Arterial Hypertension prevalence (HTN) has significantly increased in paediatric patients, mainly in older children and teenagers. In these subjects the most common type is essential or primary HTN. However, in infants HTN prevalence is significantly lower and is almost always due to secondary causes, which can be potentially severe. Hence the importance of its detection, in order to establish an etiological diagnosis and provide an appropriate treatment, which usually requires a specialist physician. In addition to the technical difficulties of blood pressure measurement in infants, the lack of recommendations to perform a systematic screening in this age range and the absence of well-established normal values turns infancy-onset HTN into a diagnostic and therapeutic challenge for the physician. By means of the exposition of three infancy-onset HTN cases, the aim is to increase the paediatrician’s awareness of this pathology and also to provide information about its diagnostic and therapeutic approach, dealing also with pharmacological measures of treatment.
La prevalencia de la hipertensión arterial (HTA) ha aumentado considerablemente en la edad pediátrica como consecuencia principal de su detección en niños mayores y adolescentes, en quienes predomina la causa esencial (primaria). En otras etapas de la edad pediátrica la HTA es menos frecuente, en especial en lactantes. En este grupo la HTA obedece casi siempre a causas secundarias, en ocasiones potencialmente graves, por lo que resulta imprescindible detectarla, llevar a cabo un proceso diagnóstico etiológico adecuado y proporcionarle un adecuado tratamiento, que suele requerir de un profesional especializado. A las dificultades técnicas propias de la medición de la presión arterial en los lactantes se añade la ausencia de recomendaciones específicas relacionadas con su identificación sistemática y de valores de normalidad bien establecidos en este rango de edad, por lo que su manejo constituye un reto diagnóstico y terapéutico para el profesional. A través de la exposición de 3 casos de hipertensión detectada en etapa de lactante, se pretende sensibilizar al pediatra sobre esta patología, así como proporcionar información sobre su orientación diagnóstica y terapéutica, incidiendo asimismo en las medidas farmacológicas.
The prevalence of hypertension (HTN) in the paediatric age group has been increasing in recent decades mainly on account of older children and adolescents, and generally in the form of primary HTN and increasingly in association with overweight and obesity.1
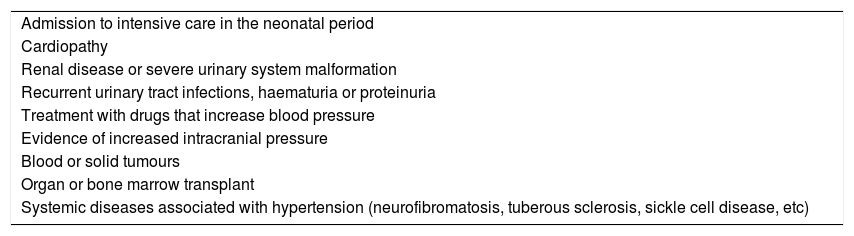
In infants and young children, HTN is significantly less frequent and usually secondary to identifiable causes. In contrast to the firmly established guidelines for routine screening of HTN in children aged more than 3 years, screening in younger children is only recommended in specific cases with known risk factors2 (Table 1). Furthermore, the difficulties involved in obtaining accurate measurements of blood pressure (BP) hinders detection of HTN in this age group.
Indications for measurement of blood pressure before age 3 years.
| Admission to intensive care in the neonatal period |
| Cardiopathy |
| Renal disease or severe urinary system malformation |
| Recurrent urinary tract infections, haematuria or proteinuria |
| Treatment with drugs that increase blood pressure |
| Evidence of increased intracranial pressure |
| Blood or solid tumours |
| Organ or bone marrow transplant |
| Systemic diseases associated with hypertension (neurofibromatosis, tuberous sclerosis, sickle cell disease, etc) |
By providing a brief description of 3 illustrative cases and subsequently discussing the most relevant points, this article aims to raise awareness in paediatricians of HTN in infants with the aim of increasing the knowledge on some unusual causes of HTN, facilitate its early identification and discuss some clinical aspects related to its management.
