A new paediatric multisystem inflammatory syndrome, linked to SARS-CoV-2 (MIS-Paed), has been described. The clinical picture is variable and is associated with an active or recent infection due to SARS-CoV-2. A review of the existing literature by a multidisciplinary group of paediatric specialists is presented in this document. Later, they make recommendations on the stabilisation, diagnosis, and treatment of this syndrome.
Se ha descrito un nuevo síndrome inflamatorio multisistémico pediátrico vinculado a SARS-CoV-2 (SIM-PedS). Este cuadro presenta una expresividad clínica variable y se asocia a infección activa o reciente por SARS-CoV-2. En este documento se revisa la literatura existente por parte de un grupo multidisciplinar de especialistas pediátricos. Posteriormente se realizan recomendaciones sobre estabilización, diagnóstico y tratamiento de este síndrome.
It is known infection by the novel coronavirus (SARS-CoV-2) in the paediatric population is usually mild.1,2 In Spain, paediatric patients aged less than 15 years account for 0.4% of hospital admissions, 0.7% of intensive care unit admissions and 0.15‰ of deaths to date.1 However, since the beginning of May 2020, a small number of paediatric patients have developed a hyperinflammatory syndrome with a variable presentation.3 This syndrome is characterised by clinical and laboratory features similar to those found in Kawasaki disease (KD), toxic shock syndrome (TSS) or macrophage activation syndrome (MAS).4–7 Official health agencies, such as the Centers of Disease Prevention and Control (CDC) of the United States, the World Health Organization or the Royal College of Paediatrics and Child Health (RCPCH) have defined its general characteristics.5–7
This novel syndrome seems to be associated with active or recent infection by SARS-CoV-2. Most affected patients have positive IgG antibody tests and elevated inflammatory markers, which suggests immune dysregulation as opposed to a direct pathogenic effect of the virus.6,7 In this document, we will use the term paediatric multisystem inflammatory syndrome – temporally associated with SARS-CoV-2 (PIMS-TS) to refer to this condition, also known widely as multisystem inflammatory syndrome in children (MIS-C).
Definitions and differential diagnosisThe definition of PIMS-TS differs slightly between these public health agencies (Table 1).4–6 This diagnosis should be contemplated in areas with a current or recent high incidence of cases of SARS-CoV-2 or coronavirus disease 2019 (COVID-19).8 It has significant overlap with the different clinical forms of KD (complete KD, incomplete KD, KD shock syndrome [KDSS]). Therefore, some experts recommend considering PIMS-TS in patients meeting the diagnostic criteria for any of the forms of KD.9
Case definition of PIMS-TS of the World Health Organization (WHO), Centres for Disease Control and Prevention (CDC) and Royal College of Paediatrics and Child Health (RCPCH).
| WHO | Patient aged ≤ 19 years with fever ≥ 3 daysAND at least 2 of the following criteria: Rash, bilateral nonpurulent conjunctivitis, or mucocutaneous inflammation signs (oral, hands, or feet) Hypotension or shock Cardiac dysfunction, pericarditis, valvulitis, or coronary abnormalities (including echocardiographic findings or elevated troponin/BNP) Evidence of coagulopathy (prolonged PT or PTT; elevated D-dimer) Acute gastrointestinal symptoms (diarrhoea, vomiting, or abdominal pain)AND elevated markers of inflammation (eg, ESR, CRP, or procalcitonin)AND no other obvious microbial cause of inflammation, including bacterial sepsis and staphylococcal/streptococcal toxic shock syndromesAND Evidence of SARS-CoV-2 infection (positive SARS-CoV-2 RT-PCR, serology, antigen test) or contact with an individual with COVID-19 |
|---|---|
| CDC | Patient aged < 21 with fever > 24 hAND evidence of severe disease requiring hospital admission with involvement of more than 2 organs or systems (cardiovascular, respiratory, haematologic, gastrointestinal, dermatologic or neurologic) laboratory evidence of inflammation (elevated CPR, ESR, fibrinogen, PCT, D-dimer, ferritin, LDH or IL-6, neutrophilia, lymphopenia or hypoalbuminaemia)AND lack of alternative plausible diagnosisAND evidence of recent or current SARS-CoV-2 infection or exposure (positive SARS-CoV-2 RT-PCR, serology, antigen test) or of COVID-19 exposure within the 4 weeks prior to the onset of symptoms |
| RCPCH, UK | Persistent feverAND signs of inflammation (neutrophilia, elevated CRP and lymphopenia)AND evidence of single or multiple organ dysfunction (shock, cardiac, respiratory, renal, gastrointestinal or neurologic disorders), with additional features (see table in the case definition). It may include children fulfilling full or partial criteria for Kawasaki diseaseExclusion of any other microbial cause, including bacterial sepsis, staphylococcal or streptococcal shock syndromes, infections associated with myocarditis such as enterovirus SARS-CoV-2 PCR testing may be positive or negative |
BNP, B-type natriuretic peptide; COVID, coronavirus disease; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IL-6, interleukin 6; LDH, lactate dehydrogenase; PCT, procalcitonin; PPT, partial prothrombin time; PT, prothrombin time; RT-PCR, reverse transcription-polymerase chain reaction.
