The suspected allergy to beta-lactam antibiotics, especially penicillin and amoxicillin, is the most frequent reason for consultation in Child Allergy Units. In this consensus document, the clinical and diagnostic criteria of allergic reactions are described, as well as alternative antibiotic treatment for the most common infections diagnosed in paediatrics for patients with known or suspected allergy.
La sospecha de alergia a antibióticos betalactámicos, especialmente penicilina y sobre todo amoxicilina, suponen el motivo de consulta más frecuente en las Unidades de Alergia Infantil. En este documento de consenso se describe la clínica y los criterios diagnósticos de las reacciones alérgicas, así como el tratamiento antibiótico alternativo de las infecciones más habituales en pediatría, para los pacientes con sospecha diagnóstica o confirmación de la alergia.
Beta-lactam antibiotics account for approximately 80% of visits to paediatric specialty clinics due to allergies to medication. Penicillins are the most frequent family involved, of which aminopenicillins (amoxicillin) are currently the leading group.1–3
The prevalence of self-reported beta-lactam allergy in children ranges from 1.7% to 5.2%,4,5 although only a minority of these children (<20%) receive an actual diagnosis of allergy.6 Anaphylactic reactions occur in 0.01–0.05% of the population, and they are rare and usually less severe in children.1
When penicillin is metabolised, different allergenic determinants are released, such as benzylpenicilloyl (95% of the total), which is the major determinant and is responsible for most allergic reactions, and minor determinants that cause a minority of reactions, which can nevertheless be just as severe.2,3
Semisynthetic penicillins (ampicillin, amoxicillin) also produce unique antigenic determinants located in their side-chains.1,2
The most frequent reactions are selective responses to amoxicillin (its side chain) in the absence of penicillin allergy. When the allergic reaction occurs in response to the major determinant of penicillin, all penicillins are involved.7
Suspicion of beta-lactam allergyImmediate allergic reactions, which manifest with urticaria, angioedema, bronchospasm or laryngeal oedema within an hour from antibiotic administration, are easy to recognise. However, many reactions may be delayed (occurring hours or days after administration) and present only with nonpruritic maculopapular or morbilliform rashes.1
The prevalence of viral infections that cause rashes is high in children, so it is important to differentiate between these two conditions. They are most commonly confused in cases of roseola (which may present with maculopapular rash or hives, or even with eyelid oedema [Berliner sign]), infectious mononucleosis (due to the frequent development of bilateral eyelid oedema and of rash following administration of amoxicillin) and infectious urticaria. The rational use of antibiotics, avoiding their administration in cases of fever without source or pharyngotonsillitis with negative results in the rapid test for streptococcus, is the best strategy to avoid such confusions. Adverse reactions that are not immune-mediated, such as vomiting or diarrhoea developed during treatment, should not be considered allergic reactions.
Taking an accurate history is of the essence when allergy to penicillin or amoxicillin is suspected, with a thorough investigation of the clinical manifestations, the time elapsed since antibiotic administration, and the previous use of the drug or other drugs with a similar antigenic structure. The child (regardless of age) must be referred to the allergy clinic.
During the evaluation, the suspected drug and other drugs with which it may be cross-reactive will be avoided.1–3,7 The suspected allergy to the involved drug must be visibly noted in the chart of the patient, removing the flag once it is ruled out.8
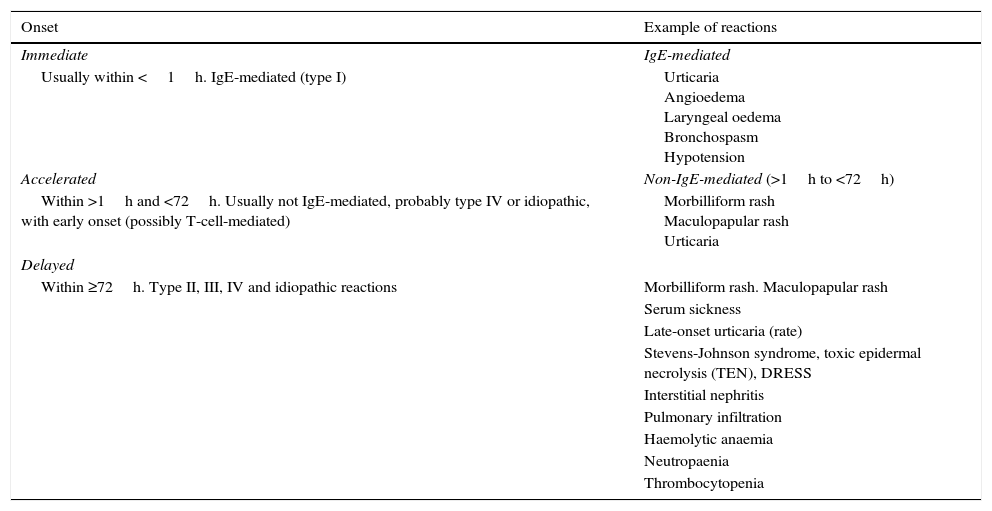
Types of reactionsAt present, reactions are classified into immediate and non-immediate1–3,9–11 for the purposes of clinical assessment and determining the underlying mechanism (Table 1).
