After the publication of the recommendations, agreed by all the scientific societies through the ILCOR, at the end of 2020, the GRN-SENeo began a process of analysis and review of the main changes since the last guidelines, to which a specific consensus positioning on controversial issues, trying to avoid ambiguities and trying to adapt the evidence to our environment. This text summarizes the main conclusions of this work and reflects the positioning of that group.
Tras la publicación de las recomendaciones, consensuadas por todas las sociedades científicas a través del ILCOR, a finales del año 2020, el GRN-SENeo inició un proceso de análisis y revisión de los principales cambios desde las últimas guías, a los que se añadió un posicionamiento específico de consenso en temas controvertidos, tratando de evitar ambigüedades, y procurando adaptar la evidencia a nuestro medio. El presente texto, resume las principales conclusiones de este trabajo y refleja el posicionamiento de dicho grupo.
The International Liaison Committee on Resuscitation (ILCOR) reviews and discusses the current evidence on subjects of particular interest in assessment, stabilization and cardiopulmonary resuscitation to then develop what are known as consensus on science and treatment recommendations (CoSTR).1 The various resuscitation councils and societies across the world then take into account these recommendations to develop guidelines appropriate for specific contexts or settings.1–4
Since 2004, the Neonatal Resuscitation Group (NRG) of the Sociedad Española de Neonatología (Spanish Society of Neonatology, SENeo) has been publishing national recommendations for neonatal stabilization and life support in the delivery room in Anales de Pediatría7 with the aim of facilitating the application of the available evidence of highest quality to clinical practice in Spain in addition to standardising the care delivered by any professional involved in childbirth in Spain.
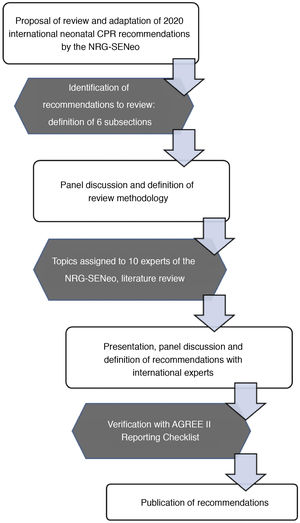
MethodsThe NRG-SENeo used the CoSTR as the foundation to develop the recommendations presented here, using a specific review methodology for subjects considered a priority for adaptation to the Spanish neonatal setting. A total of 6 subjects were prioritised and analysed until a consensus was reached in a process that involved a literature review, exposition of the findings and a group debate. We also applied the AGREE II Reporting Checklist (available at https://www.agreetrust.org/resource-centre/agree-reporting-checklist/), which was designed to improve the completeness and transparency of reporting in the development of high-quality practice guidelines.5
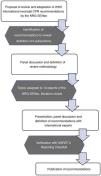
The review for each of the selected subjects followed the same structure: background, summary of the scientific evidence, recommendations given in other guidelines and position of the NRG-SENeo. Another 4 subjects were given secondary priority and the members of the NRG-SENeo simply worked on reaching a consensus. Lastly, once all authors approved the recommendations, the group proceeded to the writing of the present document (Fig. 1).
Flowchart of the methods used by the NRG-SENeo to perform the review and adaptation of international neonatal resuscitation guidelines.5.
Both the briefing and the subsequent debriefing of the resuscitation team can be useful tools in resuscitation.
What does the evidence say?One randomised controlled trial and 3 observational studies contribute limited evidence that briefing6 and debriefing (with or without the help of video recordings)6,7 improve short-term performance outcomes. We found no evidence on their long-term effect on resuscitation team performance or their impact on infant clinical outcomes.8
What are the 2020 guidelines on the subject?The ILCOR concluded that briefing and debriefing may improve clinical and performance outcomes in the short term. The European Resuscitation Council (ERC) recommends briefing with role allocation to improve performance and teamwork in addition to the use of checklists during briefings to improve team communication and process.