Clinical casesCase 1PresentationBoy aged 2 months admitted to the paediatric intensive care unit of our hospital due to respiratory failure in the context of bronchiolitis. During the hospital stay, we observed persistently high BP values of around 150/80 mmHg (95th percentile for age and height, 95/50 mmHg). The tests in the initial evaluation, which included serum urea and creatinine levels, urinary catecholamine levels, hormone levels (thyroid, cortisol, renin and aldosterone), dilated fundus examination, echocardiography and an abdominal angiogram, did not detect significant abnormalities. Treatment started with hydralazine (1.3 mg/kg/day) and 10 days after, post discharge, oral enalapril was added to a maximum dose of 0.5 mg/kg/day.
Clinical course and outcomeIn the months that followed the patient exhibited adequate BP control, so treatment with hydralazine was suspended at age 5 months, maintaining enalapril as monotherapy. During the followup, a special phenotype became apparent (macrocephaly and coarse facial features: prominent brow, wide nasal bridge with anteverted nostrils and synophrys), associated with stunted growth, feeding difficulties, psychomotor retardation and generalised hypotonia. This led to performance of diagnostic tests, which detected urinary levels of glycosaminoglycans of 52 mg/mol of creatinine (normal range, 0.92–16.25). Genetic testing at 12 months post birth identified a homozygous pathogenic variant (p.Trp402) in the IDUA gene. The patient underwent haematopoietic stem cell transplantations twice (at age 17 months and at age 19 months after failure of the initial graft), and enzyme replacement therapy with laronidase was initiated. Antihypertensive therapy was withdrawn 18 months after the second transplant. At present, at age 5 years, the patient exhibits normal renal function and BP.
DiagnosisMucopolysaccharidosis (MPS) type i in its most severe form (Hurler syndrome). Mucopolysaccharidoses are rare lysosomal storage diseases with an autosomal recessive pattern of inheritance, with the exception of MPS type ii, which is X-linked. Their global incidence is estimated at 1 in 20 000 live births.3,4 They are caused by a deficient glycosaminoglycan metabolism, with accumulation of partially degraded fragments in lysosomes, resulting in progressive cell damage and multi-organ dysfunction. Mucopolysaccharidosis (MPS) type is caused by α-l-iduronidase deficiency and the resulting accumulation of heparan sulphate and dermatan sulphate.
Case 2PresentationNewborn girl delivered at 36 weeks (birth weight, 2410 g) with prenatal diagnosis of oligohydramnios and bilateral nephromegaly that developed respiratory distress due to right-sided pneumothorax that required drainage and non-invasive ventilation in the immediate neonatal period. In the first week of life, she experienced oligoanuria, elevation of serum creatinine peaking at 1.05 mg/dL and sustained high BP (peaking at 110/85 mmHg, while the 95th percentile for gestational age is 92/65 mmHg). The initial treatment consisted of amlodipine and captopril. The abdominal ultrasound examination revealed multiple cysts in the liver and bilateral nephromegaly with loss of corticomedullary differentiation and small cysts in the renal cortex and medulla. Genetic testing confirm 2 heterozygous likely pathogenic variants (p.Thr36Met [c.107C > T] and p.Arg3240Gln [c.9719 G > A]) in the PKHD1 gene.
Clinical course and outcomeHypertension persisted past the neonatal period, requiring continuation of antihypertensive therapy with enalapril and amlodipine, both at doses of 0.2 to 0.3 mg/kg/day. From age 3 months, the patient developed proteinuria, which eventually reached nephrotic levels (peak protein/creatinine ratio, 10.2 mg/mg), and mild left ventricular hypertrophy.
At present, at age 20 months, the patient has stage 3A chronic kidney disease (estimated glomerular filtration rate, 55 mL/min/1.73 m2), the proteinuria has decreased significantly following optimization of the dose of enalapril, although it remains on the pathological range (0.6−1 mg/mg) and the left ventricular hypertrophy persists, although it is not haemodynamically significant.
