Surgical closure of patent ductus arteriosus in premature neonates is an aggressive technique and is not free of complications. A study was designed with the aim of describing our experience with a less invasive technique, the extra-pleural approach via a posterior minithoracotomy, and to compare the results with the classic transpleural approach.
Patients and methodsA retrospective cohort study was conducted on premature neonates on whom surgical closure of the ductus was performed during a ten-year period (March 2005 to March 2015). A comparison was made of the acute complications, the outcomes on discharge, and follow-up, between the extra-pleural approach and the classic transpleural approach. The study included 48 patients, 30 in the classical approach and 18 in the extra-pleural group.
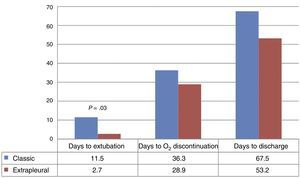
ResultsThe demographic and pre-operative characteristics were similar in both groups. No differences were found between the 2 groups in the incidence of acute post-operative complications (56.6 vs. 44.4%), on the dependence on oxygen at 36 weeks (33.3 vs. 55.5%), or in hospital mortality (10 vs. 16.6%). As regards the short-term progress, the extra-pleural group required fewer days until the withdrawal of supplementary oxygen (36.3 vs. 28.9) and until hospital discharge (67.5 vs. 53.2), although only the time until extubation achieved a statistically significant difference (11.5 vs. 2.7, p=.03).
ConclusionsThe extra-plural approach by posterior minithoracotomy for the surgical closure of ductus in the premature infant is viable and could bring some clinical benefits in the short-term.
El cierre quirúrgico del ductus arterioso persistente en el prematuro es una técnica agresiva y no exenta de complicaciones. Diseñamos un estudio con el objetivo de describir nuestra experiencia con una técnica menos invasiva, el abordaje extrapleural vía minitoracotomía posterior, y de comparar sus resultados con los del abordaje clásico transpleural.
Pacientes y métodosEstudio de cohortes retrospectivo de los neonatos prematuros a los que se les realizó cierre quirúrgico del ductus en un periodo de 10 años (marzo de 2005-marzo de 2015). Se compararon las complicaciones agudas, los resultados al alta y en el seguimiento entre los grupos de abordaje extrapleural y abordaje clásico transpleural. Se incluyó a 48 pacientes, 30 en el grupo de abordaje clásico y 18 en el grupo extrapleural.
ResultadosLas características demográficas y preoperatorias fueron similares en ambos grupos. No se encontraron diferencias entre los 2 grupos en la incidencia de complicaciones postoperatorias agudas (56,6 vs. 44,4%), en la dependencia de oxígeno a las 36 semanas (33,3 vs. 55,5%), ni en la mortalidad hospitalaria (10 vs. 16,6%). En la evolución a corto plazo, el grupo extrapleural precisó menos días hasta la retirada del oxígeno suplementario (36,3 vs. 28,9) y hasta el alta hospitalaria (67,5 vs. 53,2), aunque solo el tiempo hasta la extubación alcanzó una diferencia estadísticamente significativa (11,5 vs. 2,7, p = 0,03).
ConclusionesEl abordaje extrapleural por minitoracotomía posterior para el cierre quirúrgico del ductus en el prematuro es factible y podría conllevar algunos beneficios clínicos a corto plazo.