The following features are particularly relevant for suspicion of PIMS-TS: diagnostic criteria for complete or incomplete KD at any age, presence of gastrointestinal manifestations (vomiting, nausea, abdominal pain, diarrhoea), elevation of acute phase reactants, shock, hypotension, myocardial dysfunction, lymphopenia, anaemia and thrombocytopenia (Table 2).10
Most frequent clinical manifestations and laboratory findings.
| Clinical manifestations |
|---|
| Fever (in nearly 100% of cases); fever > 3 days (a shorter duration does not rule out PIMS-TS) |
| Gastrointestinal symptoms (> 50%): abdominal pain, vomiting, diarrhoea |
| Rash (scarlatiniform rash, erythroderma, erythema multiforme, livedo reticularis), non-exudative conjunctivitis, mucosal abnormalities, peripheral abnormalities (>2/3 patients) |
| Shock, tachycardia, hypotension, hypoperfusion (approximately half of patients) |
| Headache, meningism, confusion (10−20%) |
| Respiratory symptoms: cough, dyspnoea (30-60%) |
| Laboratory findings |
| Complete blood count: leucocytosis with lymphopenia, neutrophilia and thrombocytopenia |
| Inflammatory markers: elevation of CRP, ESR, ferritin, fibrinogen, LDH, IL-6. Normal or elevated PCT normal (in absence of bacterial infection) |
| Coagulation: fibrinogen, D-dimer elevation |
| Blood chemistry: hyponatremia, hypoalbuminemia, transaminase elevation (ALT, AST) |
| Cardiac markers: high elevation of NT-proBNP (> 200 ng/L), elevation of cardiac enzymes (troponin-I, CK-MB) |
ALT, alanine aminotransferase; AST, aspartate transaminase; CK-MB: creatine kinase myocardial band; CPR, C-reactive protein; ESR, erythrocyte sedimentation rate; IL-6, interleukin 6; LDH, lactate dehydrogenase; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, procalcitonin; PPT, partial prothrombin time; PT, prothrombin time.
The differential diagnosis should always include the following:
- -
Bacterial sepsis.
- -
Other viral infections (adenovirus, enterovirus, measles in unvaccinated individuals).
- -
Acute abdomen.
- -
Streptococcal or staphylococcal TSS.
- -
Myocarditis caused by other microorganisms.
- -
KD unrelated to SARS-CoV-2.
- -
Drug hypersensitivity reaction (Stevens-Johnson syndrome).
- -
Other systemic rheumatic disorders (systemic onset juvenile idiopathic arthritis and other autoinflammatory or autoimmune diseases).
- -
Primary or secondary haemophagocytic lymphohistiocytosis (HLH).
Initial care and stabilization will follow the ABCDE approach. The airway is usually patent, unless the patient presents with altered level of consciousness. Administer supplemental oxygen as needed. Monitor the oxygen saturation (SatO2), along with the expired carbon dioxide (EtCO2) when possible. Prepare the equipment and drugs needed for rapid sequence intubation.