DiagnosisAutosomal recessive polycystic kidney disease (ARPKD). This disorder is caused by mutations in the PKHD1 gene of chromosome 6 that encodes fibrocystin, also known as polyductin, a membrane protein expressed in cortical and medullary collecting ducts and the epithelial cells of bile ducts.5 These genetic abnormalities result in the formation of cysts in the renal cortex and medulla. This is followed by development of interstitial fibrosis, which causes progressive impairment of the renal function.
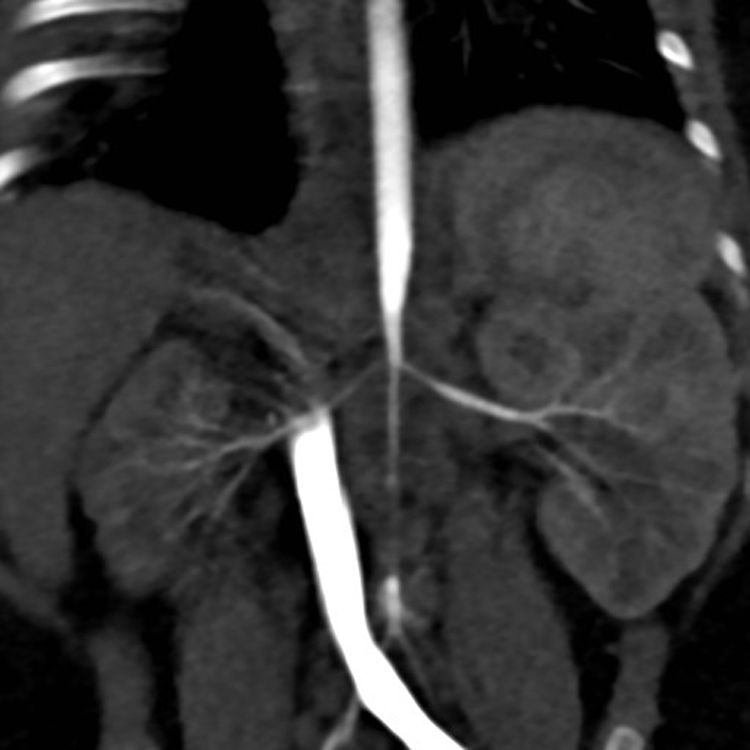
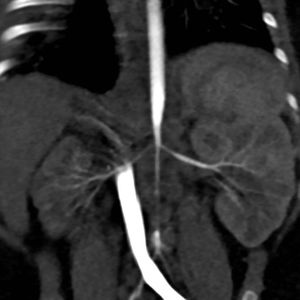
Case 3PresentationFemale infant aged 4 months with a prenatal diagnosis of hypertrophic cardiomyopathy (initially attributed to intrauterine closure of the ductus arteriosus), admitted to the paediatric intensive care unit due to a hypertensive crisis (at-home BP of 180/110 mmHg). Absence of a palpable femoral pulse or audible bowel sounds in the physical examination. A renal scintigraphy and CT angiogram of the abdominal aorta revealed a hypoplastic right kidney with decreased function (differential renal function, 9.7%) and a substantially decreased diameter of the abdominal aorta below the level of the superior mesenteric artery and narrowing of the renal arteries at the origin (Fig. 1).
Clinical course and outcomePharmacotherapy was initiated with amlodipine and propranolol, and the patient was referred to an international reference centre, where she underwent an angioplasty at age 6 months. After the surgery, there were signs of improvement of the hypertrophic cardiomyopathy. The follow-up angiogram found that the calibre of the renal arteries had increased (diameters of 2.5 and 2 mm vs previous diameters of 2 and 1.3 mm). At age 20 months, the patient underwent a second angioplasty. Currently, at age 3 years, the patient exhibits adequate control of BP with a single drug (atenolol).
DiagnosisMid-aortic syndrome. This term refers to the segmental narrowing of the abdominal aorta and its branches, chiefly the renal arteries.6 The associated HTN is usually severe and poses a therapeutic challenge that may require multiple drugs and open or endovascular surgery.
DiscussionThe 3 cases presented reflect particularities of infant HTN that are relevant for paediatricians:
- I)
All were diagnosed in the hospital setting, either as inpatients or in specialty clinics, which demonstrates that HTN in this age group is not usually identified in general paediatric care, although paediatricians should keep in mind the warning signs and risk factors for which BP measurement is recommended before age 3 years (Table 1).