What does the NRG-SENeo recommend?Before resuscitation, we recommend clear role allocation, escalation of care and use of checklists. We also recommend debriefing following resuscitation, ideally in short but frequent sessions, supported by objective data (notes/video recordings) and guided by a professional experienced in neonatal resuscitation and debriefing capable of establishing trust and safety in the process.
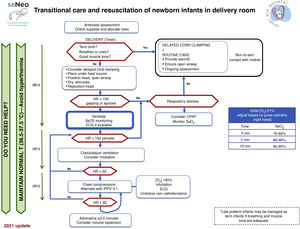
Umbilical cord managementBackgroundThere is evidence that delayed cord clamping offers several clinical benefits,3,9 so, while there is no universal definition of what it constitutes, it has been widely recommended in the past years in the context of an uncomplicated birth.
The controversy emerges when it comes to newborn infants requiring resuscitation. Different alternatives have been described in the literature aimed at preserving the potential benefits of delayed cord clamping: initiation of ventilation while the cord is still intact followed by clamping (physiological-based cord clamping [PBCC]), milking the umbilical cord from the placenta to the infant while the cord is intact, or milking the cord after it has been cut (cut umbilical cord milking [C-UCM]).
What does the evidence say?Umbilical cord milking is associated with oscillating swings in cerebral blood flow10 and may be associated with an increased incidence of intraventricular haemorrhage in neonates born before 28 weeks’ gestation.11 At present, there is insufficient evidence to support alternative strategies to milking like C-UCM. As for PBCC, its benefits in humans are still unclear, but there is some evidence suggesting that it may be as effective as delayed cord clamping.12
What are the 2020 guidelines on the subject?The ILCOR did not review umbilical cord management in the most recent recommendations, although it recently published a meta-analysis of 41 studies that concluded that delayed cord clamping could be beneficial in neonates delivered before 34 weeks’ gestation.13 The corresponding CoSTR are still being developed, and a draft is currently available at the ILCOR website. In this draft, the committee takes a favourable stance toward delayed cord clamping in neonates of any gestational age that do not require resuscitation and presented cord milking as a reasonable alternative in infants delivered after 28 weeks.
The 2020 American Heart Association (AHA) guidelines recommend delaying cord clamping for at least 30 seconds until the infant is placed on the mother, dried and assessed.3 They also consider the possibility of delaying cord clamping while the first steps of resuscitation are performed in cases in which it is needed, and expressly advise against cord milking in infants delivered before 28 weeks of gestation.3 The ERC recommends delayed cord clamping (by at least 60 s) in infants that do not require resuscitation and contemplates the option of cord milking in infants delivered after 28 weeks and physiological-based cord clamping in cases in which it can be done safely.2
What does the NRG-SENeo recommend?Delayed cord clamping (at least 1 minute) should be the standard of care in term and preterm (PT) infants of any gestational age delivered vaginally or by caesarean section that do not require immediate resuscitation.
To guide delayed cord clamping in clinical practice, especially in situations in which the need of medical care is anticipated, like PT birth, it is important to develop consensus-based protocols that take into account the obstetrics and anaesthesia teams, midwives and aids.
In infants that require resuscitation, initiation of positive pressure ventilation (PPV) should be prioritised. A possible option is to initiate stimulation manoeuvres while the cord is still intact in the context of a local-level consensus-based protocol, or even initiation of ventilation while the cord is intact if it is considered feasible and safe, assessing the risk for the mother and the infant and documenting the intervention (weak recommendation, very low-quality evidence). For example, a possible arrangement would involve placement of a resuscitation table by the mother’s bed to allow mother-side initiation of resuscitation. The evidence on these intact-cord strategies is limited, so their use needs to be individualised and integrated in the concept of the “golden minute” of newborn resuscitation.
We do not currently recommend cord milking, and expressly advise against its use in infants delivered before 28 weeks.
Positive pressure ventilationBackgroundIn neonates with apnoea and/or bradycardia, the immediate priority is to establish adequate lung ventilation. The 2015 ILCOR guideline remarked on the wide variability of the settings applied in clinical practice.
