Recombinant human growth hormone (rhGH) is the first biosimilar drug approved by the European Medicines Agency in 2006, using the biosimilar registration process. It was authorised for the treatment of growth hormone deficiency, and growth disorders associated with Turner's syndrome, chronic renal failure, Prader-Willi syndrome, and growth disorders in children/adolescents born small for gestational age, and replacement therapy in adults with pronounced growth hormone deficiency.
Materials and methodsThis review is focused on the scientific evidence published about this drug in the last ten years, including the clinical trials on which the approval of the regulatory authority is based, and the most relevant studies evaluating the clinical impact of the drug in clinical practice.
ResultsThe equivalence between biosimilar and original product has been confirmed in the clinical trials published by Romer et al. and López-Siguero et al. Furthermore, studies carried out in real-life conditions confirm its long-term efficacy and safety, as well as the absence of clinical impact by switching treatment from the original to the biosimilar product.
ConclusionThe number of patients receiving this medication has continuously increased since its approval. Its equivalence with the original product has been verified. Preliminary data from the post-authorisation PATRO study confirm the efficacy and safety of the biosimilar product in comparison with data from clinical trials. However, final results must be evaluated at the end of the study, which will provide additional information about the long-term efficacy and safety of the biosimilar drug.
La hormona de crecimiento humana recombinante biosimilar (rhGH) fue el primer medicamento autorizado por la Agencia Europea del Medicamento (EMA), en el año 2006, por la vía de registro biosimilar. Se aprobó su uso para el tratamiento del déficit de hormona de crecimiento, trastorno de crecimiento asociado al síndrome de Turner, trastorno de crecimiento asociado a insuficiencia renal crónica, síndrome de Prader-Willi, trastorno de crecimiento en niños/adolescentes nacidos pequeños para su edad gestacional y como terapia de sustitución en adultos con deficiencia pronunciada de hormona de crecimiento.
Materiales y métodosEsta revisión se centra en las evidencias científicas publicadas en los últimos 10 años, incluyendo los ensayos clínicos utilizados para conseguir la aprobación por parte de la EMA y los estudios más relevantes que evalúan el medicamento en la práctica clínica habitual.
ResultadosLa equivalencia entre este biosimilar de rhGH y su producto de referencia ha sido demostrada en los ensayos clínicos publicados por Romer et al. y López-Siguero et al. Asimismo, los estudios del fármaco realizados en condiciones de práctica clínica habitual confirman su eficacia y seguridad a largo plazo, así como la ausencia de impacto clínico al cambiar el producto original por el biosimilar.
ConclusiónDesde su aprobación, el número de pacientes que reciben esta medicación ha experimentado un continuo crecimiento. Los datos preliminares del estudio postautorización PATRO indican que la eficacia y seguridad del fármaco se correlaciona con la obtenida en los ensayos clínicos. No obstante, aún queda pendiente evaluar los resultados definitivos del estudio que aportarán información adicional sobre la eficacia y seguridad del fármaco a largo plazo.
The European Medicines Agency (EMA) defines biosimilars as biopharmaceutical products that have been approved under a well-defined regulatory pathway and which are equivalent to biological medicines that have already been approved.1
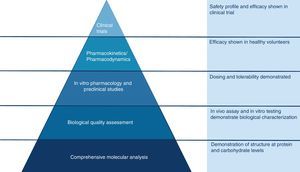
The active substance of a biosimilar medicine is similar to the one of the biological reference medicine, although there are slight differences due to the natural complexity of biological products2 and to the methods employed in their manufacture (Fig. 1).3 For biosimilar medicines to be authorised, it must be proven that any differences between them and their reference medicines do not affect the safety or efficacy of treatment.2
Schema of the development of a biosimilar medicine.