Patent ductus arteriosus (PDA) is very prevalent in preterm newborns: it is found in 30% of those born at less than 30 weeks’ gestation and more than 60% of those born at less than 28 weeks’ gestation.1 The treatment approaches that are currently available are: (1) conservative treatment, (2) pharmacological treatment with cyclooxygenase inhibitors and (3) surgical repair.2,3 Although there is variability in treatment protocols and some degree of controversy on many aspects related to the management of PDA, surgical repair is usually reserved for patients in whom pharmacological treatment is ineffective or contraindicated. Surgery is a fairly aggressive intervention in patients as fragile as preterm infants, and is not free of significant complications. Furthermore, in recent years concerns have been raised concerning the potential deleterious effects of surgical closure on respiratory and neurologic outcomes.2 Thus, there is still no consensus in the medical community on the indications for surgical closure or the optimal time window for its performance.1,4–6 In this context, technical modifications aimed at reducing the aggressiveness of surgical intervention are particularly appealing. Approaches that are supposedly less invasive have been developed, such as video-assisted thoracoscopic surgery,7,8 percutaneous closure with different devices,9 posterior minithoracotomy,10 transversal cervicotomy11 and extrapleural (EP) ligation.10,12,13 The latter offers potential advantages compared to the classic transpleural (TP) approach: (1) excellent visualisation of the PDA and associated structures, including the recurrent laryngeal nerve, (2) preservation of the integrity of the pleural space and (3) does not require insertion of a chest tube.10,12,13 The EP approach has been associated to shorter operative times and some clinical benefits,10,12,13 but to our knowledge there have been no studies comparing it to the classic TP approach in the management of PDA in preterm infants.
The aim of our study was to describe our experience in the surgical management of PDA and to compare the outcomes of the classic TP approach with those of the new EP approach with posterior minithoracotomy.
Patients and methodsWe conducted a retrospective observational cohort study, including all preterm infants that underwent surgical closure of PDA between March 2005 and March 2015. We excluded patients that underwent PDA ligation as part of a more complex surgical repair and those that had surgery after discharge from hospital. During the period under study, catheter-based percutaneous PDA closure was performed in two patients, who were also excluded from the study. The study protocol was approved by the competent Clinical Research Ethics Committee.
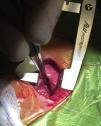
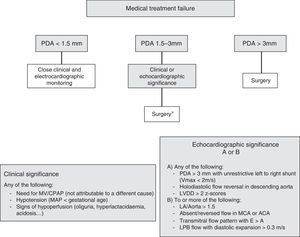
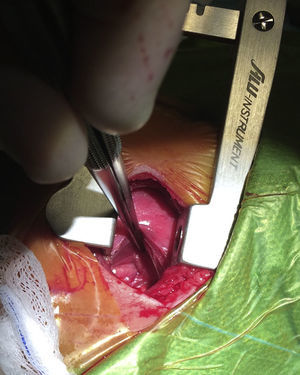
The indication for PDA ligation was determined based on clinical and echocardiographic criteria. Fig. 1 presents a flow chart of the local protocol for the management of PDA with unsuccessful pharmacological closure. All surgeries were performed at the Neonatal Intensive Care Unit by the same surgical team, under general anaesthesia and with mechanical ventilation. This is a level IIIC unit and is the regional reference unit for cardiac surgery in an area with approximately 20 000 deliveries a year in 15 different maternity units. The EP approach was performed by posterior minithoracotomy through the 4th intercostal space. The skin incision extended from below the tip of the scapula, with the patient lying with the arm extended over the head, for approximately 2cm along the superior costal facet in a posterior direction towards the spine. The pleura was dissected and separated from the chest wall without piercing it, with moderate retraction of the left lung, until the PDA became exposed (Fig. 2). The left hemiazygos vein was detached from the surrounding tissue, identifying and sparing the recurrent laryngeal nerve. Finally, the PDA was occluded with a titanium clip. Closure of the chest and lung re-expansion were performed following conventional procedure, excluding placement of a chest tube for drainage.
Flowchart of the protocol implemented in the study setting for the management of patent ductus arteriosus (PDA) in which medical treatment was indicated and was not effective. ACA, anterior cerebral artery; LA, left atrium; LPB, left pulmonary branch; LVVD, left ventricular diastolic diameter; MAP, mean arterial pressure; MCA, middle cerebral artery; MV, mechanical ventilation. *In select cases, percutaneous closure with a device was contemplated. In recent years, serial measurements of NT-ProBNP were used as an additional marker of significance.
