Tuberculosis (TB) screening in pregnancy using tuberculin skin test (TST) is recommended in case of symptoms of TB disease, close contact with a patient with infectious TB, or high risk of developing active disease. The new interferon gamma release assay (IGRA) tests are recommended in BCG-vaccinated pregnant women with positive TST and no known risk factors for TB, and in those immunocompromised, with clinical suspicion of TB but negative TST. TB diagnosis is difficult due to the non-specific symptoms, the increased frequency of extrapulmonary disease, the delay in radiological examinations, and the high rate of tuberculin anergy. Neonatal TB can be acquired in utero (congenital TB), or through airborne transmission after delivery (postnatal TB). Congenital TB is extremely rare and does not cause foetal malformations. It may be evident at birth, although it usually presents after the second week of life. In newborns with no family history of TB, the disease should be considered in cases of miliary pneumonia, hepatosplenomegaly with focal lesions, or lymphocytic meningitis with hypoglycorrhachia, especially in those born to immigrants from high TB-burden countries. TST is usually negative, and IGRAs have lower sensitivity than in older children. However, the yield of acid-fast smear and culture is higher, mostly in congenital TB. Molecular diagnosis techniques enable early diagnosis and detection of drug resistance mutations. There is a substantial risk of disseminated disease and death.
El cribado de tuberculosis (TB) gestacional mediante la realización de la prueba de tuberculina (PT) se recomienda a las embarazadas con síntomas compatibles, contacto íntimo con TB bacilífera o riesgo de progresión a formas activas. Las nuevas técnicas de diagnóstico interferon gamma release assay (IGRA) están indicadas en gestantes sin factores de riesgo, con PT positiva y antecedente de vacunación BCG, y en inmunodeprimidas con sospecha clínica y PT negativa. El diagnóstico de enfermedad es difícil, ya que los síntomas pueden ser inespecíficos y hay más formas extrapulmonares, por el retraso en las exploraciones radiológicas y por la mayor tasa de anergia a la tuberculina. La TB neonatal puede adquirirse de forma intrauterina (TB congénita) o por vía respiratoria tras el parto (TB posnatal). La TB congénita es excepcional, no produce malformaciones fetales y, aunque puede estar presente en el nacimiento, suele iniciarse a partir de la segunda semana de vida. En ausencia de antecedentes familiares, la TB neonatal debe sospecharse en caso de neumonía con patrón miliar, hepatoesplenomegalia con lesiones focales o meningitis linfocitaria con hipoglucorraquia, especialmente si la madre procede de áreas de alta endemia de TB. La PT es habitualmente negativa y la sensibilidad de los IGRA es inferior a la de niños de más edad. Sin embargo, la baciloscopia y el cultivo de jugo gástrico tienen una rentabilidad superior, especialmente en la TB congénita. Las técnicas de diagnóstico molecular permiten un diagnóstico precoz y la detección de mutaciones de resistencia farmacológica. El riesgo de formas diseminadas y la mortalidad son elevados.
The epidemiology of tuberculosis (TB) has changed drastically in the past five years, with a mean worldwide incidence of 130 cases per 100,000 inhabitants, which according to the WHO 2012 report1 corresponds to a considerable reduction in developed countries, a greater burden of disease in Asia and Africa, and an increased incidence among individuals of younger ages or with risk factors. Thus, there are a greater number of cases among young adults, including women of reproductive age, which increases the probability of TB in pregnant women and neonatal TB. The mean incidence of TB in Spain in 2011 was 14.74 cases per 100,000 inhabitants, and while there are no data for pregnant women, women of reproductive age account for 39% of the total cases.2
The prevalence of latent tuberculosis infection (LTBI) in pregnant women reflects the prevalence in the general population. In the United States, 4.2% of pregnant women have a LTBI, but it is estimated that the prevalence is tenfold for pregnant women born outside the country. It is difficult to estimate the prevalence of LTBI in highly endemic areas, as many women do not have access to the healthcare system during pregnancy3; small-scale studies have calculated that between 18% and 34% of pregnant women in India and nearly half of pregnant women with HIV in South Africa have a LTBI.4,5
Tuberculosis in pregnant women is rare in developed countries (4–39new cases/100,000 inhabitants), but the incidence is much higher in endemic areas (>60new cases/100,000 inhabitants).6 There is evidence of an increase in the prevalence of TB during pregnancy and post partum in women in the United States and the United Kingdom, which currently ranges between 0.13% and 1%,3 clearly lower than prevalences in developing countries (3.4% in India and 2.8% in Kenya), especially among women infected by HIV (10% in Tanzania and 7.9% in Rwanda).