The earliest treatments for patients with growth hormone deficiency (GHD) employed growth hormone extracted from cadaveric human pituitary glands (pit-GH). Although this achieved significant improvements in growth in treated patients, the use of pit-GH caused problems related to Creutzfeldt-Jakob disease. In the late 1980s, a biosynthetic growth hormone (GH) derived from recombinant DNA replaced pit-GH, providing a safe product without the previously observed problems and, being produced by biosynthesis, without the manufacturing restrictions that affected pit-GH, which depended on the availability of human pituitary glands. However, this biosimilar recombinant human growth hormone (rhGH) entailed a substantial increase in treatment cost.4
At present, most countries are facing restrictions in health care spending, and escalating health care costs are largely driven by novel high-cost biopharmaceutical agents. Thus, the main reason for the use of biosimilars is reducing treatment costs and facilitating access to these drugs. The uptake of biosimilars will depend on the degree to which cost savings are required by health care systems and the absolute savings that could be gained by switching from original drugs.1
The rhGH biosimilar was the first biosimilar approved by the EMA, which granted the authorisation in 2006 based on the quality, safety and efficacy demonstrated in bioequivalence studies comparing it to the reference product.1,5–7
Based on the recommendations for the use of rhGH developed by the EMA, the biosimilar rhGH is authorised in Europe for the same indications as the reference product: GHD, growth disorders associated with Turner syndrome (TS), growth disorder associated with chronic renal insufficiency, Prader-Willi syndrome (PWS), growth disorder in children or adolescents born small for gestational age (SGA), and replacement therapy in adults with pronounced GHD.8
Materials and methodsWe performed a review of the available literature on the clinical impact of biosimilar rhGH from its authorisation in 2006 to its current use in everyday clinical practice.
To do so, we performed a literature search in the PubMed database with the following criteria: articles published since 2010, studies in humans, in English or Spanish. We included the published scientific evidence with the highest impact, reviewed the clinical trials performed to obtain the authorisation for the drug, and the most relevant articles on the use of the drug in everyday clinical practice, as well as the post-marketing study on the long-term outcomes obtained with the drug (PATRO study).
We completed the search with the most relevant information on the biosimilar rhGH published by the EMA and the information presented at both the congress of the Sociedad Española de Farmacia Hospitalaria (Spanish Society of Hospital Pharmacy [SEFH]) and the congress of the Sociedad Española de Endocrinología Pediátrica (Spanish Society of Paediatric Endocrinology [SEEP]).
ResultsPre-authorisation studiesIn accordance to the requirements of the EMA for the approval of a biosimilar rhGH, Romer et al.4 carried out a clinical trial that succeeded in achieving the first authorisation for the marketing of a biosimilar after presenting the results of the first 9 months of the trial, although the trial continued for a total followup of 7 years, demonstrating the long-term efficacy and safety of the product.8 Later on, López-Siguero et al.6 conducted another multicentre study, this time in Spain, which confirmed the findings of Romer et al.4 as to the equivalence, efficacy and safety of rhGH.
Study by Romer et al.The study conducted by Romer et al.4 demonstrated the efficacy and safety of the biosimilar rhGH through a phase III open-label multicentre randomised controlled trial. It included 89 treatment-naïve patients with GHD with heights at least 2 standard deviations below the mean for sex and age, and growth velocities at least 1 standard deviation below the mean.4
The study had 3 parts carried out in the same group of patients (Fig. 2). Part 1 compared the efficacy and safety of the biosimilar rhGH, administered in a lyophilised formulation, with those of the reference rhGH. There were no significant differences between the two treatment groups, which allowed the authorisation of the biosimilar.4
Part 2 demonstrated that the response to treatment was not affected by switching from the reference rhGH to the biosimilar. Part 3 lasted 69 months. In this part, treatment was switched from the biosimilar rhGH lyophilisate to the biosimilar rhGH solution. The decision to switch formulations was based on the increased development of anti-GH antibodies associated with the use of the lyophilisate. The study did not find significant differences between groups in any of the auxological parameters under study.4
Treatment with the biosimilar rhGH proved to be as safe as treatment with the reference drug, and there were no significant differences in the incidence, distribution, severity or outcome of adverse events. A total of 323 adverse events associated with the drug were reported during the 84 months of followup. Most of these events were mild to moderate. The main difference in regards to safety was the development of anti-GH antibodies in the first 9 months of treatment with the lyophilised formulation.4