We collected data by reviewing electronic health records, surgery reports and echocardiographic images. We included baseline demographic characteristics, preoperative variables, acute complications and outcomes during the followup. The acute complications included pneumothorax, necrotising enterocolitis, chylothorax, post ligation cardiac syndrome and intraventricular haemorrhage. Post ligation cardiac syndrome was defined as the need for inotropic support in the first five days post surgery. We created a composite outcome variable defined by the presence of any of the aforementioned acute complications. The followup variables were mortality in the first seven days post surgery and at discharge, time to extubation in days, time to discharge in days, duration of continuous intravenous analgesia, need for supplemental oxygen at 36 weeks’ corrected age, and need for supplemental oxygen in the home.
We estimated that a sample size of 50 patients (30 in the TP group and 20 in the EP group) would make differences of 50% to 15% in the incidence of postoperative complications—composite outcome variable—statistically significant, with a power of 80% and a 95% confidence interval.
We have summarised quantitative variables as mean±standard deviation and median and interquartile range. We used the chi square test or Fisher's exact test as appropriate to compare discrete variables, and the Mann–Whitney U test for continuous variables. We performed the statistical analysis with the software SPSS 19.0 for Windows. We defined statistical significance as a p-value of less than 0.05 in any of the tests.
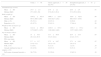
ResultsDuring the period under study, 48 patients underwent surgical closure of PDA, 30 via a conventional transpleural approach (TP group) and 18 via a posterior minithoracotomy extrapleural approach (EP group). Both groups had comparable demographic and preoperative baseline characteristics (Table 1).
Demographic and preoperative characteristics.
| Total, n=48 | Classic approach, n=30 (62.5%) | Extrapleural approach, n=18 (37.5%) | p | |
|---|---|---|---|---|
| Gestational age (weeks) | ||||
| Mean±SD | 27.9±2.5 | 27.9±2.2 | 28.0±2.9 | .91 |
| Median (IQR) | 27.5 (26.3–29) | 27.6 (26.3–29.2) | 27.2 (25.6–28.6) | |
| Birth weight (g) | ||||
| Mean±SD | 1045.2±363.8 | 1066.3±325.4 | 1010±428.1 | .60 |
| Median (IQR) | 947.5 (800–1260) | 1010 (827–1282) | 890 (748.7–1070) | |
| Male, n (%) | 25 (52.1) | 16 (53.3) | 9 (50.0) | .82 |
| Ibuprofen, n (%) | 42 (87.5) | 25 (83.3) | 17 (94.4) | .26 |
| Weight at time of surgery (g) | ||||
| Mean±SD | 1221.2±408.8 | 1244.0±433.3 | 1183.3±373.2 | .62 |
| Median (IQR) | 1170 (882.5–1455) | 1180 (880–1480) | 1137.5 (882–1295) | |
| Age at surgery (days) | ||||
| Mean±SD | 31±54.3 | 33.7±68.0 | 26.4±15.6 | .65 |
| Median (IQR) | 20 (14.2–29.7) | 19 (15–26.2) | 22 (14–33.7) | |
| PDA size (mm) | ||||
| Mean±SD | 2.7±0.7 | 2.75±0.87 | 2.71±0.61 | .87 |
| Median (IQR) | 2.8 (2–3.2) | 2.6 (2–3.5) | 2.8 (2–3) | |
| IVH, n (%) | 5 (10.4) | 4 (13.3) | 1 (5.5) | .39 |
| Already intubated at time of surgery, n (%) | 22 (45.8) | 16 (53.3) | 6 (33.3) | .17 |
| Full course of prenatal steroids, n (%) | 36 (75.0) | 21 (70.0) | 15 (83.3) | .30 |
IQR, interquartile range; IVH, intraventricular haemorrhage; PDA, patent ductus arteriosus; SD, standard deviation.