Neonatal TB can result from foetal infection in utero or during the passage through the birth canal (congenital TB), or from airborne transmission from the mother or other sources of contagion in the early days of life (postnatal TB).7 Differentiating between congenital and postnatal TB carries significant implications from an epidemiological standpoint in terms of identifying the source of contagion, but not from a therapeutic standpoint, as the treatment is the same for both.
Diagnosing tuberculosis in pregnant womenFrom the second half of the XX century, most studies showed that neither pregnancy nor puerperium were factors that influenced the course of TB nor the progression from LTBI to active TB.8–11 However, recent studies suggest that immunologic changes that take place during pregnancy and the postpartum period may increase the susceptibility to LTBI or to TB reactivation.3,12 Thus, it is important that clinicians consider the possibility of TB in pregnancy, especially in women that belong to high-risk groups (HIV-positive, immunosuppressed, or that have immigrated from endemic countries), as untreated active TB is an important cause of maternal morbidity and mortality and is associated with a higher risk of miscarriage, intrauterine growth restriction, preterm birth and foetal death.3 However, the clinical diagnosis is complicated, as the symptoms may be nonspecific, extrapulmonary forms of disease and tuberculin anergy are more frequent, and radiological testing is usually delayed.
Tuberculosis screening during pregnancyIt is only indicated in patients at high risk of developing TB during pregnancy and that could benefit from immediate treatment.3,10,13,14 This includes pregnant women with clinical manifestations compatible with TB and those exposed to a case of infectious TB. It is also indicated in pregnant women with risk factors for progression to active forms of disease if they had not been screened for TB before pregnancy (Table 1). A positive diagnostic test should be followed by a chest X-ray to assess for active disease and rule out HIV coinfection. On the other hand, TB screening must be performed in pregnant women that have tested positive for HIV.
Indications for diagnostic testing for tuberculosis infection during pregnancy.
| Clinical suspicion of tuberculosis |
| Suspicion of recent infectiona: contact with case of infectious tuberculosis |
| Risk of progression to active diseaseb: HIV infection, immunosuppression; IVDU,c congregate living settings and social exclusion |
In case of recent contact and negative TST or interferon gamma release assay (IGRA) results, it is recommended that the test is repeated at 8–12 weeks.15
It is the standard test for the diagnosis of TB infection and it is considered safe and valid in pregnant women.10,13 The antigens used in this test are called purified protein poner en cursiva (PPD), and the PPD variant used in Spain is RT-23. The test consists of the intradermal injection of 2 TU in the ventral surface of the forearm. The reaction is read 48–72h after by measuring the transversal diameter of the induration. In Spain, the test is considered positive if the diameter is 5mm or greater in individuals that have not been vaccinated with the BCG vaccine.15 In immunosuppressed patients (with HIV infection, transplant recipients, undergoing corticosteroid therapy) the reaction may be smaller, and any amount of induration in the TST is considered positive.15 A clear threshold has yet to be established for vaccinated individuals, as the potential effect of the vaccine cannot be ruled out. However, it is believed that previous vaccination against TB should be disregarded in groups at high risk of having the disease (exposure to patient with infectious disease, residual lesions in chest radiography with negative bacteriology, HIV infection), in whom a TST induration of 5mm or more should be considered a positive result.
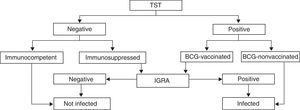
Interferon gamma release assaysTechniques for detecting interferon gamma (IFN-γ) released in the blood in response to the stimulation of T-cells sensitised to specific Mycobacterium tuberculosis antigens have been developed recently (interferon gamma release assays [IGRAs]). The two IGRA products that are currently commercially available for the diagnosis of TB infections measure the levels of IFN-γ (QuantiFERON-TB-Gold®-In tube) or the number of lymphocytes that produce it (T-SPOT.TB®). These techniques do not interfere with the BCG vaccine and have a higher sensitivity than the TST in immunosuppressed patients.15 They also have the advantage of not requiring a second visit for obtaining the results. Their cost is higher, but it is compensated by having a lower false positive rate than the TST, thus reducing the need for chest radiographs and treatment, something of particular importance during pregnancy. The CDC considers IGRAs an alternative to TST, especially in patients that have been vaccinated against TB or in whom followup would be difficult.16 These assays are considered safe during pregnancy and they are probably as effective in diagnosing LTBI as they are outside of pregnancy, but there are few studies on the matter, and they have yielded contradictory results.17,18 However, the use of IGRAs is recommended in selected patients, as it is outside of pregnancy, based on recent expert opinion.3,19 It would be mainly indicated in pregnant women with no risk factors, a positive TST and a history of BCG vaccination, and in immunosuppressed pregnant women with symptoms suggestive of TB and a negative TST20 (Fig. 1).
