Takayasu arteritis (TA) is a granulomatous large vessel vasculitis that is infrequent in children. It usually has onset with headache, fever, abdominal pain or hypertension (HTN). In rare cases, onset occurs with heart failure, which has only been described in 18% of paediatric cases. There has been recent evidence of the involvement of interleukin-6 (IL-6) in its pathogenesis, whose levels appear elevated in serum and the arterial wall.1
The diagnosis of TA requires the presence of angiographic abnormalities in addition to at least 1 out of 5 criteria (EULAR/PRINTO/PRES criteria, Table 1).2 Although computed tomography angiography (CTA) is the gold standard of imaging, as it allows visualization of blood flow and the extent of collateralization, it does not provide any information about the arterial wall. Thus, a magnetic resonance angiography (MRA) is also useful to assess abnormalities of the vessel wall, and its results correlate to clinical manifestations and inflammatory marker levels. Positron emission tomography/computer tomography (PET-CT) is not indicated for routine assessment, but it may be useful in patients with negative inflammatory markers.2
EULAR/PRINTO/PRES criteria for childhood Takayasu arteritis.
| Angiographic abnormalities (conventional, CT or MR imaging) of the aorta or its main branches and pulmonary arteries showing aneurysm/dilatation, narrowing, occlusion or thickened arterial wall not due to fibromuscular dysplasia or similar causes (mandatory criterion) plus one of the following 5 criteria: |
| 1. Pulse deficit or claudication: lost/decreased/unequal peripheral artery pulses or claudication (focal muscle pain induced by physical activity) |
| 2. Blood pressure discrepancy: discrepancy of four limb systolic blood pressure> 10mmHg difference in any limb |
| 3. Bruits: audible murmurs or palpable thrills over large arteries |
| 4. Hypertension: systolic/diastolic blood pressure greater than 95th percentile for height |
| 5. Acute phase reactants: ESR>20mm per first hour or any value of CRP above normal (according to the local laboratory) |
Treatment is delivered in 2 phases: induction and maintenance. In case of haemodynamic instability, the induction phase consists of steroids delivered by intravenous pulse initially followed by oral administration combined with an immunosuppressive agent (cyclophosphamide or methotrexate).3 The agents used in the maintenance phase include methotrexate, mycophenolate mofetil or azatioprine.3 In recent years, new treatments have been proposed for refractory cases.2,4–6
We present the case of a patient with TA with onset of heart failure that eventually required biological therapy with anti-IL6.
The patient was a girl aged 11 years presenting with vomiting and abdominal pain with onset 10 days prior associated with asthenia of a few weeks’ duration. The physical examination revealed pallor in the skin and mucosae, acral coldness, difficulty breathing, hypoventilation and bibasilar crackles, a gallop rhythm (heart rate, 140 bpm), an oxygen saturation (SatO2) of 90% and hepatomegaly. The left radial and brachial pulses were absent, with a blood pressure (BP) of 80/50 in the left arm (below the 50th percentile) and 140/100 in the right arm (above the 99th percentile). The salient laboratory features were a haemoglobin concentration (Hb) of 9.9g/dL, a platelet count of 636000/mm3 and a C-reactive protein (CPR) level of 20mg/L. The chest radiograph revealed cardiomegaly with signs of acute pulmonary oedema, and the electrocardiogram featured signs of atrial enlargement and left ventricular hypertrophy.
The patient was admitted to the PICU with a diagnosis of acute heart failure and assessed by means of an echocardiogram that revealed severe systolic failure (forced expiratory volume in 1 second [FEV1], 20%) and mild mitral valve regurgitation. Haemodynamic support was initiated with milrinone, diuretics and levosimendan, and respiratory support with BIPAP, to which the patient responded favourably.
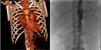
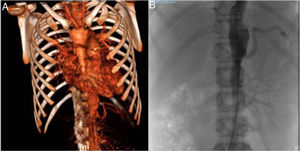
An abdominal ultrasound examination revealed signs of aortitis, and since TA was suspected, the investigation was completed with a CT angiogram (Fig. 1A) that showed thickening of the left subclavian artery with significant stenosis and narrowing at the outlet of the left vertebral artery with collateralization, as well as thickening in segments of the thoracic and abdominal aorta and stenosis of the right renal arteries. Magnetic resonance angiography confirmed the acute involvement of the subclavian and vertebral arteries, and the chronic involvement of the aorta and its branches. The patient received a diagnosis of TA type V and started treatment with boluses of methylprednisolone at a dose of 30mg/kg for 3 days (followed by prednisone at a dose of 2mg/kg/day) and intravenous cyclophosphamide at a dose of 500mg/m2 every 3 weeks, which achieved normalization of laboratory parameters after the second dose. The patient's blood pressure remained above the 99th percentile despite a combination of 6 drugs, so, following performance of an arteriogram (Fig. 1B), the patient underwent a right renal artery angioplasty that allowed the discontinuation of 4 drugs and achieved adequate control of the blood pressure. Two months later, there was evidence of elevation of CRP (46mg/L), the ESR (77mm/h) and serum levels of IL-6 (29.4pg/mL), so after administration of 5 doses of cyclophosphamide, treatment was initiated with anti-IL-6 (tocilizumab iv 8mg/kg every 2 weeks). The patient exhibited a good response after 2 doses, with normalization of laboratory tests and a decrease in the levels of IL-6 (35pg/mL). Twelve months later, the patient was asymptomatic, with a FEV1 70% without treatment with antihypertensive drugs, so, on confirmation of stabilization by imaging, the interval between doses of tocilizumab was increased to 3 weeks.
(A) Computed tomography angiogram. Stenosis of left subclavian and vertebral arteries, abdominal aorta and right renal arteries. (B) Arteriogram of the aorta. Right kidney with renal artery bifurcation and critical stenosis in the ostium, and left kidney with no signs of stenosis of the renal artery.
In addition to the control of HTN, which may be complicated and in some cases require invasive interventions such as renal artery angioplasty,4 the patient must remain under strict monitoring of other cardiovascular risk factors, such as hypercholesterolemia and hypercoagulability. Our patient required rosuvastatin and acetylsalicylic acid.
The challenge in the management of TA is to differentiate between the active and chronic phases, as there are no specific markers of disease activity. The sensitivity and the specificity of CRP and ESR values are low, so other biomarkers are currently being investigated, such as matrix metalloproteinases (MMPs), vascular cell adhesion proteins (VCAM), the inverted CD4/CD8 ratio and pentraxin 3.5 However, there is evidence of a correlation between the levels of IL-6 and disease activity, which suggests that this cytokine may be a useful target for treatment.1,5,6
Although conventional treatment continues to be widespread, several case series have been published that demonstrate the effectiveness of biological agents such as anti-TNF-α (infliximab/adalimumab) or anti-IL6 (tocilizumab).4–6 These studies have reported a mean time to resolution of symptom of 3 months in the absence of severe side effects, in addition to allowing a reduction of the steroid dose.
Please cite this article as: Cubiles Arillo Z, Núñez Cuadros E, Martínez Rivera V, González Gómez JM, Cuenca Peiró V. Arteritis de Takayasu de presentación atípica. Tocilizumab como alternativa terapéutica. An Pediatr (Barc). 2019;91:411–413.