This increase in the development of anti-GH antibodies was likely due to the presence of a higher level of the host cell protein in the lyophilised formulation. Based on these findings, the manufacturing process was modified to add 2 purification steps, which did not change any other of the product characteristics.4
Study by López-Siguero et al.In our country, López-Siguero et al.6 published another of the key studies that confirmed the long-term efficacy and safety of the biosimilar rhGH for the treatment of children with GHD. Like the one by Romer et al.,4 it was a phase III multicentre open-label trial, and it was conducted in Spanish hospitals. It included 70 treatment-naïve patients with GHD and lasted 5 years. In this study, all patients received the biosimilar rhGH in solution, the dose of which was adjusted based on the weight of the patient. The duration of initial treatment was a minimum of 2 years. Participants that completed them continued in treatment when the biosimilar rhGH was introduced in the market, and treatment was discontinued when the patient reached a satisfactory height or epiphyseal fusion had occurred (Fig. 3).6
Treatment with biosimilar rhGH had the greatest impact in the expected final height after 4 years of treatment, as it was 10cm greater than the expected height in untreated individuals with GHD. When it came to safety, the incidence of adverse events was comparable to the one reported by Romer et al.4 Therefore, the authors concluded that long-term treatment with the biosimilar rhGH was well tolerated and that the development of anti-GH antibodies was low and within the range described for other GH therapies.6
Clinical experienceSince the year that the EMA authorised the biosimilar, the number of patients treated with this drug has been increasing. The introduction of this drug in routine clinical practice called for pharmacovigilance studies to support its use.9
Real-world evidence (RWE): PATHH study and switching studiesWhen it comes to the assessment of the impact of the biosimilar in routine clinical practice, it is worth highlighting the PATH5 study conducted in Spain. It was a multicentre, observational, retrospective follow-up study in a subgroup of the patients who participated in the Phase III clinical trial of López-Siguero et al.6 (which we just reviewed above) that assessed the long-term efficacy and safety of the biosimilar, with followup of the patients until they discontinued treatment.5
In every routine visit made by patients, the patient's height was recorded along with any adverse events experienced by the patient. Treatment efficacy was assessed based on height, height z-score and height velocity (in cm/year).5
The results showed that treatment with biosimilar rhGH achieved normalisation of height values, with a mean height velocity during the followup of 11.6cm in female patients and 18.9cm in male patients Thus, the cohort in this followup study showed a positive response to the biosimilar,5 and reached adult heights within the normal range for the Spanish population.10 The study did not report any adverse events related to the biosimilar.5
Several studies in the literature have extrapolated data on the safety and efficacy of the biosimilar based on the outcomes obtained after switching treatments. In 2014, Rashid et al.11 published a retrospective study of routine clinical practice that assessed the effectiveness of switching to the biosimilar in children with GHD, TS or idiopathic short stature. The patients in this study were aged less than 18 years at the time of the switch, had been previously treated with rhGH for a minimum of 15 months and were treated with the biosimilar for at least another 15 months. The data showed that growth rates after the switch remained unchanged relative to the original medication.11
A single-centre study was conducted in Sweden in 2009 to analyse the impact of a change in the treatment plan for patients requiring rhGH. The new protocol consisted of switching treatment with originator rhGH to biosimilar rhGH with the agreement of all involved parties.1
As part of this teamwork approach, all patients were informed about the reasons for the switch. They were also provided with a several resources for information and support in case they had concerns regarding the biosimilar.1
The data obtained after the switch showed the continuity of the effects obtained with the original medication, with no negative impact on growth rate. In addition, there were no unexpected or severe adverse events. This experience suggests that this approach may be helpful when medicines are switched in clinical practice.1
According to the position statement of several Spanish scientific societies, a biological agent should only be switched under the supervision of the responsible prescribing physician.12 Data collected in the context of clinical practice have shown that switching to a biosimilar is efficacious and safe.1,11
PATRO childrenDeveloped in the framework of the risk management plan for the biosimilar rhGH, the PATRO study is a post-marketing study designed to assess the long-term efficacy and safety of the biosimilar medicine in children and adolescents with growth problems treated with this biosimilar in the context of routine clinical practice. It is a multicentre, observational, open-label longitudinal study conducted in 14 countries where the drug has been authorised. The study included patients currently treated with biosimilar rhGH (those previously treated with other type of GH before the biosimilar were also eligible), and treatment was maintained until they reached their adult height. This study is still underway.7