Classification of allergic reactions to beta-lactam antibiotics based on the timing of onset.
| Onset | Example of reactions |
|---|---|
| Immediate | IgE-mediated |
| Usually within <1h. IgE-mediated (type I) | Urticaria Angioedema Laryngeal oedema Bronchospasm Hypotension |
| Accelerated | Non-IgE-mediated (>1h to <72h) |
| Within >1h and <72h. Usually not IgE-mediated, probably type IV or idiopathic, with early onset (possibly T-cell-mediated) | Morbilliform rash Maculopapular rash Urticaria |
| Delayed | |
| Within ≥72h. Type II, III, IV and idiopathic reactions | Morbilliform rash. Maculopapular rash |
| Serum sickness | |
| Late-onset urticaria (rate) | |
| Stevens-Johnson syndrome, toxic epidermal necrolysis (TEN), DRESS | |
| Interstitial nephritis | |
| Pulmonary infiltration | |
| Haemolytic anaemia | |
| Neutropaenia | |
| Thrombocytopenia | |
Adapted from Lagace-Wiens and Rubinstein.9
Immediate reactions: develop immediately following antibiotic use, generally within an hour. They are IgE-mediated reactions, can progress rapidly, are potentially fatal and tend to increase in severity with repeated exposure.1 They include urticaria, angioedema and anaphylaxis with its most severe features (laryngeal oedema, bronchospasm and hypotension).
Non-immediate reactions: they develop after a variable period of time that ranges between hours and days. They include accelerated reactions (>1 to <72h) and delayed reactions (days to weeks), and manifest with urticaria and rashes that are usually not IgE-mediated and more rarely with severe presentations such as Stevens–Johnson syndrome, toxic epidermal necrolysis (TEN) or drug reaction with eosinophilia and systemic symptoms (DRESS). Other manifestations that have been associated with these reactions are erythroderma, acute generalised exanthematous pustulosis, haemolytic anaemia, interstitial nephritis and vasculitis.
Most non-immediate reactions present with nonpruritic morbilliform and maculopapular rashes. They develop in 3–7% of children that take amoxicillin.1,6,10 These reactions are usually mediated by T-cells (type IV hypersensitivity).10 Some of the children that develop these rashes will have another reaction when they take the antibiotic again, and these are the patients that are truly allergic; the rest (a majority) will tolerate it well.
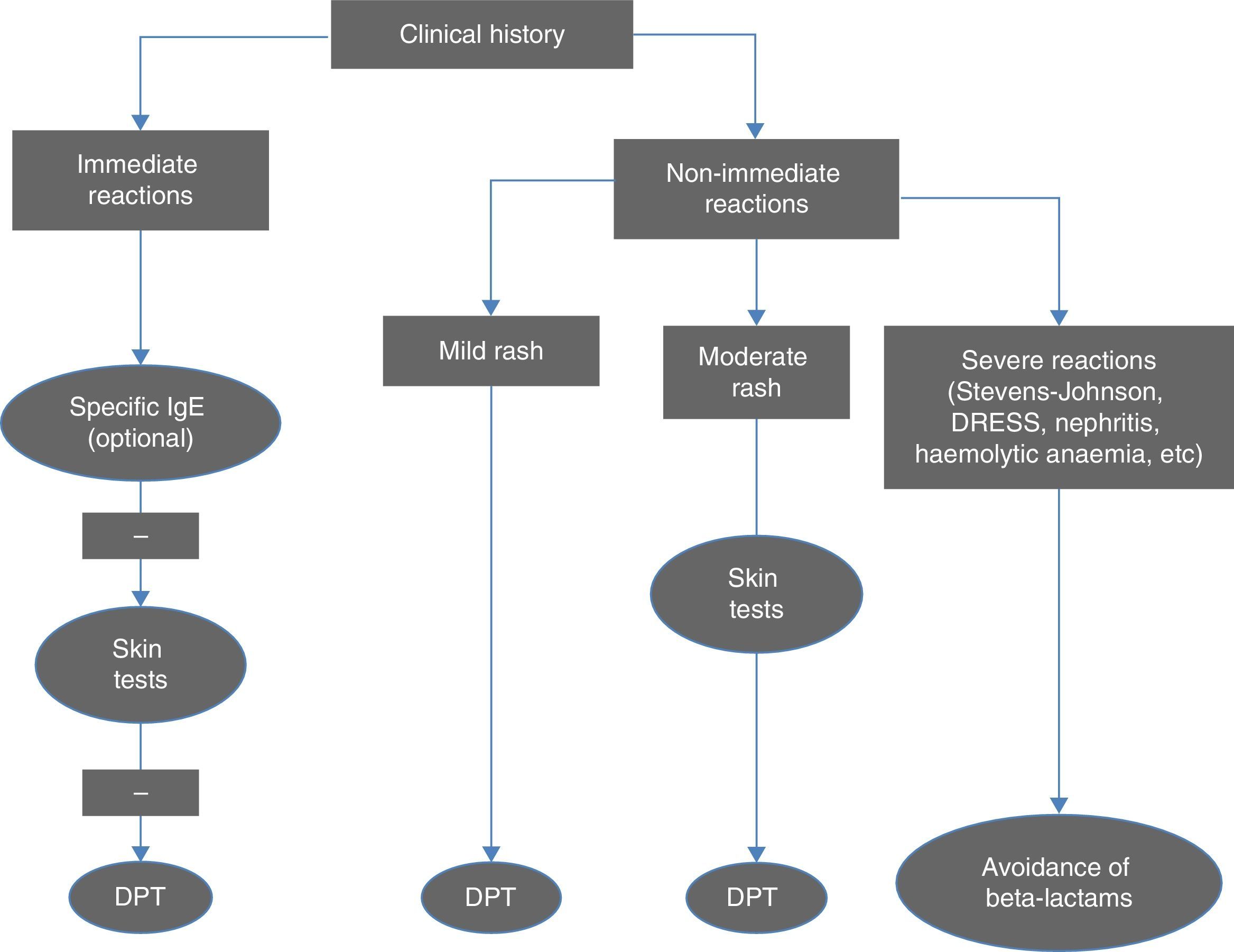
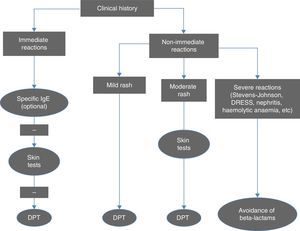