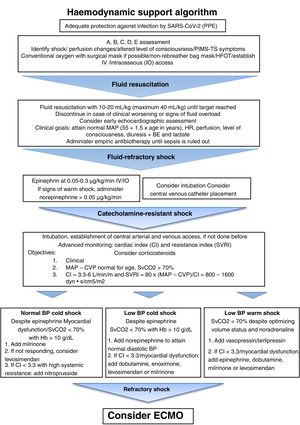
Monitor the respiratory rate (RR). Tachypnoea without breathing difficulty is a possible feature in these patients (as compensation of the metabolic acidosis associated with shock). Rule out lung infection or cardiogenic pulmonary oedema. Measure and document the blood pressure (BP), heart rate (HR) and peripheral perfusion (capillary refill time, skin temperature and colour, perfusion index). Establish peripheral vascular access (ideally, 2 lines). In case it cannot be establish, consider a peripheral catheter (ideally, 2). If this fails, consider placement of an intraosseous catheter. Fig. 1 presents the recommended algorithm for management of haemodynamic support. The level of consciousness, pupillary reflex, glycaemia and severity of pain must be assessed in every patient. It is also necessary to assess for the presence of exanthema and petechiae and measure the temperature.11
Algorithm for haemodynamic support in patients with PIMS-TS. BE, base excess; CI, cardiac index; CVP, central venous pressure; ECMO, extracorporeal membrane oxygenation; HFOT, high-flow oxygen therapy; HR, heart rate; IV, intravenous; MAP, mean arterial pressure; PPE, personal protective equipment; RR, respiratory rate; SBP, systolic blood pressure; SvCO2, central venous oxygen saturation; SVRI, systemic vascular resistance index.
Adapted from the American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Critical Care Medicine 2017;45:1062-1093.
Any patient with suspected PIMS-TS should be transferred to a hospital allowing multidisciplinary management. In severe cases, transfer to a hospital with a paediatric intensive care unit (PICU) is recommended.4–6,12
Diagnostic tests unrelated to SARS-CoV-2Alternative infectious agents that could be causing the disease must be ruled out. Table 3 details the recommended diagnostic tests and the most frequent abnormalities found in patients with suspected PIMS-TS.10–13 Patients with shock may present with lymphopenia, anaemia and thrombocytopenia and increased levels of ferritin, LDH and D-dimer D (DD).10 Patients with coronary aneurysms may present with even lower white blood cell and lymphocyte counts associated with elevation of C-reactive protein (CPR).10–13,14
Recommended diagnostic tests and most frequent abnormalities.
| Test | Abnormalities |
|---|---|
| Complete blood count | Leucocytosis (usually < 20 000/mm3) with lymphopeniaAnaemia variableMild thrombocytopenia (normally > 50 000/mm3) |
| Electrolyte panel | Hyponatraemia |
| Liver panel and albumin | Transaminase elevationHypoalbuminemia |
| Cardiac markersa | Elevation of pro-BNP (> 35 pg/mL) or NT-proBNP (> 125 pg/mL) and ultrasensitive troponin (> 14 ng/L) |
| Blood gases | Metabolic/respiratory acidosis, depending on clinical condition |
| Inflammatory markersa | Elevated CPR (> 20 mg/L), PCT (> 0.5 µg/mL), IL-6 (> 8.5 pg/mL) and ferritin (> 120 mg/dL) |
| Coagulation study | Increased fibrinogen (> 400 mg/dL)Significant D-dimer elevation D (> 500 ng/mL)Abnormal PT/PPT |
| Blood culture | Usually negative |
| Other | Coinfections |
| [0,1–2]Urine culture | |
| [0,1–2]PCR respiratory panel in nasal aspirate sample | |
| [0,1–2]Stool culture/PCR for detection of pathogens in stool | |
CPR, C-reactive protein; IL-6, interleukin 6; PCT, procalcitonin; PPT, partial prothrombin time; PT, prothrombin time.
