Preterm infants at high risk for retinopathy of prematurity (ROP) should undergo regular retinal examinations,1 which may be painful.2,3 Assessing pain accurately in newborns continues to be a challenge, and there is a need for more objective and reliable tools.4
The aim of our study was to evaluate the changes in skin conductance (SC) during eye examinations conducted for ROP screening and to study their association with changes in heart rate and oxygen saturation.
We carried out a prospective observational study from January to May 2017. We included infants born at 32 or fewer weeks’ gestation that were candidates for retinal screening according to national guidelines.5 We used both pharmacological and nonpharmacological pain relief measures, including swaddling, facilitated tucking, a quiet environment, oral paracetamol, sucrose, non-nutritive sucking and topical anaesthetic drops. We measured skin conductance with a Med-Storm® monitor (Med-storm Innovation; Oslo, Norway) and a 3-electrode system applied to the sole of the foot. We recorded the number of SC fluctuations per second (NSCF) in peaks/sec. More extensive descriptions of the SC monitor and the NSCF have been published previously.6 We recorded the maximum NSCF at 4 predefined time points: baseline, at placement of the lid speculum, at scleral indentation and at the end of the procedure. We also recorded all episodes of tachycardia (>180bpm), bradycardia (<100bpm) and oxygen desaturation (saturation<85% for >10s). The analysis included a maximum of 3 examinations per patient. We obtained the written consent of the parents, and the study protocol was approved by the local research ethics committee.
The sample included 30 eye examinations performed in 19 patients (8 girls, 11 boys) born at a mean gestational age of 30.1 weeks (standard deviation [SD], ±1.1) and a mean birth weight of 1226.3g (SD, ±259.6). None of the patients required mechanical ventilation, while 3 (15.7%) required supplementary oxygen.
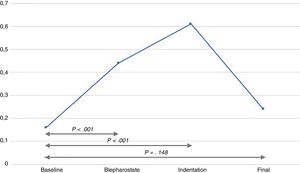
The infants experienced episodes of tachycardia in 19 out of the 30 examinations (63.3%), bradycardia in 6 (20%) and desaturation in 15 (50%). There was a significant increase in the NSCF from baseline to both lid speculum insertion and to scleral indentation (Fig. 1). The mean maximum NSCF during the examination was 0.64peaks/s (SD, ±0.4), most frequently at the time of scleral indentation.
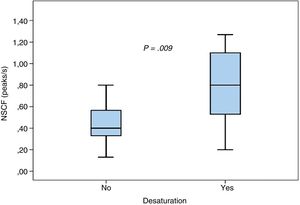
We found no statistically significant differences in the mean maximum NSCF between patients with and without tachycardia (0.74±0.52 vs 0.58±0.31; P=.3) or between patients with and without bradycardia (0.84±0.62 vs 0.59±0.32; P=.17). Episodes of desaturation were associated with a higher NSCF (0.83±0.46 vs 0.44±0.19; P=.009), as can be seen in Fig. 2.
Skin conductance methods are based on stress-induced sweating and reflect increases in sympathetic nervous system activity. This sweating is a reaction to any of various emotional stimuli, such as nervousness, anxiety, fear, stress, or pain. When palmar and plantar sweat glands fill as a result of sympathetic nerve firing, skin resistance decreases; and when the sweat is reabsorbed, skin resistance increases. This outcome gives rise to the peaks in SC that are used to evaluate pain and stress (the NSCF).
In our study, we recorded marked changes in SC during an examination already known to be painful, illustrating that SC may be a good marker of acute pain. To our knowledge, no prior studies on SC have been performed in this setting. We ought to mention that the NSFC values that we observed during the eye examination were higher compared to those reported in association with other painful procedures (such as heel prick and venepuncture),6 which suggests that the level/intensity of pain is higher during a ROP evaluation.
Our study involves several limitations. First, we cannot exclude that uncontrolled confounders have influenced SC responses. Second, our study was designed as a pilot study aiming to evaluate feasibility and changes in the NSCF; as such, we did not use any clinical scales to assess pain responses. An observational study is currently underway to investigate the correlation between clinical scales and SC.
Our results show a marked increase in the NSFC during eye examinations for ROP screening coupled with frequent changes in heart rate and desaturation episodes. We conclude that these changes in NSFC during ROP screening could be related at least partially to pain, but further research should investigate whether SC values are correlated to validated pain scores and determine whether SC can be a helpful tool for the detection of pain during ROP screening. Our data could serve as a reference in such future studies.
Please cite this article as: Avila-Alvarez A, Vazquez Gomez L, Sucasas Alonso A, Romero Rey H, Cabana Vazquez M. La conductancia de la piel para evaluar el dolor y el estrés durante el cribado de la retinopatía de la prematuridad. An Pediatr (Barc). 2020;92:365–366.