Diagnostic criteriaFig. 1 summarises the diagnostic algorithm for cases of suspected allergy to beta-lactam antibiotics. The clinical history is essential in guiding the decision of which tests to perform. Serology tests for IgE antibodies specific to amoxicillin, ampicillin and penicillin V and G are particularly relevant in cases of anaphylactic reaction, and should be performed before skin tests (STs).1 The cut-off point for positive reactions is 0.35kU/L and levels above 0.1 are indicative of sensitisation. In order to reduce the likelihood of obtaining a false negative, serology tests should not be performed until four to six weeks after the reaction, and ideally should be done before a year has elapsed. Their sensitivity is low (except in cases of anaphylactic shock), but their specificity is high. Antibody levels decrease over time and may become undetectable in half of the patients when two or three years have passed since the reaction.
Algorithm for the diagnosis of immediate and non-immediate reactions to beta-lactam antibiotics. DPT, drug provocation test. Adapted from Fernández et al.10
Skin tests (skin-prick and intracutaneous tests) have a very high specificity for the diagnosis of immediate reactions to beta-lactam antibiotics, and an acceptable sensitivity, which reaches up to 80% when combining the major and minor determinants in penicillin and amoxicillin, with intracutaneous tests giving positive results more often than skin-prick tests.10 The results of STs also become negative over time, even in cases of persistent allergy. The negative predictive value of STs for beta-lactams in immediate reactions is high (94%), and in patients with false negative results, the reactions that develop in challenge tests are not severe.10
In delayed reactions, which are more frequent in children, STs (delayed reaction in intracutaneous and patch tests) offer a low yield, and a drug provocation test (DPT) is required to confirm the diagnosis. More than 95% of children with non-immediate reactions are diagnosed by means of a DPT. These data support the option of proposing quicker diagnostic protocols for cases considered to be low-risk.12,13
Drug provocation tests are performed following negative allergy tests either to confirm or rule out the diagnosis, or to confirm tolerance to an alternative drug other than the one that caused the reaction. Based on clinical experience, these tests are positive in 7–10% of suspected cases.1,3,6
Routine performance of STs and DPTs is not recommended in cases of severe delayed reactions (haemolytic anaemia, interstitial nephritis, TEN, Stevens-Johnson syndrome, DRESS), and the use of beta-lactam antibiotics should be avoided indefinitely in these patients.