Algorithm for the use of the tuberculin skin test (TST) and interferon gamma release assay (IGRA) methods for the diagnosis of tuberculosis during pregnancy (adaptation for pregnant women of the algorithm proposed by the SEPAR for the general population).20
Radiological testing will be performed with adequate abdominal protection if the results of the TST or the IGRA are positive and in cases presenting with characteristic symptoms (haemoptysis, persistent cough lasting more than 3 weeks, fever of unknown source). Radiological findings are interpreted as they are interpreted in nonpregnant women.
Microbiological diagnosisThe diagnostic methods applied during pregnancy are the same as those performed in the rest of the adult population.10
- -
Microscopy or bacilloscopy. Staining methods (Ziehl–Neelsen, auramine–rhodamine) for quick and easy identification of the bacillus. At least 3 sputum samples must be tested every time. A positive respiratory test is evidence of active TB, but a negative bacilloscopy is not sufficient to rule out the disease. Bacilloscopy can be used during followup to assess the effectiveness of the treatment.15
- -
Culture. A mycobacterium culture, which is the gold standard for the diagnosis of TB, should be performed in any pregnant woman in whom the disease is suspected. It allows for the identification of the species and antituberculosis drug susceptibility testing. It has the drawback that it takes two to four weeks to get the definitive result. The main criterion for the microbiological cure of TB is the negativization of cultures during treatment (2 consecutive negative cultures in a 2-month period).3,15
- -
Molecular techniques. Polymerase chain reaction (PCR). These methods are based on the amplification of M. tuberculosis gene fragments. They have a high specificity and a sensibility slightly greater than that of culture. Their main advantage is that results become available in a few hours. The Gene-Xpert® MTB/RIF automated test also allows for the detection of mutations associated with rifampicin resistance, which is usually a marker of multidrug resistance. The new molecular diagnosis techniques allow the simultaneous detection of mutations for resistance to isoniazid and rifampicin and to second-line drugs for the identification of multidrug-resistant and extensively drug-resistant strains. The cost of molecular techniques is high, so they are used as supplementary tests in cases with a high suspicion of active disease or drug resistance.15
- -
Exposure to tuberculosis. Asymptomatic patient with a known history of exposure and negative TST/IGRA results.
- -
Latent tuberculosis infection. Asymptomatic patient with a positive TST or IGRA result and a normal chest X-ray. The patient may have risk factors for progression to active TB if she meets any of the criteria presented in Table 1.
- -
Active tuberculosis. Patient with clinical manifestations and abnormal radiological findings suggestive of TB and a positive TST or IGRA result or isolation of M. tuberculosis from a clinical sample. In pregnant women, some specific signs of the disease may be masked (anorexia, weight loss) or misinterpreted as pregnancy symptoms (general malaise, asthenia, fatigue).3,21 Furthermore, the disease may have an atypical presentation, with a greater prevalence of extrapulmonary forms, which makes diagnosis difficult. Genital TB is usually asymptomatic or manifests with nonspecific symptoms (abdominal or pelvic pain, menstruation abnormalities) and is a frequent cause of tubal factor infertility in developing countries. It must be considered before initiating assisted reproductive therapies in women with tubal factor infertility, especially in immigrant women from countries with a high TB burden. On the other hand, genital TB must be suspected whenever congenital TB is diagnosed in the newborn of an asymptomatic mother, and ruled out by testing of endometrial samples.