Historically, the peak inspiratory pressure (PIP) recommended for the first few inflations was 20 to 25 cmH2O in PT infants and 25 to 30 cmH2O in term infants. Although the PIP is used for initial lung inflation, maintenance of ventilation to avoid alveolar collapse, improve gas exchange and lung compliance and achieve an adequate functional residual capacity (FRC) depends to a greater extent on the optimization of positive end-expiratory pressure (PEEP).14
What does the evidence say?When it comes to the optimal initial PIP, we found evidence obtained in experimental studies that analysed volutrauma secondary to excessively high tidal volumes (Vt)15–16 and clinical trials that monitored Vt values in the first few minutes post birth.15–17 There was variability in the PIP values identified by these studies for achieving a Vt of 4 to 6 mL/kg.
As for sustained inflation (SI), recent studies and meta-analyses have not found clear evidence that it achieves a reduction in mortality, bronchopulmonary dysplasia or the length of stay, while they found an increased risk of mortality in the first 48 hours.18–19
What are the 2020 guidelines on the subject?The recommended initial PIP values vary between the 2020 international guidelines, with the AHA and the ILCOR recommending maintenance of a PIP between 20 and 25 cmH2O in PT infants and 25 cmH2O in term infants, while the ERC recommends starting directly with 25 cmH2O in PT infants and 30 cmH2O in term infants.1–3 When it comes to the use of SI, the 2020 ILCOR guideline, maintaining the position held in 2015, does not recommend its use in term or PT infants in the delivery room. The AHA recommendations are more specific, stating that inflations must adhere to the conventional pattern with a shorter inspiratory time (Ti) (< 1 s). The PEEP is beneficial in the respiratory stabilization of PT infants, but it has not been possible to establish the optimal PEEP since all studies in neonates have applied a PEEP of 5 cmH2O. T-piece resuscitators allow control of PEEP and PIP and can maintain more consistent tidal volumes.20
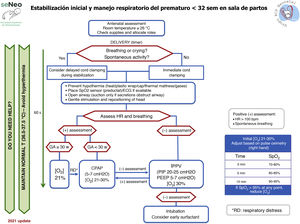
What does the NRG-SENeo recommend?Ideally, intermittent positive-pressure ventilation (IPPV) will be delivered through a ventilator with a heated and humidified mixture of gases and without interruption for a minimum of 30 seconds. We recommend a rate of 40 to 60 bpm (with an initial Ti < 1 s), a PEEP of 5 to 7 cmH2O and a PIP of 20 to 25 cmH2O in PT infants and 25 to 30 cmH2O in term infants, adjusting these settings as early as possible based on changes in heart rate (HR). Suctioning of secretions may be necessary if adequate aeration cannot be achieved, and must be performed under direct vision. Until evidence emerges to justify performance of SI, we recommend avoiding this practice and focusing ventilation and lung recruitment on the optimization of the PIP and PEEP.
Oxygen therapy in the delivery roomBackgroundThe first studies on resuscitation with room air were published in the 1990s, demonstrating that the approach could be efficacious as using 100% oxygen in addition to reducing mortality and the impact of hyperoxaemia.21 In 2010, recommendations were updated in favour of using room air in infants born at or after 35 weeks, a stance that was maintained in 2015. At the same time, other studies compared the use of a fraction of inspired oxygen (FiO2) of 1 with lower FiO2 values in very PT infants and did not find any evidence of harm, so in 2015 the ILCOR recommended initiating resuscitation in infants with a gestational age of less than 35 weeks with low FiO2 values (0.21-0.3) and advised against the use of high FiO2 values (0.65-1).