Bacterial drug resistance in the most common paediatric community-based infectionsRespiratory tract infectionBacterial pharyngotonsillitis is usually caused by Streptococcus pyogenes. This bacterium is not resistant to beta-lactam antibiotics, but between 5% and 20%14,15 of the strains isolated in children are resistant to macrolides. Unlike what happens with Streptococcus pneumoniae, of which nearly all strains exhibit the MLS-resistance phenotype (with cross-reactivity to all macrolides and clindamycin), 60% of S. pyogenes isolates have an M phenotype14 (resistant to 14- and 15-atom macrolides, but not 16-atom macrolides or clindamycin), and josamycin, midecamycin or clindamycin are the drugs of choice if beta-lactam antibiotics cannot be used.
S. pneumoniae, nontypeable Haemophilus influenzae and Moraxella catarrhalis are the main pathogens involved in pneumonia, otitis media and sinusitis. Antimicrobial resistance in S. pneumoniae exhibits geographical and ecological variations depending on circulating serotypes that are also affected by the commercialization of pneumococcal vaccines.15–18
While third-generation cephalosporins (cefotaxime/ceftriaxone) delivered by the parenteral route are active against these three bacteria, the activity of oral cephalosporins varies depending on the group. We can divide oral cephalosporins into two main groups: “old” or first-generation cephalosporins (cefadroxil, cephalexin) and the so-called “advanced-generation” cephalosporins, which include three subgroups: I (cefachlor, cefuroxime), II (cefixime, ceftibuten) with activity against enterobacteria but not against methicillin-sensitive staphylococci (MSSA), and III (cefpodoxime, cefditoren), which are useful against respiratory pathogens and MSSA.19H. influenzae and M. catarrhalis are highly susceptible to oral cephalosporins. Conversely, in S. pneumoniae, penicillin resistance (25–30% of isolates with intermediate resistance)20 influences the response to cephalosporins, with cefixime, cefachlor and cefuroxime showing the least activity and cefpodoxime and cefditoren being most efficacious.21,22 In serotype 19A, the most prevalent of the resistant pneumococcus serotypes currently circulating in Spain (although it may disappear or decrease with vaccination), the susceptibility rate of strains that have intermediate resistance to penicillin is 33.8% for cefuroxime, 47% for cefpodoxime, 96% for cefotaxime and 100% for cefditoren.23 Although cefditoren has the highest activity of all cephalosporins against S. pneumoniae (including strains with high-level resistance to penicillin),24 its use is not approved in children aged less than 12 years.
Macrolides (erythromycin, azithromycin, clarithromycin) are active against H. influenzae and M. catarrhalis, while in Spain there has been a progressive decline in the rates of resistance of S. pneumoniae from 49% to 20%.25 The percentage of pneumococcal isolates resistant to levofloxacin has remained stable in Spain (2.3%),26 and the rates of susceptibility to levofloxacin in serotype 19A penicillin-intermediate and penicillin-resistant strains are 99.5% and 100%, respectively.23
Skin infectionsStaphylococcus aureus and S. pyogenes are the two bacteria most frequently involved in skin and soft-tissue infections.
Methicillin-sensitive S. aureus strains are also sensitive to cephalosporins except the cefixime/ceftibuten group. Methicillin resistance (found in approximately 10% of community-acquired infection isolates) confers cross-resistance to all beta-lactam antibiotics except ceftaroline.27 These strains are generally susceptible to clindamycin and trimethoprim-sulfamethoxazole.27,28 Other effective antibiotics include rifampicin, fusidic acid and fosfomycin, but they should not be used as monotherapy as resistant strains could easily undergo selection.