It occurs only when the mother develops active disease during pregnancy, although the maternal TB can be asymptomatic (especially in cases of genital TB) or manifest after childbirth.22 The rate of vertical transmission ranges between 0% and 16%, and it is very rare when the mother has exclusively pulmonary TB and has received adequate treatment before labour, and more frequent in miliary and genital forms of TB.23,24 It is a rare disease, with less than 300 cases published in the scientific literature, and its prevalence is lower than that of postnatal TB. A study conducted in the Hospital Universitario La Paz of 343 children diagnosed with TB over a 24-year period (1988–2011) identified six cases of congenital TB, which amounted to 1.75% of TB cases in the paediatric age group.25
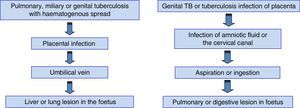
Routes of transmissionFig. 2 summarises the routes of transmission of TB infection and the localisation of the lesions. Each of these routes accounts for approximately 50% of the cases.10 The only pathognomonic lesion in congenital TB is the presence of a primary hepatic complex with caseating granulomas.26
Clinical featuresCongenital TB should be suspected in any newborn of a mother with a history of disseminated or extrapulmonary TB during pregnancy or with active disease during labour or the postpartum period, even if the physical examination at birth is normal.27 Intrauterine infection may cause spontaneous miscarriage, but there have been no reports of associated foetal abnormalities in the literature. The onset of symptoms in the first week of life is diagnostic, but symptoms usually start in the second or third week of life, although there have been cases of late onset (up to 3 months of life).26 Preterm birth and low birth weight for gestational age are common. Most cases involve the lungs, and miliary dissemination with central nervous system and liver and spleen involvement occurs frequently (Table 2).7 Congenital TB is associated with a high mortality rate of approximately 50% that is influenced by its difficult diagnosis and a low degree of clinical suspicion.10 Its prognosis is better in cases with an early diagnosis.22
Clinical manifestations suggestive of tuberculosis in neonates.
| – Pneumonia with a miliary, nodular, interstitial or lymphadenopathic pattern with progressive clinical worsening despite broad-spectrum antibiotic treatment |
| – Hepatosplenomegaly, focal lesions in the liver and spleen, abdominal swelling, adenopathies, or ascites of unknown origin |
| – Meningitis with lymphocyte predominance, with hypoglycorrhachia and hyperproteinorrhachia or focal neurologic signs of unknown aetiology, especially with cranial nerve palsy |
| – Sepsis with negative cultures and an unfavourable progression despite treatment with antibiotics |
| – Prolonged nonspecific symptoms of unknown aetiology (pertussis-like coughing, progressive breathing difficulties, persistent ear discharge, jaundice, apnoeic pauses, lethargy or irritability, failure to thrive, refusal to feed), especially associated to persistent fever or low-grade fever, leukocytosis with left shift, and elevated acute phase reactants |
The criteria currently used are those proposed by Cantwell in 1994,26 which are based on the microbiological confirmation of TB in the newborn or infant (PCR or culture) plus at least one of the following:
- 1.
Onset of symptoms in the first week of life.
- 2.
Evidence of a primary hepatic complex or caseating hepatic granulomata.
- 3.
TB infection of the placenta or the maternal genital tract.
- 4.
Exclusion of the possibility of postnatal transmission by exhaustive investigation of contacts (including the staff of the maternity ward).
The treatment is the same as for postnatal TB.
Diagnosing tuberculosis in the neonateAnamnesisDiagnosing neonatal TB requires a high degree of suspicion, as the clinical presentation tends to be atypical and it carries a high morbidity and mortality. A history of maternal LTBI or TB during pregnancy or the postpartum period is very important in guiding the diagnosis.7 In the absence of such a history, a detailed anamnesis must be performed to assess for the presence of constitutional symptoms or productive cough in family members, and to ask about close contacts with pneumonia, sepsis or meningitis with negative bacterial cultures.28
Clinical manifestationsThe clinical manifestations of neonatal TB are varied and nonspecific (Table 2). Neonates with suggestive manifestations or with an epidemiological history of confirmed TB should be admitted for evaluation.
Diagnostic tests (Table 3)First-tier tests- -
Microbiological and histopathological testing of the placenta and amniotic fluid. They are very useful for the diagnosis of congenital infection, and they must be performed whenever there is a history of maternal TB during pregnancy. Infection of the placenta is not sufficient for diagnosis, as the foetus is not always infected even in cases of massive chorioamnionitis.10
- -
Complete blood count and biochemistry with liver function tests. There are usually abnormal findings for all three series, with leukocytosis combined with low levels of neutrophils, anaemia and thrombocytopaenia. Biochemistry tests reveal hypertransaminasaemia and elevated C-reactive protein.22
- -
Tuberculin skin test. It is usually negative in the first month of life,7,22 but it can be positive in up to 20% of neonates, a fact that may prove very useful in diagnosis.