What does the evidence say?A recent meta-analysis of the ILCOR concluded that resuscitation with a FiO2 of 0.21 as opposed to a FiO2 of 1 is associated with a lower mortality in infants delivered at or after 35 weeks, with no difference in the severity of hypoxic-ischaemic encephalopathy or neurodevelopmental outcomes.22
Another meta-analysis compared resuscitation with a FiO2 of 0.5 or lower compared to greater than 0.5 in infants born before 35 weeks.23 The authors concluded that initiating ventilation with low FiO2 values did not add any benefits or risks. Within this review, we ought to highlight the To2rpido study, which found an increase in mortality in infants under 28 weeks of gestational age resuscitated with room air.24 On the other hand, an assessment of oxygen saturation (SpO2) patterns in infants under 32 weeks found that the target SpO2 was achieved within 5 minutes in only one fourth of the infants, and that a SpO2 of less than 80% at 5 minutes was associated with increases in the frequency of severe intraventricular haemorrhage and of mortalitly.25
The ILCOR has not reviewed the use of supplemental oxygen during chest compressions. A meta-analysis on the subject published in 2018 that included 8 animal studies found no differences in mortality, the return of spontaneous circulation, oxidative damage or neurologic outcomes with a FiO2 of 0.21 compared to 1.26
The ILCOR also has not reviewed SpO2 targets, so the targets published by Dawson continue to apply.27 In the context of the introduction of delayed cord clamping, one study analysed SpO2 curves in healthy term neonates subject to this approach.28 Compared to the Dawson curves (early clamping), the authors found higher SpO2 values in the first 5 minutes post birth.
What are the 2020 guidelines on the subject?In infants ≥ 35 weeks, the ILCOR recommends administration of a FiO2 of 0.21, and discourages a FiO2 of 1. In infants < 35 weeks, it recommends starting with low FiO2 values (0.21-0.3). The AHA recommends an initial FiO2 of up to 0.3, and the ERC low FiO2 values based on gestational age (0.21 for GA ≥ 32 weeks; 0.21-0.3 for GA of 28-31 weeks and 0.3 for GA < 28 weeks). If administered with chest compressions, both the AHA and the ERC recommend a FiO2 of 1. As for the SpO2 targets, the ERC uses the 25th percentile (P25) and the AHA targets around the P25-median in the Dawson charts.27
What does the NRG-SENeo recommend?In infants ≥ 35 weeks, we recommend initiation of resuscitation with a FiO2 of 0.21. In the group < 35 weeks, we recommend initiation of resuscitation with room air in infants ≥ 30 weeks and infants < 30 weeks without distress, starting with a FiO2 of 0.3 in infants < 30 weeks with respiratory distress. In infants < 28 weeks, a FiO2 of 0.3 or even 0.4 can be contemplated in the most extremely premature independently of the presence or absence of distress. If infants need chest compressions, we recommend increasing the FiO2 to 1 and reducing it after the return of spontaneous circulation.
The SpO2 is used to adjust the oxygen dose thereafter to reach a target SpO2 greater than the P25 in the Dawson charts45 and avoiding saturations greater than 90%. The current evidence is insufficient to support the use of other charts, such as charts obtained in the context of delayed cord clamping.
Ethical considerations: humane care and termination of resuscitation effortsBackgroundHistorically, there have been concerns about how parents may be affected by witnessing resuscitation and the potential impact of their presence on the performance of health care staff. Another ethical dilemma concerns the timing to terminate resuscitation efforts. The 2010 ILCOR recommendations called for terminating resuscitation after 10 minutes of an undetectable heart rate if advanced resuscitation had been provided correctly, a limit that the ILCOR maintained in 2015, although at this time the authors recommended individualising the decision taking into account factors like the place of birth, available resources, team experience, possibility of induced hypothermia and communication with the family.29 In recent years, the need to maintain this limit has been questioned.