Urinary tract infectionEighty-five percent of urinary tract infections are caused by enterobacteria, especially by Escherichia coli. The resistance profile of enterobacteria exhibits variations due to multiple factors, such as the type of sample, the type of patient or geographical region, so prior knowledge of local resistance rates is needed to make empiric treatment recommendations for infections caused by these microorganisms.29 Resistance to third-generation cephalosporins (especially through production of extended-spectrum beta-lactamases [ESBLs]) is found in 5–12% of isolates.25 The percentage of E. coli isolates resistant to gentamicin is 15%.25 Fosfomycin is a good alternative for the management of lower urinary tract infection in outpatient settings,30 as more than 90% E. coli isolates are susceptible to it, even among ESBL-producing strains.
Empiric antibiotherapy in children allergic to penicillin/amoxicillinImmediate allergic reactionAvoiding cephalosporins is recommended,10,31 even though the risk of cross-reactivity is less than 10% and probably closer to 0.5%.32 However, since type I hypersensitivity reactions can be severe, this risk is considered to outweigh the potential benefits of using a cephalosporin. In severe infections for which there are no adequate alternative treatments, STs can be performed and, should they be negative, followed by a DPT with cephalosporins that do not have the same side chain as penicillin or amoxicillin. If performance of a ST is not possible and the past reaction to penicillin was not severe (anaphylaxis), a cephalosporin with a different side chain or a carbapenem can be administered in a graded challenge.10
In bacterial infections of the upper respiratory tract with no complications or risk factors, macrolides will be the first line of treatment.33–35 Although pneumococcus may be resistant to therapy, these infections are associated with a high rate of spontaneous resolution. The widespread use of antibiotics with a broader spectrum could lead to the quick development of antimicrobial resistance in a population, making it difficult to treat children with more severe forms of disease.
In children with complicated respiratory infections, at risk of severe infection or in whom treatment has failed, levofloxacin is the best treatment option, as its activity against pneumococcus is much higher than that of ciprofloxacin. Quinolones are safe drugs that are well tolerated by children, and only lead to the development of osteoarticular adverse effects in exceptional cases.36 Parents can prepare the dose by crushing and dissolving 500mg tablets, although it is also available in pharmacies as a compounded dosage form (50mg/mL). The use of levofloxacin is off-label, so it requires the informed consent of the parents, which should be documented in the medical record. Its use should be restricted to severe infections (consultation with a specialist in infectious disease should be considered).
Clindamycin is a good alternative for the treatment of skin infections,37 as it has exhibited good activity against the two major causative pathogens, S. aureus and S. pyogenes, in addition to being effective against the toxins produced by both bacteria.
Urinary tract infections can be treated with the drugs that are used customarily, which are easy to administer, have good antimicrobial activity, and are usually well tolerated: gentamicin for pyelonephritis and fosfomycin, nitrofurantoin or trimethoprim-sulfamethoxazole for lower urinary tract infections. As noted above, if the rate of resistance to a given antibiotic exceeds 15%, other options should be considered. Although nitrofurantoin is poorly tolerated and may cause vomiting, its use may be necessary in these cases, especially in young children.
Broad-spectrum antibiotics should be used to treat severe community-acquired infections. Cross-reactivity to carbapenems (imipenem, meropenem, ertapenem) is infrequent (0.9%),38 so they could be used after verifying its absence by means of a negative ST or a DPT. Monobactams (aztreonam) can be used due to their weak and very infrequent cross-reactivity, as can glycopeptides (vancomycin, teicoplanin) and oxazolidinones (linezolid). The combination used most frequently in cases of sepsis or meningitis is aztreonam with vancomycin.
Delayed allergic reactionCephalosporins are the first-line treatment, although those that have similar or the same side chains as penicillin and ampicillin (cefadroxil, cefprozil and cefachlor) should be avoided.10,31 Cases of severe delayed reactions, in which the use of beta-lactams is contraindicated, are exceptions to be treated following in the same way as immediate reactions. Tolerance to penicillin should be tested at the allergy clinic by means of a DPT, following negative ST results.7
When there is a clear history of nonsevere, nonurticarial rashes that unquestionably establishes risk, and especially in young children and in the context of viral infection, it would be feasible to perform a DPT with a first full dose under direct supervision9,12,13 (after informed consent is given and signed), followed by two hours’ observation and treatment for up to five days,10 which could even be carried out in the primary care setting to avoid unnecessary restrictions and referrals.