Table 3.Diagnostic tests in the newborn.
First tier 1. Complete blood count, biochemistry, C-reactive protein 2. Tuberculin test 3. Eye fundus examination 4. Interferon gamma release assay (IGRA) - Quantiferon®-TB-Gold In-Tube - T-SPOT®.TB 5. Chest X-ray (chest CT if the diagnosis is uncertain) 6. Abdominal ultrasound 7. Gastric aspirate samples (3) for immediate PCR, BK and culture 8. Histopathological and microbiological investigation of the placenta and amniotic fluid if there is a history of tuberculosis during pregnancy Second tier 1. Cerebrospinal fluid (cytochemistry, ADA, PCR, BK and culture) 2. Cranial ultrasound/MRI 3. Other invasive tests (bronchoscopy, tissue biopsy) if previous tests were inconclusive and there is a strong clinical suspicion ADA, adenosine deaminase; BK, bacilloscopy; CT, computed tomography; PCR, polymerase chain reaction; MRI, magnetic resonance imaging.
- -
Eye fundus examination. Choroidal tubercles may be observed in cases of disseminated TB.29
- -
Interferon gamma release assays (IGRAs). The immaturity of the immune system of newborns and young infants results in a lower production of interferon in response to antigenic stimuli.30 Few data are available on the diagnostic yield of IGRAs in neonates, but they seem to have a high specificity and a lower sensitivity, with a higher percentage of indeterminate results than in older children.31,32 However, as happens in other populations, their sensitivity seems to be higher than that of the TST and positive results can be obtained before the onset of symptoms. Positive results have been reported both for cases of neonatal TB and for the investigation of contacts of newborns that have been exposed to TB.33,34 If the initial result is negative, the TST and the IGRA should be repeated 12 weeks later.35
- -
Gastric aspirate. PCR, bacilloscopy and culture should be performed for 3 fasting samples taken on consecutive days to be processed immediately. Samples of tracheal aspirate are used in intubated newborns. Bacillary loads are greater in congenital TB, so the yield of rapid diagnostic tests and culture is higher than in postnatal TB cases. PCR-based molecular diagnostic methods allow for early diagnosis and the simultaneous detection of potential mutations for resistance to isoniazid and rifampicin.36
- -
Chest radiography. It is abnormal in most cases, revealing a miliary pattern in half of the patients, although there can be forms with interstitial, nodular or lymph node involvement. In some cases it is normal at the outset, but shows rapid radiological progression if the disease is not treated.22 If chest radiography is normal but there is a high clinical suspicion or the patient has a history of close contact, the possibility of performing a chest CT scan should be considered.37
- -
Abdominal ultrasound. It may reveal hepatosplenomegaly, multiple nodular lesions in the liver and spleen, mesenteric lymphadenopathies and ascites.22
Once first-tier tests have been conducted, if the case is highly suggestive of TB or the infection has been confirmed, the investigation should be completed with a lumbar puncture and a cranial ultrasound. Meningeal involvement is present in 20–30% of neonatal TB cases,22 so cytochemistry tests, staining, culture and PCR for M. tuberculosis should be performed in cerebrospinal fluid samples. If the central nervous system is involved, cranial ultrasonography or magnetic resonance imaging may show ventriculomegaly, meningeal thickening and calcifications, tuberculomas and infarctions.38 Invasive tests may need to be done in cases with high suspicion and an inconclusive diagnosis (bronchoscopy, transbronchial, liver, or lymph node biopsy) to confirm the diagnosis.39
Differential diagnosisSince the symptoms are nonspecific, the differential diagnosis must include bacterial sepsis, neonatal pneumonia (viruses, bacteria, Candida, P. jirovecii)10 and other vertically transmitted infections (toxoplasmosis, cytomegalovirus, rubella, herpes, HIV).28
Investigation of contactsIf there is no documented history of TB in the mother and the child is diagnosed in the first month of life, the mother should undergo a TST and chest X-ray. An endometrial biopsy should be performed if the TST were positive in a mother with a normal chest X-ray and no known TB contacts in the household (this test is particularly indicated in patients with a history of in vitro fertilisation due to tubal infertility). If maternal TB is ruled out, an exhaustive investigation of family members should follow, consisting of a TST, a chest X-ray and a sputum bacilloscopy if the X-ray shows abnormal findings suggestive of TB. If the family investigation are negative, the investigation should be extended to the known out-of-household contacts of the child, including health workers.40
Conflicts of interestThe authors have no conflicts of interest to declare.