What does the evidence say?Parents want to participate in making decisions regarding resuscitation or the withdrawal of life-sustaining treatment.30
A 10-minute Apgar score of 0 or 1 is a strong predictor of morbidity and mortality, although recently cases of infants with favourable outcomes following successful resuscitation and therapeutic hypothermia have been described (survival of 20% of the total, with 37% of survivors free of moderate to severe neurodevelopmental sequelae).31 It takes at least 20 minutes to complete and consolidate every step of resuscitation,51 after which the probability of survival with an undetectable heart rate is 13%.32
What are the 2020 guidelines on the subject?The ERC guidelines2 recommend that parents be present during resuscitation of the infant in the delivery room, but this aspect is not addressed by the AHA3 or ILCOR guidelines.1
The ILCOR has established that 20 minutes is a reasonable time to consider termination of resuscitation efforts.1
What does the NRG-SENeo recommend?We recommend that parents be present during neonatal resuscitation in the delivery room whenever possible. Parents must be informed of the resuscitation manoeuvres being performed and the reason they are necessary. At the same time, efforts should be made to achieve early skin-to-skin contact or at least visual or tactile contact under supervision of the neonatal care team in infants that are going to be moved to the neonatal unit. In case of termination or withholding of resuscitation efforts, care should focus on the comfort of the infant and the wellbeing of the parents, allowing them to be with the infant if they so desire.
It is difficult to establish a specific time at which to terminate resuscitation. If all the steps of resuscitation have been completed and the heart rate is still undetectable (ECG is a reliable means for this purpose), it is reasonable to discuss the possibility with the care team and inform the family that resuscitation efforts will be terminated after approximately 20 minutes. The decision should be individualised taking into account factors such as pre-existing foetal illness, perinatal circumstances, gestational age (no evidence available for infants < 36 weeks) and the availability of therapeutic hypothermia.
Border of viabilityBackgroundIn recent years, the literature has reflected the increase in the survival of preterm infants.33 This trend is most marked in infants born at the lowest GAs (22-23 weeks) due to the more proactive approach toward these subset of infants that has been adopted in some countries.34,35 However, there have not been clear improvements in the long-term outcomes in this population.36
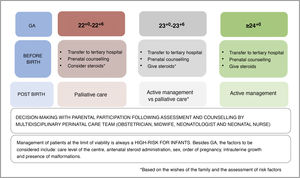
In the latest recommendations of the NRG-SENeo,29 we advocated for active treatment starting at 24+0 week of gestation and palliative care through 22+6. In infants delivered between 23+0 and 23+6, an interval considered a grey zone, the decision whether to provide active treatment would be made jointly by the medical team and the family.
What does the evidence say?There is no evidence on this subject, only guidelines or consensus recommendations published by scientific societies at the national level.37 There are 2 main trends: countries that advocate for active treatment in patients born from 22+0 weeks, such as the United Kingdom, United States, Australia and Sweden37–39 (after careful consideration of risk factors and the opinion of the parents) and countries that offer palliative care (Canada and France).40,41 Nearly all countries agree on offering active treatment from 23+0 weeks taking into account the wishes of the family.
What are the 2020 guidelines on the subject?Neither the ILCOR1 nor the ERC2 have stated a particular position on the approach to infants at the limit of viability. The ERC mentioned that each hospital should have an established approach for counselling parents in different high-risk situations, including birth at the border of viability.2
What does the NRG-SENeo recommend?In each centre, obstetrics and neonatal care teams must determine limit of viability in a shared decision-making and treatment-planning process, taking into account past outcomes of these infants in the centre and the wishes of the family.
We continue to recommend active treatment from 24+0 weeks of postmenstrual age. Between 23+0 y las 23+6 weeks, we recommend decision-making in collaboration with the family (after informing them of the morbidity and mortality risk) following assessment of the risk factors present in the perinatal period, but in infants with favourable perinatal conditions, we consider active treatment an acceptable approach in these patients.
In the case of delivery between 22+0 and 22+6 weeks, we continue to believe that palliative care is the correct approach, although a more proactive approach can be taken if families request it and the perinatal conditions are favourable, especially in infants closer to 23 weeks. We believe that antenatal steroid administration and transfer to the mother to a tertiary care hospital are imperative in the event of threatened preterm labour from 22+0 weeks of gestation to ensure the possibility of adequate assessment and prenatal counselling by experienced multidisciplinary care teams; this allows the family to make decisions after being informed of the current evidence and the development of a treatment plan based on the decision that has been reached (Fig. 2).