In general, at the outpatient level, cefuroxime should be used to treat infections by gram-positive bacteria, and third-generation cephalosporins (cefixime, ceftibuten) for infections caused by gram-negative bacteria. Clindamycin is an excellent option in infections with abscess formation mediated by toxins or with anaerobic involvement. The risk of cross-reactivity between penicillin or amoxicillin and first-generation cephalosporins with the same side chain is approximately 1%.39 However, one study found that cross-reactivity to cefadroxil can reach up to 27%. For this reason, there is an ongoing debate in the literature and among the experts that drafted this consensus document, who did not reach a unanimous agreement regarding the use of cefadroxil in patients with pharyngotonsillitis, adenitis and cutaneous infections. It could be considered an appropriate option (and would be the one with the narrowest spectrum) if it were certain that the patient had selective delayed hypersensitivity to penicillins with mild symptoms and not mediated by IgE. Therefore, we feel compelled to recommend that appropriate diagnostic tests be performed whenever an allergy is suspected in order to be able to use the best possible treatment in each case. Very severe infections that require admission to the hospital (mastoiditis, periorbital cellulitis, complicated pneumonia,40 sepsis and meningitis) can be treated parenterally with a third-generation cephalosporin (cefotaxime or ceftriaxone) as monotherapy or combined with vancomycin.
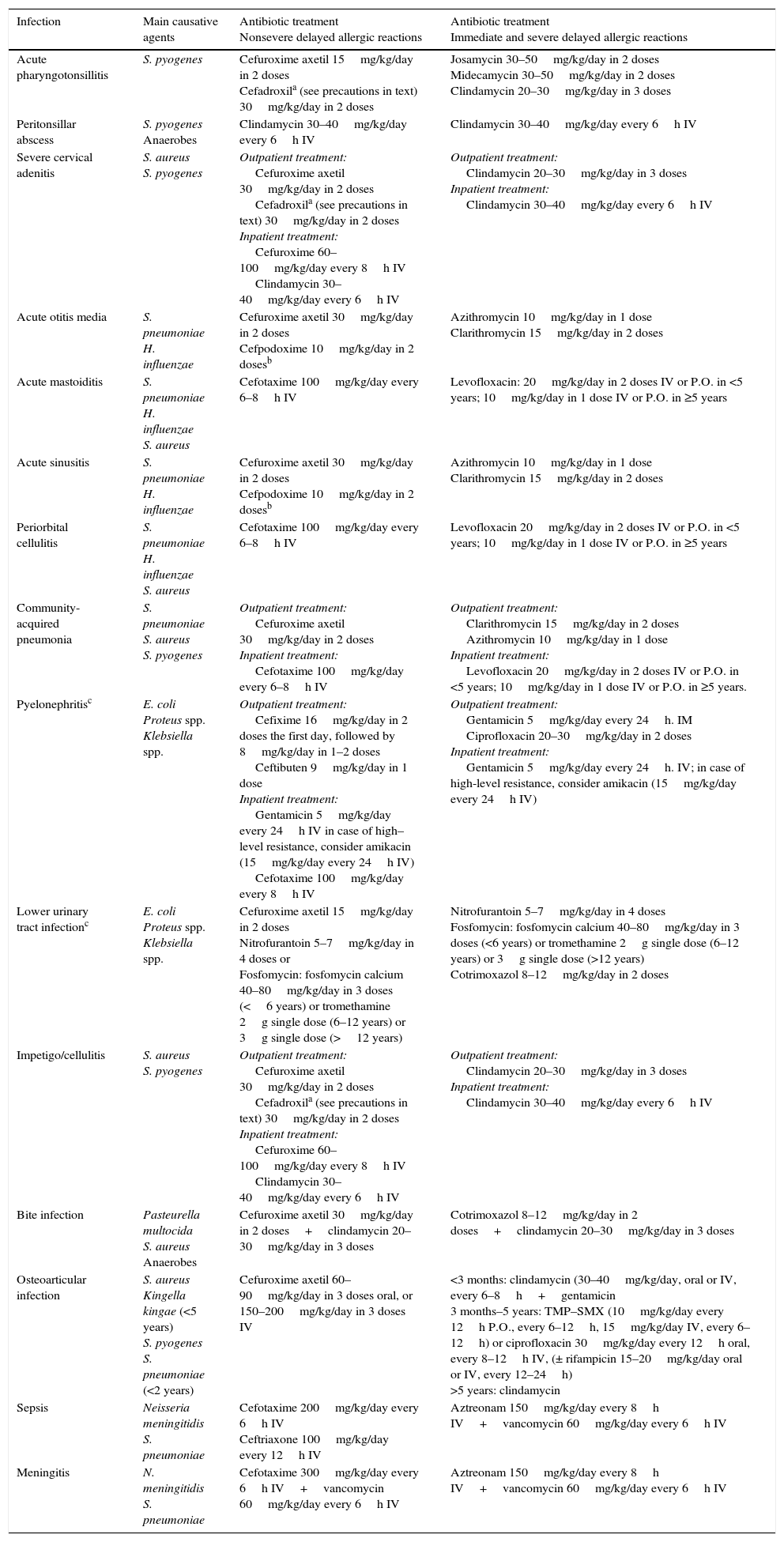
Table 2 summarises the most frequent community-acquired infections in the paediatric age group and the alternative treatments for these infections based on the type of penicillin hypersensitivity.
