg/mL.
Compounded formulation of pyrazinamide (other). At which concentration?: free text.
Pyrazinamide 250mg tablets, crushed and mixed with water or juice.
FDC tablets, crushed and mixed with water or juice.
Describe the form in which you most frequently prescribe pyrazinamide to children unable to swallow pills (multiple choice, single answer):
- –
Compounded formulation of pyrazinamide 100mg/mL.
- –
Compounded formulation of pyrazinamide (other). At which concentration?: free text.
- –
Pyrazinamide 250mg tablets, crushed and mixed with water or juice.
- –
FDC tablets, crushed and mixed with water or juice.
Ethambutol
Describe the form(s) in which you usually prescribe ethambutol to children unable to swallow pills (multiple choice, multiple answer):
Compounded formulation of ethambutol 50mg/mL.
Compounded formulation of ethambutol (other). At which concentration?: free text.
Coated tablets of ethambutol 400mg crushed and mixed with water or juice.
FDC tablets crushed and mixed with water or juice.
Describe the form in which you most frequently prescribe ethambutol to children unable to swallow pills (multiple choice, single answer):
- –
Compounded formulation of ethambutol 50mg/mL.
- –
Compounded formulation of ethambutol (other). At which concentration?: free text.
- –
Coated tablets of ethambutol 400mg crushed and mixed with water or juice.
- –
FDC tablets crushed and mixed with water or juice.
Availability of compounded preparations in the hospital
Availability of compounded preparations of isoniazid in your centre (multiple choice, multiple answer):
- –
Available at a concentration of 1mL=10mg.
- –
Available at a concentration of 1mL=100mg.
- –
We have compounded preparations at a different concentration. Which?: free text.
- –
No compounded preparation of isoniazid is available.
- –
We do not know.
- –
Available at a concentration of 1mL10mg.
- –
Available at a concentration of 1mL=100mg.
- –
We have compounded preparations at a different concentration. Which?: free text.
- –
No compounded preparation of pyrazinamide is available.
- –
We do not know.
- –
Available at a concentration of 1mL=50mg.
- –
Available at a concentration of 1mL=100mg.
- –
We have compounded preparations at a different concentration. Which?: free text.
- –
No compounded preparation of ethambutol is available.
- –
We do not know.
- –
Water.
- –
Milk.
- –
Juice.
- –
Other: free text.
- –
We do not crush tablets.
- –
We have no preference.
Assuming you use crushed tablets, do you recommend a particular type of splitter/crusher?
- –
Yes.
- –
No.
- –
We do not crush tablets.
In regards to the administration of medication under fasting conditions:
All medications are administered under fasting conditions.
Only rifampicin is administered under fasting conditions.
Medications are not administered under fasting conditions, but with food.
Not administered fasting or with food.
Other: free text.
Do you think that a compounded preparations or FDC with the following combination of antituberculosis agents: 150mg H, 200mg R, 350mg Z±200mg E (or else 75mg H, 100mg R, 175mg Z±100mg E) would be useful in the treatment of tuberculosis in children?
Yes, it would be ideal.
No, FDCs with those concentrations are already available.
There are no FDCs with those concentrations, but the FDCs that are available suffice to adequately treat children.
Please cite this article as: Piñeiro Pérez R, Santiago García B, Fernández-Llamazares CM, Baquero Artigao F, Noguera Julian A, Mellado Peña MJ, et al. El reto de la administración de antituberculosos en lactantes y preescolares. Proyecto Magistral de pTBred. An Pediatr (Barc). 2016;85:4–12.
Previous presentations: This is an original work that has not been previously presented in meetings, conferences or symposia.