Other controversial subjectsDocumentation during resuscitationCorrect documentation is an important part of the resuscitation process, but the quality of records taken in the delivery room could be improved.42 Whenever possible, a specific team member not directly involved in resuscitation should be allocated the task of documenting events. The records should include any performed manoeuvres and their exact timing, vital signs and support settings (pressures, FiO2). It is important to record the SpO2 and Apgar score at 5 minutes due to their strong predictive value for the outcome of stabilization. While awaiting consensus-based guidelines, we propose using templates or standard forms used locally, in print or alternative formats.
Use of supraglottic devices (laryngeal mask airways)Their use may be considered in infants > 34 weeks (BW > 1500-2000 g) when ventilation with a facemask is ineffective or intubation is not possible or not considered safe due to a congenital anomaly, lack of equipment or lack of qualifications.2,43 Training on their use should be encouraged, especially for staff that is not used to performing advanced neonatal resuscitation manoeuvres or intubation.
Monitoring systemsRespiratory function monitors (RFMs) provide quick and accurate estimates of the Vt, which facilitates decision-making. The use of lung ultrasound can also improve the quality of cardiopulmonary resuscitation during ventilation and heart rate detection.25 Both techniques may be useful in resuscitation, but we have yet to review the evidence on the subject.
Induced hypothermia at 33 to 34 °C and neuroprotectionExcept for the AHA guidelines,3 international guidelines do not specify the gestational age from which therapeutic hypothermia is indicated for neuroprotection. The AHA considers that it is indicated from 36 weeks of postmenstrual age. In Spain, since 2011, infants ≥ 35 weeks are considered eligible,44 extending the indication to infants as young as 34 weeks of GA on a case-by-case basis, as there is limited evidence of its long-term benefits.
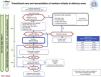
Neonatal resuscitation algorithmThe general complete neonatal resuscitation algorithm of the SENeo (Fig. 3) provides a graphic representation of the sequence of infant resuscitation in the delivery room. Starting from this general algorithm, the NRG-SENeo proposes an update of the specific algorithm for the stabilization and respiratory management of preterm infants born before 32 weeks of postmenstrual age (Fig. 4).
The ILCOR is constantly reviewing the literature, which may give rise to updates on different aspects of resuscitation published regularly after these recommendations.
Conflicts of interestThe authors have no conflicts of interest to declare.
Enrique Salguero García; Department of Neonatology, Hospital Regional Universitario de Málaga, Malaga, Spain.
Miguel Sánchez Mateos; Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, Spain.
Asunción Pino Vázquez; Hospital Clínico Universitario de Valladolid, Spain.
Elena García Victori; Department of Neonatology, Hospital Universitario Virgen del Rocío, Seville, Spain.
Eva González Colmenero; Hospital Álvaro Cunqueiro, Vigo, Spain.
Dolores Elorza Fernández; Department of Neonatology, Hospital Universitario La Paz de Madrid, Spain.
Josefa Aguayo Maldonado; Department of Neonatology Hospital Universitario Virgen del Rocío, Seville, Spain.
Máximo Vento; Department of Neonatology, Hospital Universitario y Politécnico La Fe and Instituto de Investigación Sanitaria La Fe, Valencia, Spain.
Mata Thió Lluch; Newborn Research Centre & Neonatal Services, The Royal Women’s Hospital Melbourne, Melbourne, Australia.
Appendix Adetails the members of the Neonatal Resuscitation Group of the Sociedad Española de Neonatología (NRG-SENeo).
Please cite this article as: Zeballos Sarrato G, Ávila-Álvarez A, Escrig Fernández R, Izquierdo Renau M, Ruiz Campillo CW, Gómez Robles C, et al. Guía española de estabilización y reanimación neonatal 2021. Análisis, adaptación y consenso sobre las recomendaciones internacionales. An Pediatr (Barc). 2022;96:145.