Treatment of the most common community-acquired infections in children allergic to penicillin.
| Infection | Main causative agents | Antibiotic treatment Nonsevere delayed allergic reactions | Antibiotic treatment Immediate and severe delayed allergic reactions |
|---|---|---|---|
| Acute pharyngotonsillitis | S. pyogenes | Cefuroxime axetil 15mg/kg/day in 2 doses Cefadroxila (see precautions in text) 30mg/kg/day in 2 doses | Josamycin 30–50mg/kg/day in 2 doses Midecamycin 30–50mg/kg/day in 2 doses Clindamycin 20–30mg/kg/day in 3 doses |
| Peritonsillar abscess | S. pyogenes Anaerobes | Clindamycin 30–40mg/kg/day every 6h IV | Clindamycin 30–40mg/kg/day every 6h IV |
| Severe cervical adenitis | S. aureus S. pyogenes | Outpatient treatment: Cefuroxime axetil 30mg/kg/day in 2 doses Cefadroxila (see precautions in text) 30mg/kg/day in 2 doses Inpatient treatment: Cefuroxime 60–100mg/kg/day every 8h IV Clindamycin 30–40mg/kg/day every 6h IV | Outpatient treatment: Clindamycin 20–30mg/kg/day in 3 doses Inpatient treatment: Clindamycin 30–40mg/kg/day every 6h IV |
| Acute otitis media | S. pneumoniae H. influenzae | Cefuroxime axetil 30mg/kg/day in 2 doses Cefpodoxime 10mg/kg/day in 2 dosesb | Azithromycin 10mg/kg/day in 1 dose Clarithromycin 15mg/kg/day in 2 doses |
| Acute mastoiditis | S. pneumoniae H. influenzae S. aureus | Cefotaxime 100mg/kg/day every 6–8h IV | Levofloxacin: 20mg/kg/day in 2 doses IV or P.O. in <5 years; 10mg/kg/day in 1 dose IV or P.O. in ≥5 years |
| Acute sinusitis | S. pneumoniae H. influenzae | Cefuroxime axetil 30mg/kg/day in 2 doses Cefpodoxime 10mg/kg/day in 2 dosesb | Azithromycin 10mg/kg/day in 1 dose Clarithromycin 15mg/kg/day in 2 doses |
| Periorbital cellulitis | S. pneumoniae H. influenzae S. aureus | Cefotaxime 100mg/kg/day every 6–8h IV | Levofloxacin 20mg/kg/day in 2 doses IV or P.O. in <5 years; 10mg/kg/day in 1 dose IV or P.O. in ≥5 years |
| Community-acquired pneumonia | S. pneumoniae S. aureus S. pyogenes | Outpatient treatment: Cefuroxime axetil 30mg/kg/day in 2 doses Inpatient treatment: Cefotaxime 100mg/kg/day every 6–8h IV | Outpatient treatment: Clarithromycin 15mg/kg/day in 2 doses Azithromycin 10mg/kg/day in 1 dose Inpatient treatment: Levofloxacin 20mg/kg/day in 2 doses IV or P.O. in <5 years; 10mg/kg/day in 1 dose IV or P.O. in ≥5 years. |
| Pyelonephritisc | E. coli Proteus spp. Klebsiella spp. | Outpatient treatment: Cefixime 16mg/kg/day in 2 doses the first day, followed by 8mg/kg/day in 1–2 doses Ceftibuten 9mg/kg/day in 1 dose Inpatient treatment: Gentamicin 5mg/kg/day every 24h IV in case of high–level resistance, consider amikacin (15mg/kg/day every 24h IV) Cefotaxime 100mg/kg/day every 8h IV | Outpatient treatment: Gentamicin 5mg/kg/day every 24h. IM Ciprofloxacin 20–30mg/kg/day in 2 doses Inpatient treatment: Gentamicin 5mg/kg/day every 24h. IV; in case of high-level resistance, consider amikacin (15mg/kg/day every 24h IV) |