The primary objective of the study was to assess the long-term safety of treatment, with particular emphasis on the diabetogenic potential of rhGH therapy in children born SGA, the occurrence of malignancies in treated patients, respiratory and diabetogenic side effects in treated patients with PWS and the clinical repercussions of anti-GH antibodies. The secondary objective was to assess the long-term efficacy of treatment.7
Early data from the study up to 2012 confirmed the results of previous phase III trials. The study did not find signs of an increase in diabetogenic potential or increased risk of developing tumours or anti-GH antibodies. Furthermore, the results suggested that the drug could be efficacious for treatment of other indications that had not been studied in previous clinical trials, such as PWS, chronic renal insufficiency, children born SGA and TS.7
As of October 2013, participating hospitals in Spain had enrolled 34 patients (19 with GHD, 12 born SGA and 3 with other indications) and there had been no cases of de novo diabetes mellitus, impaired glucose tolerance or changes in fasting glucose levels. Due to the short duration of followup at that time point, it was not possible to assess efficacy yet.13
In the future, the PATRO study, with its large cohort and long duration of followup, will contribute additional data on the diabetogenic potential and risk of malignancy associated with this drug. It will also provide relevant information on the outcome of treatment in patients with PWS as well as the clinical impact of the development of anti-GH antibodies.7
PATRO adultsThe PATRO study in adults, whose design is identical to the PATRO study in children, is also underway and aimed at assessing the safety and efficacy of treatment with biosimilar rhGH in adults in the context of routine clinical practice. Its primary objective is to obtain additional data on the long-term use of the drug with particular emphasis on safety, especially as regards the development of diabetes mellitus and malignant tumours.14
Metabolic syndrome is a significant risk factor for the development of type 2 diabetes.14 Other pharmacovigilance studies conducted in patients treated with rhGH have found a greater prevalence of metabolic syndrome in patients with GHD compared to the general population.15,16 On the other hand, GH and IGF-1 both have mitogenic effects, so the risk of developing malignant tumours is one of the most important aspects to consider when assessing safety, be it in terms of the recurrence of hypothalamic-pituitary tumours or the development of de novo tumours. The studies published to date have not found an increased risk of recurrence of hypothalamic-pituitary tumours in association with GH therapy.14
ConclusionsDue to the global economic situation, governments have needed to cut health care spending, a circumstance that has promoted research and development of biosimilar medicines.1 In 2006, the EMA approved the first biosimilar rhGH, and from this moment, the number of patients treated with this medicine has growth progressively.9
The efficacy and safety of this drug have been assessed in equivalence trials, which demonstrated that they were comparable to those of the reference product and constituted the basis for the application for marketing authorisation submitted to the EMA.4,7
The introduction of the biosimilar in routine clinical practice required performance of pharmacovigilance studies to confirm the conclusions of previous phase III trials and prove that the long-term safety and efficacy of this medicine were the same as those of the reference medicine under real-life conditions.7,9 In this regard, we would like to highlight the PATH study conducted in Spain, which confirmed the efficacy of the biosimilar in children with GHD (the most frequent recipients of this type of drug) with an adequate safety and tolerability comparable to those of the reference medicine.5
Furthermore, the two PATRO observational studies, one in children and one in adults, are currently underway and will provide additional data on the long-term efficacy and safety of this biosimilar in the context of routine clinical practice. They will be particularly relevant in relation to certain aspects for which there is still insufficient data, such as the diabetogenic potential of rhGH therapy, its potential association with tumour development and the clinical impact of anti-GH antibodies. Although these studies have yet to conclude, their preliminary data suggest that the long-term efficacy and safety of the biosimilar are consistent with the data obtained in previous studies, meeting expectations.7,14
FundingThis study has been funded by Sandoz Farmacéutica SA.
Conflicts of interestJPLS and MP have no conflicts of interest to declare. MPG, EMB and FJR are full-time employees of Sandoz Farmacéutica SA.
Please cite this article as: López-Siguero JP, Palla García M, Martínez Busto E, Rebollo FJ, Pombo M. Diez años de experiencia con el primer biosimilar autorizado de hormona del crecimiento recombinante humana en la práctica clínica habitual. An Pediatr (Barc). 2018;88:209–215.