- II)
They evince the secondary nature of the HTN and the need of testing with the purpose of ruling out underlying diseases, mainly systemic, renal and vascular.
- III)
The diagnosis of HTN in the first weeks or months of life requires a reliable measurement method and specific reference values, which we will discuss later in the article. However, BP values found in cases of secondary HTN are frequently high enough to eliminate most of the diagnostic uncertainty and even cause target organ damage, although the persistence of HTN and the potential role of transient factors, such as stress, pain and, of course, of measurement errors, must also be assessed.
- IV)
The treatment of HTN must both address the underlying disease, in some cases by multidisciplinary teams in reference centres, and achieve a reduction of BP, for which the paediatrician must be knowledgeable of the most effective drugs and the use of drugs for which data on the paediatric population are available.
The first case corresponds to a patient with HTN that was a chance finding in the context of a respiratory infection, leading to diagnosis of a metabolic disorder, MPS type i (Hurler syndrome). Adequate treatment of this disease achieved normalization of BP and the patient has not required antihypertensive drugs since. Hypertension has been described in patients with Hurler syndrome, although it is not among its most frequent manifestations.7–9 The underlying mechanism of HTN in this syndrome remains unclear, although it is believed to involve the obstruction of the aorta and the renal arteries caused by the deposition of mucopolysaccharides10,11 and chronic upper airway obstruction.
In the second case, HTN was diagnosed in a patient with primary renal disease (ARPKD). In these patients, HTN usually develops in the first months of life and is difficult to control, although it tends to exhibit a transient improvement after infancy.12,13 The pathogenesis of HTN is not well established; it may be caused by local activation of the renin-angiotensin-aldosterone system and an increase in sodium reabsorption, and therefore angiotensin-converting enzyme (ACE) inhibitors may be a good therapeutic option, although careful consideration must be given to their potential use in neonates or preterm infants.14,15 Poor control of HTN may accelerate the loss of renal function and cause cardiovascular and central nervous system complications.16 In these patients, BP should be measured frequently in the early months of life. Home BP monitoring with a validated oscillometric device and an appropriate cuff, while not used routinely, can be contemplated in select patients.17
In the last case, the development of refractory HTN led to diagnosis of severe renovascular HTN associated with mid-aortic syndrome, which posed a significant diagnostic and therapeutic challenge. Renovascular HTN is a form of HTN secondary to renal artery stenosis. The decreased renal blood flow results in decreased glomerular filtration, which stimulates the renin-angiotensin-aldosterone system. The vasopressor effect of angiotensin II and the retention of sodium and water and the renal level induced by aldosterone cause an increase in BP.18 In this case, HTN was detected during the followup of a cardiopathy, so this patient, like the patient with ARPKD, belonged to one of the risk groups in which screening for HTN is indicated.
These cases illustrate that early detection of HTN in infants is crucial. The problems involved in BP measurement in this age group and the lack of guidelines regarding routine screening of BP in infants add to the difficulties hindering the correct identification of these patients. Measuring BP in infants is indeed difficult and readings are commonly affected by a variety of external factors (equipment, measurement setting, measurement during crying or sleep, position of the infant, etc). In clinical practice, adequate measurements continue to be hardly attainable, and many facilities have yet to introduce protocols adapted to patients aged less than 1 year.19 A specific protocol has been published to standardise BP measurement in infants,20 and its use has been supported by a recent systematic review.21 It recommends the following:
- •
Method: oscillometric, to be used routinely. Invasive intraarterial BP monitoring for confirmation as needed in select cases.
- •
Cuff with inflatable bladder and a width of approximately 50% the circumference of the middle of the arm of the child.
- •
Measurement site: right arm.
- •
With the oscillometric method, the mean BP value is the best value to compare with reference values.
- •
Take at least 3 measurements at least 2 min apart.