| Lower urinary tract infectionc | E. coli Proteus spp. Klebsiella spp. | Cefuroxime axetil 15mg/kg/day in 2 doses Nitrofurantoin 5–7mg/kg/day in 4 doses or Fosfomycin: fosfomycin calcium 40–80mg/kg/day in 3 doses (<6 years) or tromethamine 2g single dose (6–12 years) or 3g single dose (>12 years) | Nitrofurantoin 5–7mg/kg/day in 4 doses Fosfomycin: fosfomycin calcium 40–80mg/kg/day in 3 doses (<6 years) or tromethamine 2g single dose (6–12 years) or 3g single dose (>12 years) Cotrimoxazol 8–12mg/kg/day in 2 doses |
| Impetigo/cellulitis | S. aureus S. pyogenes | Outpatient treatment: Cefuroxime axetil 30mg/kg/day in 2 doses Cefadroxila (see precautions in text) 30mg/kg/day in 2 doses Inpatient treatment: Cefuroxime 60–100mg/kg/day every 8h IV Clindamycin 30–40mg/kg/day every 6h IV | Outpatient treatment: Clindamycin 20–30mg/kg/day in 3 doses Inpatient treatment: Clindamycin 30–40mg/kg/day every 6h IV |
| Bite infection | Pasteurella multocida S. aureus Anaerobes | Cefuroxime axetil 30mg/kg/day in 2 doses+clindamycin 20–30mg/kg/day in 3 doses | Cotrimoxazol 8–12mg/kg/day in 2 doses+clindamycin 20–30mg/kg/day in 3 doses |
| Osteoarticular infection | S. aureus Kingella kingae (<5 years) S. pyogenes S. pneumoniae (<2 years) | Cefuroxime axetil 60–90mg/kg/day in 3 doses oral, or 150–200mg/kg/day in 3 doses IV | <3 months: clindamycin (30–40mg/kg/day, oral or IV, every 6–8h+gentamicin 3 months–5 years: TMP–SMX (10mg/kg/day every 12h P.O., every 6–12h, 15mg/kg/day IV, every 6–12h) or ciprofloxacin 30mg/kg/day every 12h oral, every 8–12h IV, (± rifampicin 15–20mg/kg/day oral or IV, every 12–24h) >5 years: clindamycin |
| Sepsis | Neisseria meningitidis S. pneumoniae | Cefotaxime 200mg/kg/day every 6h IV Ceftriaxone 100mg/kg/day every 12h IV | Aztreonam 150mg/kg/day every 8h IV+vancomycin 60mg/kg/day every 6h IV |
| Meningitis | N. meningitidis S. pneumoniae | Cefotaxime 300mg/kg/day every 6h IV+vancomycin 60mg/kg/day every 6h IV | Aztreonam 150mg/kg/day every 8h IV+vancomycin 60mg/kg/day every 6h IV |
The duration of treatment is the usual applied to the various diseases.
The authors have no conflict of interests to declare.
María Teresa Muñoz Giner (Sociedad Española de Inmunología Clínica y Alergia Pediátricas).
Roi Piñeiro Pérez (Sociedad Española de Infectología Pediátrica).
Fernando Alvez (Sociedad Española de Infectología Pediátrica).
Santiago Alfayate (Sociedad Española de Infectología Pediátrica).
María José Cilleruelo (Sociedad Española de Infectología Pediátrica).
Antonio José Conejo Fernandez (Sociedad Española de Infectología Pediátrica).
Members of the Senior Management Group Ambulatory infections occur in Appendix A.
Please cite this article as: Baquero-Artigao F, Michavila A, Suárez-Rodriguez Á, Hernandez A, Martínez-Campos L, Calvo C, et al. Documento de consenso de la Sociedad Española de Infectología Pediátrica, Sociedad Española de Inmunología Clínica y Alergia Pediátricas, Asociación Española de Pediatría de Atención Primaria y Sociedad Española de Pediatría Extrahospitalaria y Atención Primaria sobre antibioterapia en alergia a penicilina o amoxicilina. An Pediatr (Barc). 2017;86:99.el–99.e9.