Compared to KD or TSS, PIMS-TS usually presents with a greater elevation of inflammatory markers.10 Specifically, compared to complete KD, PIMS-TS is associated with higher levels of CRP, ferritin, inflammatory cytokines and N-terminal pro b-type natriuretic peptide (NT-proBNP) and a higher frequency of lymphopenia and thrombocytopenia, with no differences in DD levels.14–17
Another frequent feature is an increased neutrophil-lymphocyte ratio. There is a decrease in the CD4+, CD8+ and natural killer (NK) T cell counts. This phenomenon could be associated with the infiltration of the extracellular space by these innate immune system lymphocytes.18 When it comes to the cytokines, PIMS-TS manifests with elevation of interleukin (IL)-1 in its soluble form (IL-1β) and IL-6.19–21
Diagnostic tests related to SARS-CoV-2Most patients with PIMS-TS have positive results in one or more diagnostic tests for detection of acute or past infection by SARS-CoV-2. In every patient with suspected PIMS-TS, at least 1 respiratory sample should be obtained to perform a reverse-transcriptase polymerase chain reaction (RT-PCR) test. It is widely accepted that the samples that offer the highest sensitivity for detection of SARS-CoV-2 infection are bronchoalveolar lavage or endotracheal aspirate samples. Nasal swab, throat swab or nasal wash samples are also acceptable.22,23 If the RT-PCR test is negative and the clinical suspicion is high, we recommend repetition of the test in the next 24–48 hours. In case of clinical worsening requiring invasive mechanical ventilation, we recommend collection of an endotracheal aspirate sample.22
We also recommend serologic testing of all patients, independently of RT-PCR test performance or results. The yield of serology is greater after a minimum of 10 days from the onset of symptoms. In patients with negative results of both RT-PCR and serology with a high clinical suspicion, we recommend repetition of serologic testing 3–4 weeks after admission. Previous studies have reported that 26 to 55% of patients with PIMS-TS have positive RT-PCR tests and up to 90% have positive for IgG antibody tests.10–17 Antibodies can be detected from 10 to 15 days after infection, and cumulative seroconversion peaks at about 16–21 days.22,23
Imaging testsPerformance of plain chest radiograph or a lung ultrasound scan is indicated in patients with respiratory symptoms or to assess placement of indwelling devices (endotracheal tube or central lines).24 The chest radiograph can detect abnormalities in a high percentage of cases.8–25 Some of the radiographic features reported in the literature included pulmonary infiltration, patchy consolidation, ground glass opacities or interstitial changes, atelectasis and pleural effusion. The lung ultrasound scan can detect patterns compatible with pneumonia or a buildup of fluid in the lungs. Routine performance of a thoracic computed tomography (CT) scan is not recommended, except in patients with complicated pneumonia, significant worsening or who are immunocompromised.
An abdominal ultrasound or CT scan is indicated in patients with manifestations suggestive of acute abdomen to detect potential complications and assess the need of surgery. Ileocolitis has been described in some patients with PIMS-TS.
Cardiac function testsWe recommend performance of an echocardiographic study in all patients with PIMS-TS. Echocardiographic abnormalities are not usually found in patients with mild forms. The abnormalities described in patients with more severe disease include left ventricular systolic dysfunction, right ventricular dysfunction, mitral valve insufficiency, pericardial effusion and coronary artery (CA) dilatations or aneurysms.13 The assessment of the CAs includes calculation of CA size z-scores and classification based on the National Consensus Document on Kawasaki Disease.26 A high proportion of patients exhibit elevation of troponin I or T (55-68%) and BNP/NT-proBNP (83-100%), with significantly higher values compared to patients with shock.3,10–19
The electrocardiographic features are generally nonspecific. Patients with PIMS-TS may exhibit abnormalities suggestive of myocardial involvement, such as low voltage, ST segment abnormalities and prolongation of the T wave or the QT interval. Different degrees of atrioventricular block and supraventricular and ventricular arrhythmias have also been reported.9–13 Cardiovascular magnetic resonance imaging (MRI) is not indicated during the acute phase of disease. Performance of MRI should be contemplated based on the suspicion of cardiac involvement, patient safety during the test and availability of MRI in the hospital.
Hospital admission and careAll patients with suspected PIMS-TS should be admitted to the ward for observation and treatment, if applicable. All efforts must be made to keep the staff that enters in contact with the patient to the minimum necessary. The staff in contact with a patient with PIMS-TS must wear, at minimum, a FFP2 mask, disposable gowns, gloves and eye protection until the patient has at least 2 negative RT-PCR test results. A high percentage of patients will have negative RT-PCR results. This is indicative of a low or no risk of transmission. In patients with potential active infection despite a negative RT-PCR test, a second RT-PCR test must be done. Fig. 2 summarizes the recommendations on isolation measures, monitoring and treatment.
Critical patientsIf admission to PICU is necessary, the patient should be placed in an isolation room, preferably one with negative pressure.27 If performance of aerosol-generating procedures is necessary, the care staff will wear FFP3 masks, a full-face powered-air-purifying respirator, eye goggles, disposable coveralls or caps, a water-resistant disposable gown or, if the gown is not water-resistant, a water-resistant apron, and gloves. If intubation is required, use double gloves.
The patient will be accompanied by one family member or caregiver that will adhere to the isolation and hygiene measures as directed by staff and will wear, at minimum, a surgical mask, gown and gloves. If the RT-PCR is negative, modification of the required protective measures will be considered, always maintaining contact and droplet precautions.