Another challenge in infants is the very definition of HTN. It is known that there is a physiological increase in BP with increasing age and weight from birth, so the definition of HTN in paediatrics is based on the distribution of BP values in the healthy population and not in the morbidity and mortality associated with specific BP values, contrary to the practice in adults. Hypertension is defined as a persistent elevation of the systolic and/or diastolic BP above the 95th percentile for sex, age and height in at least 3 separate measurements. Values between the 90th and 95th percentile are considered indicative of prehypertension (United States guidelines2) or high-normal BP (European guidelines1).
On the other hand, there are other factors (gestational age, postnatal age and postconceptional age, birth weight, weight for gestational age etc) that must be taken into account to define the normal range of BP in early life. In 2012, Dionne et al17 compiled the data available to date on BP in newborn infants22–31 and produced a table summarising BP values obtained in neonates between 26 and 44 weeks of postconceptional age to allow classification of HTN in term and preterm neonates as it is done in older children. To date, no alternative data have been published, so these values continue to be the sole available reference for neonates. When it comes to infants aged 1 month to 1 year, normal BP values were published in the Report of the second task force on blood pressure control in children–1987,32 which also continue to be the reference for this age group. In young children aged more than 1 year, the Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents of the United States, published in 20172 as an update to the Fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents,33 should be used to identify HTN.
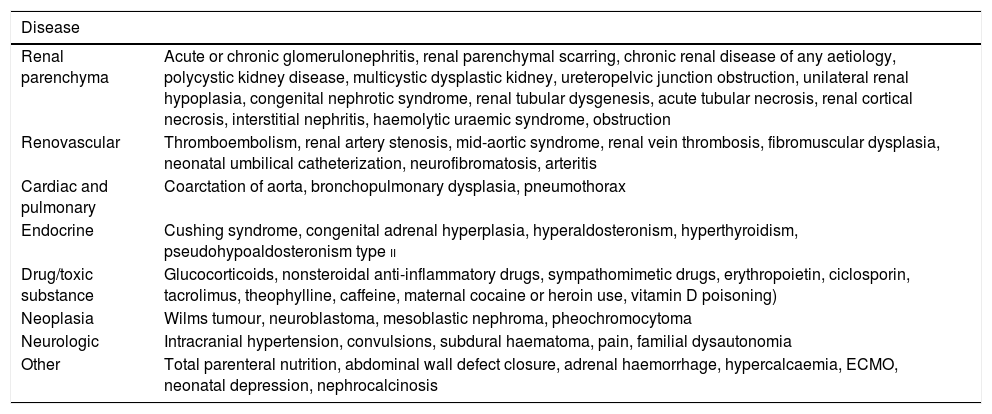
The particularity of HTN in infants that is most relevant to clinical practice involves its aetiology. In infancy, in contrast to childhood and adolescence, HTN has a potentially identifiable cause in nearly all cases, and therefore an adequate aetiological diagnosis must be performed in this age group. Renovascular HTN and diseases of the renal parenchyma (polycystic disease, urinary tract defects, etc) seem to be the most frequent causes, but non-nephrourologic forms of HTN also need to be considered. Table 2 details the possible causes of HTN in infants. A detailed physical examination and history should serve to correctly guide the selection of diagnostic tests (see Table 3).
Differential diagnosis of hypertension in infants.
| Disease | |
|---|---|
| Renal parenchyma | Acute or chronic glomerulonephritis, renal parenchymal scarring, chronic renal disease of any aetiology, polycystic kidney disease, multicystic dysplastic kidney, ureteropelvic junction obstruction, unilateral renal hypoplasia, congenital nephrotic syndrome, renal tubular dysgenesis, acute tubular necrosis, renal cortical necrosis, interstitial nephritis, haemolytic uraemic syndrome, obstruction |
| Renovascular | Thromboembolism, renal artery stenosis, mid-aortic syndrome, renal vein thrombosis, fibromuscular dysplasia, neonatal umbilical catheterization, neurofibromatosis, arteritis |
| Cardiac and pulmonary | Coarctation of aorta, bronchopulmonary dysplasia, pneumothorax |
| Endocrine | Cushing syndrome, congenital adrenal hyperplasia, hyperaldosteronism, hyperthyroidism, pseudohypoaldosteronism type ii |
| Drug/toxic substance | Glucocorticoids, nonsteroidal anti-inflammatory drugs, sympathomimetic drugs, erythropoietin, ciclosporin, tacrolimus, theophylline, caffeine, maternal cocaine or heroin use, vitamin D poisoning) |
| Neoplasia | Wilms tumour, neuroblastoma, mesoblastic nephroma, pheochromocytoma |
| Neurologic | Intracranial hypertension, convulsions, subdural haematoma, pain, familial dysautonomia |
| Other | Total parenteral nutrition, abdominal wall defect closure, adrenal haemorrhage, hypercalcaemia, ECMO, neonatal depression, nephrocalcinosis |
Diagnostic tests to identify the underlying cause of hypertension in neonates and infants.
