Congenital leukaemia (CL) is defined as leukaemia with onset in the neonatal period, and accounts for fewer than 1 % of cases of infant leukaemia. Contrary to the pattern in the rest of childhood, two thirds of cases correspond to acute myeloid leukaemia (AML). It typically presents with cytopaenia, hyperleukocytosis, hepatosplenomegaly and coagulopathy and, in up to 60 % of patients, cutaneous infiltration.1 This article presents 2 cases of CL managed in our hospital.
The first was an infant aged 2 months that had onset with fever and malaise. From the first month of life, the patient exhibited a purplish, elastic lump in the anterior fontanelle, with subsequent development of nodules in a generalised distribution. This was associated with pallor of the skin and mucosae, hepatosplenomegaly, respiratory failure and generalised hypertonia. The salient findings of blood tests were anaemia (4.4 g/dL), thrombocytopenia (66,000/mm3), leucocytosis (38,440/mm3) with atypia, elevation of lactate dehydrogenase (LDH) (2357 U/L) and hyperuricemia (7.49 mg/dL). The acute phase reactants were negative. The diagnosis of CD10-B-precursor acute lymphoblastic leukaemia (ALL) was confirmed by bone marrow aspiration. Fluorescence in situ hybridization (FISH) detected rearrangement of the MLL gene (11q23) with the t(10;11) translocation, and chromosome analysis revealed a normal 46 XX karyotype. The findings of cerebrospinal fluid (CSF) analysis, fundoscopy and transfontanellar ultrasound examination were normal. The patient received chemotherapy following the Interfant-99 protocol for the high-risk group and achieved complete remission after the induction phase with persistence of MLL+. After completing the consolidation phase, the patient underwent a pretransplantation evaluation that revealed bone marrow infiltration with 80 % of myeloid blasts (M0–M1), infiltration of the CSF and additional skin lesions. Molecular tests once again detected the t(10:11) translocation. Following cytoreductive chemotherapy (hydroxyurea, cytarabine and tioguanine), the patient was treated with intravenous fludarabine and intrathecal chemotherapy, achieving a second complete remission. Later on, the patient underwent haematopoietic stem cell transplantation (HSCT) of peripheral cord blood cells from a matched unrelated donor after conditioning with busulfan, cyclophosphamide, thiotepa and thymoglobulin. Full donor chimerism was not achieved and the patient experienced a second marrow relapse with a myeloid immune phenotype, which was treated with a FLAG-Ida regimen (idarubicin, fludarabine and cytarabine). The patient experienced recurrence of massive infiltration by lymphoid blasts during haematologic recovery, and died at age 12 months.
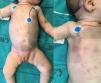
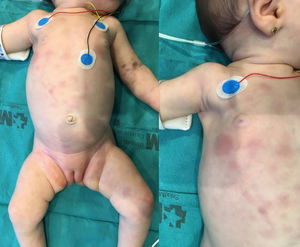
The second patient was an infant aged 7 weeks that developed indurated purplish nodular lesions in the skin with a generalised distribution starting at 20 days of age (Fig. 1), accompanied by pallor of the skin and mucosae and splenomegaly. The salient findings of blood tests were leucocytosis (32,910/mm3) with atypia, anaemia (8.7 g/dL), thrombocytopenia (100,000/mm3), elevation of LDH (661 U/L) and coagulopathy. Examination of a bone marrow aspiration sample revealed infiltration with 90 % of blasts with an immune phenotype compatible with M0 AML, and FISH revealed MLL rearrangement with no evidence of translocation. There was no central nervous system infiltration. The patient received chemotherapy following the St Jude AML 02 protocol, with resolution of the skin lesions on day 8 and complete full remission with a minimal residual disease of less than 0.1 % after the first cycle of ADE (cytarabine, etoposide, daunorubicin). After a second cycle of ADE, the patient, in first complete remission, underwent HSCT of αβ/CD19-depleted peripheral blood from a haploidentical donor (the mother). The patient had achieved complete chimerism at 30 days post transplantation, and the only complication she experienced was grade 3 acute cutaneous graft versus host disease, which has since resolved. The patient is in complete remission 15 months after the diagnosis.
The incidence of CL is estimated at 1–5 cases per million live births. Approximately 65 % of cases are cases of AML compared to 35 % of cases of ALL.1 Due to the immaturity of haematopoietic stem cells in these patients, co-expression of myeloid and lymphoid markers is frequent in the neonatal period, and so is lineage switch, as illustrated by the first case presented here. The diagnosis of CL also requires the presence of blasts in blood or bone marrow and extramedullary haematopoietic organs, ruling out other diseases such as leukemoid reactions or transient myeloproliferative disorder of Down syndrome.1,2
As observed in the presented cases, cutaneous infiltration in CL is characterised by the presence of erythematous, purple papules, plaques or nodules, usually with a generalised distribution. Due to its appearance, this rash is referred to as “blueberry muffin syndrome”, a term that can be applied to lesions of different aetiologies such as neuroblastoma metastases, Langerhans cell histiocytosis or leukemoid reactions caused by congenital infections or haemolytic anaemia, all of which must be included in the differential diagnosis.1,2 In up to 10 % of cases, leukaemia cutis is the sole manifestation of disease, with no bone marrow infiltration.2,3 Although cases of spontaneous resolution of CL have been described,4,5 cutaneous infiltration does not have an impact on the course of disease. In general, the survival of patients with CL is poor, with the most representative case series reporting survival rates of 25 % in patients with AML and up to 17 % in patients with ALL.6
Please cite this article as: Hernández MC, Catalán MA, Angulo BM, Robleda AC, López LM. Infiltración cutánea como manifestación de leucemia congénita: presentación de dos casos. An Pediatr (Barc). 2020;92:106–108.