Patients admitted to the PICU will be monitored per protocol according to their condition and disease severity. In severe cases, consider early establishment of central arterial and venous access.28
Respiratory supportRespiratory distress occurs in 70% of cases, and the need of respiratory support is usually associated with cardiovascular involvement or haemodynamic instability.24 Administer supplemental oxygen through nasal prongs under a surgical mask to maintain the SatO2 in the 94 to 98% range. If this is not sufficient due to the condition of the patient or the arterial blood gas values, consider high-flow oxygen therapy (HFOT) or non-invasive ventilation (NIV). Respiratory support must always be provided while maintaining isolation and protective measures.
In case of hypoxaemia in absence of hypercapnia, consider initiation of HFOT.
If HFOT is ineffective or as an alternative option, initiate NIV with continuous positive airway pressure (CPAP) with an oronasal mask, full-face mask or helmet (the latter provides the best seal). If the patient also has hypercapnia, consider bilevel positive airway pressure (BIPAP).29
If there is no clear improvement in clinical parameters (HR, RR, respiratory distress) and oxygenation (PaO2/FiO2, SatO2/FiO2) within a few hours, we recommend early intubation. Early intubation should also be considered in case of altered level of consciousness or fluid-refractory or catecholamine-resistant shock.29 We recommend the following initial settings for respiratory support: tidal volume of 4 to 8 mL/kg, identification of the optimal positive end-expiratory pressure to achieve adequate alveolar recruitment, plateau pressures of less than 30 cmH2O and a driving pressure of less than 15 cmH2O.22 If the patient develops moderate-to-severe acute respiratory distress, follow the recommendations for lung protection with permissive hypercapnia, use of the prone position and neuromuscular blockade. Nitric oxide is reserved for patients with refractory hypoxaemia, especially if it is associated with pulmonary hypertension.30
Haemodynamic supportHaemodynamic instability is usually associated with vasoplegic syndrome or heart failure.5,31Fig. 1 summarises the recommendations for haemodynamic support and its goals.11 In patients with heart failure administer epinephrine and consider addition of milrinone or, in case of moderate to severe heart failure, levosimendan.
PharmacotherapyImmunomodulatory therapy will be given with a stepwise approach, starting with intravenous immunoglobulin (IVIG) or corticosteroids as first-line treatment.24–32 In severe or refractory cases, we recommend combining both.
Immunomodulatory therapyIntravenous immunoglobulinIntravenous immunoglobulin should be given at a dose of 2 g/kg, especially in patients meeting the criteria for KD or TSS.12–17 Haemodynamic instability can be treated with 1 g/kg/day for 2 days. If the fever persists, consider administration of a second dose 36 h after the first.17–31
Systemic corticosteroid therapyIntravenous corticosteroid therapy is indicated both as the first step of treatment and in patients that do not respond to the initial dose of IVIG. Early administration should be considered in patients with risk factors for development of coronary aneurysms or laboratory results compatible with MAS.20–26
- -
Mild-moderate disease: intravenous methylprednisolone at 1−2 mg/kg/day for 3−5 days followed by discontinuation of methylprednisolone. In patients requiring treatment for 6 or more days or with persistent symptoms or elevation of inflammatory markers, step up to oral prednisone with tapering off over 2–3 weeks.
- -
Severe disease (shock, especially in patients requiring high doses of inotropes/vasopressors): intravenous methylprednisolone at 1−2 mg/kg/day for 3−5 days or intravenous methylprednisolone at 30 mg/kg/day for 1–3 days (maximum of 1 g). If the patient responds well, continue treatment with oral prednisone at 1−2 mg/kg/day with tapering of the dose until the inflammatory markers normalize.
- -
Kawasaki-like disease: combine corticosteroids and IVIG in patients at high risk of IVIG resistance (male, age < 12 months, CPR > 100 mg/L, platelet count < 300 000/mm3, alanine aminotransferase > 100 IU/L, neutrophil count > 80%, sodium < 133 mmol/L).9
An IL-1 receptor antagonist (anakinra) has been used successfully in patients with severe pneumonia and hyperinflammation associated with SARS-CoV-2 infection,33 MAS and KD refractory to IVIG/corticosteroid therapy. Thus, it may be beneficial to patients with PIMS-TS. It is considered a safe drug on account of its short half-life, quick action and infrequent association with bacterial superinfections. Although it is distributed in syringes for subcutaneous administration, it has been delivered intravenously in severely ill patients.5–10 The duration of treatment would be of 5–14 days depending on the clinical response (Table 4).