| Routine testing | Blood: complete blood count, creatinine, urea, electrolytes, calcium, uric acid, glucose, cholesterol, triglycerides |
| Urine: urinalysis and urine sediment, protein-creatinine ratio, albumin-creatinine ratio | |
| Echocardiogram | |
| Abdominal ultrasound with Doppler renal ultrasound | |
| Specific tests based on suspected diagnosis | Hormone tests (renin, aldosterone, adrenal steroids, thyroid hormones), urinary catecholamines, molecular/genetic testing, toxicology, chest radiograph, vascular imaging |
Certain aspects need to be considered when it comes to the treatment of HTN in infants. Due to its secondary nature, the first step of treatment should be aimed at correcting iatrogenic causes (use of pharmaceuticals, electrolyte abnormalities, such as hypercalcaemia, etc) and reversible causes (hormone replacement therapy in endocrine disorders, treatment of hypoxaemia in patients with bronchopulmonary dysplasia, surgical repair of coarctation of aorta, etc). Once this is done, the use of pharmacotherapy should be weighed carefully, especially considering the lack of adequate evidence in this age range for most of the commonly used drugs. In general, pharmacological treatment is recommended in case of persistent BP above the 99th, starting with a single drug. If the single drug does not achieve good control of BP, the dose should be increased without unnecessary delay, or another drug added, if needed, in order to attain adequate control in the shortest possible time.
The particularities of this age group entail the nearly generalised use of compounded formulations, which involves a more precise dosage of the administered drugs and facilitates adherence to treatment. On the other hand, the higher cost of compounding, the lower availability in pharmacies and the problems associated with the long-term stability of these formulations pose limitations to their use.
Different drugs may be used to lower BP in clinical practice, such as diuretics, ACE inhibitors, beta blockers calcium antagonists and direct vasodilators (hydralazine), although data on their use in this age group is scarce. Overall, the hypotensive drugs used most frequently in the paediatric age group are ACE inhibitors and calcium antagonists. The most frequent indication for prescription of ACE inhibitors is renal disease (on account of their effect on proteinuria) and heart failure, and they are contraindicated in case of hyperpotassaemia, bilateral renal artery stenosis or renal artery stenosis in patients with solitary kidney (they may cause reduced renal blood flow and thus impair renal function). In any case, their use in infancy, especially in preterm infants, is controversial due to the role of the renin-angiotensin-aldosterone system in nephrogenesis in this stage of development, on account of which it has been hypothesised that it may interfere with the late stages of nephron maturation.17 Calcium antagonists have an adequate hypotensive effect, and their use is contraindicated in case of heart failure.