Surgery achieved PDA closure in all cases, and none of the patients experienced ductal reopening after surgery. Table 2 summarises the clinical outcomes in the immediate postoperative period and during the followup. We did not find statistically significant differences in the incidence of acute complications, mortality or the need for supplemental oxygen at 36 weeks. For the overall sample, the time to extubation was 8.3±13.0 days, the time to discontinuation of supplemental oxygen was 33.7±26.3 days, and the time to hospital discharge was 62.1±46.7 days. All three times were shorter in the EP group compared to the TP group, although only the difference in the time to extubation was statistically significant: 11.5±15.4 in the classic TP group (median, 5; interquartile range, 3–17.5) vs 2.7±2.2 in the EP group (median, 2; interquartile range, 1–3.75), with a p-value of 0.03 (Fig. 3). The duration of intravenous analgesia was 67.1±105.3 hours, with no difference between the groups: 66.6±131.1 vs 68.0±36.8 hours. None of the patients developed stridor, dysphonia or any other manifestations indicative of recurrent laryngeal nerve damage during their hospital stay.
Postoperative outcomes.
| Total, n=48 | Classic approach, n=30 (62.5%) | Extrapleural approach, n=18 (37.5%) | p | |
|---|---|---|---|---|
| Acute complications n (%) | ||||
| Pneumothorax | 2 (4.2) | 2 (6.6) | 0 (0) | .52 |
| Enterocolitis | 1 (2.1) | 1 (3.3) | 0 (0) | .99 |
| Post ligation cardiac syndrome | 23 (47.9) | 15 (50.0) | 8 (44.4) | .70 |
| IVH | 1 (2.1) | 1 (3.3) | 0 (0) | .38 |
| Chylothorax | 0 (0.0) | 0 (0) | 0 (0) | – |
| Composite outcome | 25 (52.1) | 17 (56.6) | 8 (44.4) | .41 |
| Follow up n (%) | ||||
| Oxygen at 36 weeks, N=43 | 20 (46.5) | 10 (33.3) | 10 (55.5) | .10 |
| Oxygen at home, N=42 | 5 (11.9) | 2 (6.6) | 3 (16.6) | .22 |
| Death by 7 days | 2 (4.2) | 1 (3.3) | 1 (5.5) | .70 |
| Death before discharge | 6 (12.5) | 3 (10.0) | 3 (16.6) | .49 |
IVH, intraventricular haemorrhage.
Although there is no consensus on the management of PDA in preterm infants, surgical repair continues to be a significant and widely used treatment option.14,15 With the purpose of minimising the negative impact of surgery and potentially improve outcomes, new minimally invasive techniques have been developed in recent years, as well as alternative approaches with video-assisted thoracoscopy or with percutaneous incisions.16 However, very few studies have compared these new modalities with the classic transpleural approach. In this study, we studied the clinical characteristics and outcomes of 48 patients that underwent surgical repair of PDA, comparing one group operated with the conventional approach and another group operated with an EP approach via posterior thoracotomy. The postoperative complication outcomes were similar in both groups, but the patients in the EP group showed a tendency towards shorter times to discharge and discontinuation of supplemental oxygen and a statistically significant reduction in the time to extubation (11.5 vs 2.7 days; p=.03).
The EP approach for PDA ligation has been described previously and associated to a series of a priori advantages over the classic approach, such as not requiring a chest drainage tube; improved exposure of the PDA, recurrent laryngeal nerve and thoracic duct; a lower cost and some clinical benefits.10,12,13 Vicente et al. described an extrapleural approach to PDA ligation via dorsal minithoracotomy in 52 preterm infants,10 as did Demirturk et al. in 24 patients.13 Both series demonstrate that the EP approach is feasible and safe, but included very limited descriptions of the postoperative outcomes, and none of them included a comparison group. In our cohort, the EP approach to PDA closure was practised in a group of preterm infants with a mean weight at the time of surgery of 1183.3±373.2g. During the followup, 10 patients (55.5%) in the EP group still needed supplemental oxygen at 36 weeks, and three patients (16.6%) required oxygen at home. Only one patient died in the first seven days post surgery: an infant born preterm at 26 weeks’ gestation that had nosocomial sepsis with onset at the time of the surgery. We found no differences between the EP and the TP groups in the postoperative incidence of enterocolitis, intraventricular haemorrhage, need for supplemental oxygen at 36 weeks, need for supplemental oxygen at home, or mortality.