Dosage, precautions and route of administration of immunomodulator drugs.
| Drug | Dose | Dilution | Adverse effects | Precautions | |
|---|---|---|---|---|---|
| Anti- IL-1 | Anakinra | Subcutaneous2 mg/kg/day with progressive increase to 8 mg/kg/day every12 h (maximum of 400 mg/day)Intravenous2 options:1) similar to subcutaneous2) continuous infusion< 20 kg: single 2 mg/kg dose, followed by 0.02 ml/kg/h > 20 kg: single 2 mg/kg dose, followed by 0.01 mL/kg/hMaximum dose 400 mg/day | If intravenous, dilute with PS to concentration of 4−36 mg/mL | Local reaction at injection site, flu-like illness, neutropenia, headache, myalgia, higher vulnerability to infection | Local cooling at injection site. Monitor transaminase levels |
| Anti-TNF-α | Infliximab | 5 mg/kg | Dilute the reconstituted dose in 250 mL of normal saline. Administer over 2 h | Anaphylaxis, infection | Consider premedication with antihistamine and corticosteroid to prevent infusion reaction |
| Anti- IL-6 | Tocilizumab | Single dose< 30 kg: 12 mg/kg > 30 kg: 8 mg/kg(maximum of 800 mg) | < 30 kg: dilute in 50 mL of normal saline > 30 kg: dilute in 100 mL of normal salineAdminister over 1 h | Neutropenia, thrombocytopenia, hypertransaminasaemia, infections, intestinal perforation | Close monitoring of concurrent infections (prevent CPR elevation)Do not use if platelets < 100 000, neutrophils < 500 or AST/ALT > 3 times the baseline |
aIf the dose of anakinra is > 100 mg/day, administer every 8-12 h or as continuous infusion.
bThe intravenous route is preferred for doses > 100 mg/day or in patients with a platelet count < 20 000, haemorrhagic complications or severe oedema.
Infliximab has been proposed as an alternative treatment in patients with IVIG-resistant KD, but in clinical trials, while it achieved quicker improvement of symptoms and laboratory parameters, it did not improve long-term cardiovascular outcomes.10,11 There are few data on its use in patients with PIMS-TS (Table 4).
Interleukin-6 inhibitorsTocilizumab is approved for treatment of CAR T-cell-associated cytokine release syndrome and several trials are currently underway of its use in patients with SARS-CoV-2 pneumonia. It has been used in select cases of PIMS-TS, but the use of tocilizumab in patients with KD could accelerate the development of coronary aneurysms34,35 (Table 4).
Antiviral therapyAt the time of this writing, there is no evidence on the efficacy or safety of any antiviral used for treatment of SARS-CoV-2 infection in children. We recommend against the use of hydroxychloroquine36,37 or hydroxychloroquine combined with azitromicin.37,38 While the role of SARS-CoV-2 in the development of PIMS-TS remains unclear, treatment with remdesivir should be contemplated in case of active infection, high suspicion of SARS-CoV-2 or severe disease (Table 5). Remdesivir can be administered to paediatric patients in the context of a clinical trial or after obtaining authorization for expanded access.39
Dosage and adverse effects of antiviral therapy.
| Drug | Indication | Dose | Adverse effects | Monitoring |
|---|---|---|---|---|
| Remdesivir (intravenous) | Clinical trial/ compassionate use | Weight: 2.5-40 kgLoading dose: 5 mg/kg/24 h (1 bolus)Maintenance dose: 2.5 mg/kg/24 hWeight ≥ 40 kgLoading dose: 200 mg/24 h (1 bolus)Maintenance dose: 100 mg/24 h | HypertransaminasaemiaAltered renal function | TransaminasesRenal function |
Consider prophylaxis with low molecular weight heparin in the following cases39:
- a)
DD ≥ 6 times the upper limit of normal.
- b)
Immobilized patient.
- c)
Presence of giant aneurysms.
- d)
Severe left ventricular dysfunction (ejection fraction < 30%).
- e)
Personal or family history of thromboembolism.
- f)
Personal history of arterial ischaemia (peripheral, coronary or cerebrovascular).
Administer subcutaneous enoxaparin at 1 mg/kg/day (in case of renal insufficiency with a glomerular filtration rate < 30 mL/min/m2, dose of 0.25 mg/kg/12 h). Measure anti-factor Xa levels at 48−72 h (recommended range, 0.3−0.49). Continue until symptoms resolve and DD levels have normalized based on the reference values used at the specific hospital.