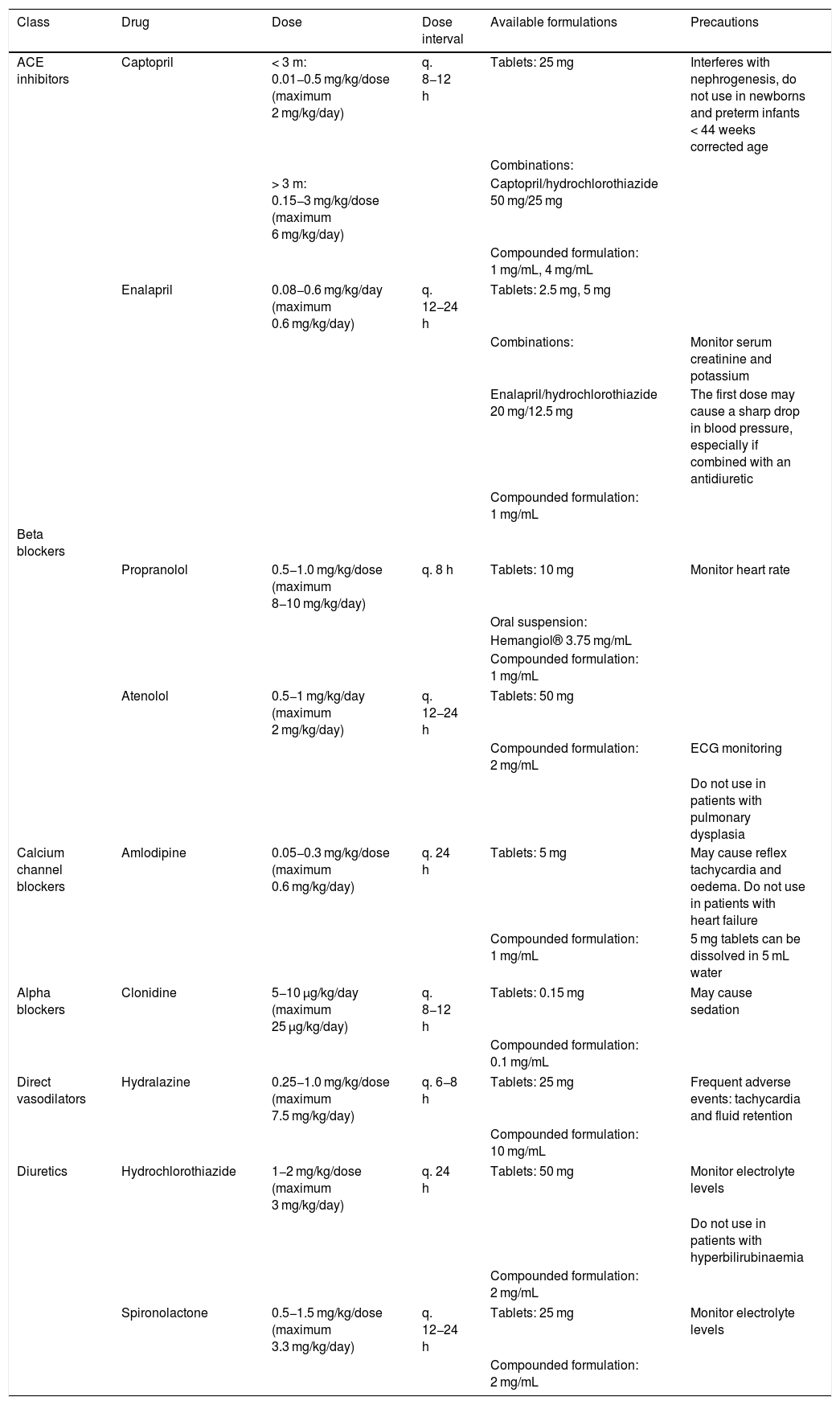
Diuretics and beta-blockers are not generally prescribed as first-line treatment in children, contrary to the practice in adult patients. Loop diuretics may be useful in patients with heart failure but are contraindicated in patients with nephrocalcinosis or hypercalciuria. Thiazides are contraindicated in case of hyperbilirubinaemia (especially in neonates), hyperglycaemia, hyperuricaemia or dyslipidaemia. Beta blockers are also indicated in patients with heart failure (except in case of decompensation) or patients with pheochromocytoma in combination with alpha blockers, and they must be avoided in patients with asthma or diabetes (with the exception of alpha and beta dual receptor blockers, such as carvedilol). Intravenous hydralazine is a good option for hypertensive crises, but it is not recommended for long-term treatment on account of its adverse effects (tachycardia, flushing).34,35 Hypotensive drugs delivered through continuous infusion (labetalol, nicardipine, nitroprusside) should be reserved for the rare hypertensive crisis. Table 4 presents the antihypertensive drugs most frequently used in neonates and infants along with their dosage, commercial formulations and precautions.