In our series, a chest tube for drainage was not placed in any of the patients in the EP group, which may have improved comfort during the postoperative period and decreased costs. We ought to note that complications such as hemithorax, chylothorax or pneumothorax were not documented in this group. The EP approach was implemented through a left posterior minithoracotomy, which has been associated with improved aesthetic outcomes and fewer complications in previous studies.10,12,17,18
When it comes to short-term respiratory outcomes, previous studies on PDA closure have found an improvement in lung compliance.19 In fact, the times to extubation and to discontinuation of supplemental oxygen in our cohort were relatively short considering the high morbidity found in this population: 8.3±13.0 and 33.7±26.3 days respectively. In a cohort study of 197 patients with baseline characteristics comparable to those of our patients, Raval et al. reported a mean time to extubation following PDA closure of 27 days.20 It is worth mentioning that in the same study, early extubators were defined as patients extubated before day 10 post surgery. In our cohort, all patients in the EP group were extubated before day 10, with a mean and standard deviation of 2.7±2.2 days. Furthermore, there was a clear trend towards shorter times to discharge and to discontinuation of oxygen in patients in the EP group, and we even found a statistically significant difference in the time to extubation between the two groups (Fig. 3).
However, these favourable short-term outcomes were not associated with an improvement in chronic lung disease markers, and there was no difference between groups in the need for supplemental oxygen at 36 weeks. The effect of PDA ligation on the subsequent development of bronchopulmonary dysplasia (BPD) has been debated extensively in the past few years. The incidence of BPD, defined as the need for supplemental oxygen at 36 weeks, was 46.5%, which is high considering the expected incidence in the total population of infants with birth weights of less than 1500g.21 However, when we compared it to the incidence reported in other series of patients with surgically closed PDA, it was clearly lower.22,23 Kang et al. reported an incidence of BPD of 88% in a series of 102 preterm infants that underwent PDA ligation,22 and Lee et al. reported an incidence of 77% in 87 infants that underwent PDA ligation.23
In this study, the comparison of the TP and EP groups did not reveal any differences in the incidence of acute postoperative complications, although it must be taken into account that sample size limited the power of the analysis, so that differences of less than 35% in the incidence of the composite outcome could not be detected. Similar to the findings of other case series,5,24 nearly half of the patients required inotropic support in the postoperative period, a phenomenon referred to as post ligation cardiac syndrome in the literature. The pathophysiology of this syndrome has yet to be fully elucidated, but it probably involves acute changes during and following ventricular loading. Therefore, we would not expect simple changes in the surgical approach to have a significant impact on this outcome.
There are various limitations to this study. The main one concerns its sample size and design. The limitations intrinsic to retrospective cohort studies carry a risk of selection bias, so that any differences found may be due to changes taking place during the period under study other than the surgery itself (in ventilatory support strategies, sedation and analgesia protocols, target oxygen saturation ranges, etc.). This is a possibility, even though the baseline characteristics of both groups were comparable and all surgeries were conducted by the same team in the same unit. Given the power that can be attained with this sample size, we undertook the study as an initial exploration of bivariate associations, and did not fit logistic regression models.
In conclusion, the EP approach via posterior minithoracotomy for PDA closure in preterm infants is feasible and safe, and may offer advantages in short-term outcomes compared to conventional surgery. We believe that the continuous updating of professional skills and the use of new strategies aimed at reducing the aggressiveness of invasive interventions in PDA are an essential element in the management of this disease.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Avila-Alvarez A, Serantes Lourido M, Barriga Bujan R, Blanco Rodriguez C, Portela-Torron F, Bautista-Hernandez V. Cierre quirúrgico del ductus arterioso persistente del prematuro: ¿influye la técnica quirúrgica en los resultados?. An Pediatr (Barc). 2017;86:277–283.