Ana Alarcón Allen, Servicio de Neonatología, Hospital Universitari Sant Joan de Déu, Esplugues de Llobregat, Barcelona and Neonatal Unit, Oxford University Hospitals NHS Trust, United Kingdom; Fernando Álvez González, Unidad de Infectología y Vacunas, GENVIP, Hospital Clínico Universitario Santiago de Compostela, La Coruña; Fernando Baquero-Artigao, Servicio de Pediatría, Enfermedades Infecciosas y Patología Tropical, Hospital Infantil La Paz, Madrid; Daniel Blázquez Gamero, Sección de Inmunodeficiencias y Niños Pequeños, Servicio de Pediatría, Hospital 12 de Octubre, Madrid; Marta Cabrera Lafuente, Servicio de Neonatología, Hospital La Paz, Madrid; José Antonio Couceiro Gianzo, Unidad de Infectología, Servicio de Pediatría, Complexo Hospitalario de Pontevedra; María de la Calle Fernández-Miranda, Unidad de Tocología de Alto Riesgo, Servicio de Obstetricia y Ginecología, Hospital La Paz, Madrid; Teresa del Rosal Rabes, Servicio de Pediatría, Enfermedades Infecciosas y Patología Tropical, Hospital Infantil La Paz, Madrid; Claudia Fortuny Guasch, Unidad de Infecciones, Servicio de Pediatría, Hospital Sant Joan de Déu, Universitat de Barcelona, Esplugues de Llobregat, Barcelona; Anna Goncé Mellgren, Servicio de Medicina Maternofetal, Institut Clínic de Ginecologia, Obstetrícia i Neonatologia, Hospital Clínic, Barcelona; Teresa Hernández-Sampelayo Matos, Sección de Enfermedades Infecciosas, Servicio de Pediatría, Hospital Gregorio Marañón, Madrid; Andrea Martín-Nalda, Unidad de Patología Infecciosa e Inmunodeficiencias de Pediatría, Hospital Materno-Infantil Vall d’Hebron, Barcelona; Leticia Martínez Campos, Unidad de Infectología Pediátrica, Hospital La Inmaculada de Huercal Overa, AGS Norte de Almería; María José Mellado Peña, Servicio de Pediatría, Enfermedades Infecciosas y Patología Tropical, Hospital Infantil La Paz, Madrid; María Méndez Hernández. Servicio de Pediatría, Unidad de Enfermedades Infecciosas e Inmunología Clínica, Hospital Universitario Germans Trias i Pujol, Universidad Autónoma de Barcelona; David Moreno Pérez, Unidad de Infectología, Hospital Materno-Infantil Carlos Haya, Málaga; María Luisa Navarro Gómez, Sección de Enfermedades Infecciosas, Servicio de Pediatría, Hospital Gregorio Marañón, Madrid; Antoni Noguera Julián, Unidad de Infecciones, Servicio de Pediatría, Hospital Sant Joan de Déu, Universitat de Barcelona, Esplugues de Llobregat, Barcelona; Félix Omeñaca Teres, Servicio de Neonatología, Hospital La Paz, Madrid; José Tomás Ramos Amador, Servicio de Pediatría, Hospital Clínico San Carlos, Madrid.
Please cite this article as: Baquero-Artigao F, Mellado Peña MJ, del Rosal Rabes T, Noguera Julián A, Goncé Mellgren A, de la Calle Fernández-Miranda M, et al. Guía de la Sociedad Española de Infectología Pediátrica sobre tuberculosis en la embarazada y el recién nacido (I): epidemiología y diagnóstico. Tuberculosis congénita. An Pediatr (Barc). 2015;83:285.
The members of the working group on gestational, congenital and postnatal tuberculosis of the Sociedad Española de Infectología Pediátrica (Spanish Society of Pediatric Infectology [SEIP]) are listed in Appendix A.