Oral antihypertensive drugs commonly used in infants.
| Class | Drug | Dose | Dose interval | Available formulations | Precautions |
|---|---|---|---|---|---|
| ACE inhibitors | Captopril | < 3 m: 0.01−0.5 mg/kg/dose (maximum 2 mg/kg/day) | q. 8−12 h | Tablets: 25 mg | Interferes with nephrogenesis, do not use in newborns and preterm infants < 44 weeks corrected age |
| Combinations: | |||||
| > 3 m: 0.15−3 mg/kg/dose (maximum 6 mg/kg/day) | Captopril/hydrochlorothiazide 50 mg/25 mg | ||||
| Compounded formulation: 1 mg/mL, 4 mg/mL | |||||
| Enalapril | 0.08−0.6 mg/kg/day (maximum 0.6 mg/kg/day) | q. 12−24 h | Tablets: 2.5 mg, 5 mg | ||
| Combinations: | Monitor serum creatinine and potassium | ||||
| Enalapril/hydrochlorothiazide 20 mg/12.5 mg | The first dose may cause a sharp drop in blood pressure, especially if combined with an antidiuretic | ||||
| Compounded formulation: 1 mg/mL | |||||
| Beta blockers | |||||
| Propranolol | 0.5−1.0 mg/kg/dose (maximum 8−10 mg/kg/day) | q. 8 h | Tablets: 10 mg | Monitor heart rate | |
| Oral suspension: | |||||
| Hemangiol® 3.75 mg/mL | |||||
| Compounded formulation: 1 mg/mL | |||||
| Atenolol | 0.5−1 mg/kg/day (maximum 2 mg/kg/day) | q. 12−24 h | Tablets: 50 mg | ||
| Compounded formulation: 2 mg/mL | ECG monitoring | ||||
| Do not use in patients with pulmonary dysplasia | |||||
| Calcium channel blockers | Amlodipine | 0.05−0.3 mg/kg/dose (maximum 0.6 mg/kg/day) | q. 24 h | Tablets: 5 mg | May cause reflex tachycardia and oedema. Do not use in patients with heart failure |
| Compounded formulation: 1 mg/mL | 5 mg tablets can be dissolved in 5 mL water | ||||
| Alpha blockers | Clonidine | 5−10 μg/kg/day (maximum 25 μg/kg/day) | q. 8−12 h | Tablets: 0.15 mg | May cause sedation |
| Compounded formulation: 0.1 mg/mL | |||||
| Direct vasodilators | Hydralazine | 0.25−1.0 mg/kg/dose (maximum 7.5 mg/kg/day) | q. 6−8 h | Tablets: 25 mg | Frequent adverse events: tachycardia and fluid retention |
| Compounded formulation: 10 mg/mL | |||||
| Diuretics | Hydrochlorothiazide | 1−2 mg/kg/dose (maximum 3 mg/kg/day) | q. 24 h | Tablets: 50 mg | Monitor electrolyte levels |
| Do not use in patients with hyperbilirubinaemia | |||||
| Compounded formulation: 2 mg/mL | |||||
| Spironolactone | 0.5−1.5 mg/kg/dose (maximum 3.3 mg/kg/day) | q. 12−24 h | Tablets: 25 mg | Monitor electrolyte levels | |
| Compounded formulation: 2 mg/mL | |||||
In short, we analysed these cases with the aim of raising awareness in the readers on HTN in infancy, as it is an infrequent problem that is difficult to detect but can lead to the diagnosis of severe and clinically relevant underlying diseases. Thus, being aware and keeping in mind this disease is essential to enable early diagnosis through screening of at-risk patients and subsequently determine its aetiology for the purpose of providing adequate care.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Díaz Anadón LR, López CG, Ordóñez Álvarez FA, Rodríguez FS. Hipertensión arterial en el lactante. Un reto diagnóstico en pediatría. An Pediatr (Barc). 2021;94:119.