In patients with thromboembolism or suspected deep vein thrombosis, administer enoxaparin at a dose of 1 mg/kg every 12 h delivered subcutaneously. When given in anticoagulant doses (0.5–1 anti-Xa), measure anti-Xa at 48 h (adjust dose based on reference value). Maintain throughout the hospital stay and consult with haematology department prior to discharge.
Acetylsalicylic acidThere are 2 possible indications:
- -
Anti-inflammatory: patients with PIMS-TS meeting the criteria for complete or incomplete KD. Prescribe along with IVIG. Start treatment with oral acetylsalicylic acid (ASA) at a dose of 30−50 mg/kg/day given at 6 -h intervals until the patient is afebrile for 48 h. At this time, reduce to a dose appropriate for antiaggregant therapy of 3−5 mg/kg/day, also by the oral route. Maintain this dose until 6–8 weeks from the onset of symptoms and after verifying normalization of the platelet count, levels of acute phase reactants and echocardiographic parameters.26
- -
Antiaggregant: in patients with PIMS-TS that are severely ill, have aneurysms, with clinical or laboratory findings indicative of inflammation or thrombocytosis with a platelet count greater than 700 000/mm3, consider administration of ASA at an antiaggregant dose for 6 weeks (confirm normalization of echocardiographic findings at the end of this period). This recommendation is based on the description of coronary abnormalities in patients that do not meet the criteria for complete or incomplete KD.10
Patients with PIMS-TS may be eligible for extracorporeal membrane oxygenation if they fail to respond to maximal conventional therapies, their condition is deemed reversible and they have no absolute contraindications for ECMO.40 These patients must be referred before their condition precludes conventional transport. When the latter occurs, consider transport under ECMO with cannulation at the referring hospital performed by qualified staff.
Discharge and followupAssess the potential risk of transmission of SARS-CoV-2 in the household. Patients with a negative RT-PCR test and a positive IgG test are not considered infectious. Also, positive detection of IgG antibodies combined with a positive RT-PCR test or seroconversion after negative PCR results is currently interpreted as detection of dead viral remnants that are not infectious. We recommend followup by a multidisciplinary team at the outpatient level, with involvement of the primary care paediatrician (Fig. 2).
Conflicts of interestJordi Antón has received research grants, speaker and consultant fees and financial support to attend medical congresses from Sobi and Roche. Esmeralda Núñez has participated in educational activities sponsored by AbbVie, Roche and Sobi. Inmaculada Calvo has participated in educational activities sponsored by Novartis, AbbVie, Sobi, Roche and GSK and collaborated in workshops offered by GSK, Novartis and Sobi. Javier Pérez-Lescure Picarzo has received fees from MSD for delivery of educational programmes. The rest of the authors have no conflicts of interest to declare.
We thank all health care professionals involved in paediatric care, patients and their families.
Sylvia Belda Hofheinz (SECIP, Hospital Universitario 12 de Octubre de Madrid), Inmaculada Calvo Penadés (SERPE, Hospital Universitario y Politécnico La Fe), Juan Carlos de Carlos Vicente (SECIP, Hospital Universitario Son Espases), Carlos Daniel Grasa Lozano (SEIP, Hospital Universitario La Paz), Susanna Hernández Bou (SEUP, Hospital Sant Joan de Déu), Rosa M. Pino Ramírez (SEPHO, Hospital Sant Joan de Déu), Esmeralda Núñez Cuadros (SERPE, Hospital Regional Universitario de Málaga), Javier Pérez-Lescure Picarzo (SECPCC, Hospital Universitario Fundación Alcorcón), Jesús Saavedra Lozano (SEIP, Hospital General Unversitario Gregorio Marañón), Diana Salas-Mera (SECPCC, Hospital Universitario La Paz), Enrique Villalobos Pinto (SEPHO, Hospital Infantil Universitario Niño Jesús).
Please cite this article as: García-Salido A, Antón J, David Martínez-Pajares J, Garcia GG, Cortés BG, Tagarro A. Documento español de consenso sobre diagnóstico, estabilización y tratamiento del síndrome inflamatorio multisistémico pediátrico vinculado a SARS-CoV-2 (SIM-PedS). Estudio beenis. An Pediatr (Barc). 2021;94:118.