The incidence of serogroup C invasive meningococcal disease (IMD) has decreased since the introduction of systematic vaccination in 2000 The aim of this study is to determine the number serogroup C IMD cases diagnosed since then and the vaccine failures.
Patients and methodsA retrospective analysis was performed on patients diagnosed with IMD by culture or polymerase chain reaction (PCR) in a maternity and childhood hospital in Barcelona between 2001 and 2018. An analysis was made of the number of vaccine doses and the age received, as well as on the medical records and vaccine cards.
ResultsThere were 128 confirmed cases of IMD (7.1 cases/year; 70.3 in <5 years). The serogroup was studied in 125 (97.6%) cases, in which 103 (82.4%) were B, 10 (8%) were C, 1 (0.8%) was 29E, and 1(0.8%) was Y, and only 10 (8%) were not able to be serogrouped. Of the 10 patients with serogroup C, 4 were not vaccinated, and in 3, the course was not complete as regards the number of doses. The other 3 received the complete course according to age and current calendar, and thus were considered vaccine failures. A total of 6 patients died (mortality rate: 4.7%), 5 due to serogroup B (mortality: 4.8%), and 1 due to serogroup C (mortality: 10%).
ConclusionsSerogroup C only represented 8% (10 cases) of IMD cases in the period studied, 3 cases due to this serogroup being vaccine failures.
La incidencia de la enfermedad meningocócica invasiva (EMI) por serogrupo C ha disminuido desde la introducción de la vacunación sistemática en 2000. El objetivo de este estudio es determinar los casos de EMI diagnosticados desde entonces y los fallos vacunales en los casos por serogrupo C.
Pacientes y métodosAnálisis retrospectivo de pacientes diagnosticados de EMI confirmada por cultivo o reacción en cadena de la polimerasa, en un hospital materno infantil de tercer nivel de Barcelona, entre 2001 y 2018. Se analizó el número de dosis de vacuna recibidas y la edad, recogidos de la historia clínica y del carnet de vacunaciones.
ResultadosSe confirmaron 128 casos de EMI (7,1 casos/año; 70,3% en <5 años). Se estudió el serogrupo en 125 casos (97,6%): 103 fueron B (82,4%), 10 fueron C (8%), 1 fue 29E (0,8%) y 1 fue Y (0,8%); solo 10 (8%) no fueron serogrupables. De los 10 pacientes con serogrupo C, 4 no estaban vacunados y en 3 la pauta fue incompleta en cuanto a número de dosis; 3 de ellos recibieron la pauta completa según la edad y el calendario vacunal vigente, por lo que se consideran fallos vacunales. Fallecieron 6 pacientes (tasa letalidad: 4,7%): 5 por serogrupo B (letalidad: 4,8%) y 1 por serogrupo C (letalidad: 10%).
ConclusionesEl serogrupo C representó solo el 8% (10 pacientes) de los casos de EMI en el periodo de estudio siendo 3 de los casos por este serogrupo fallos vacunales.
Invasive meningococcal disease (IMD) continues to be an important public health problem on account of its mortality, the high proportion of patients that develop sequelae, the significant impact on families and the social alarm that it generates.1,2
Neisseria meningitidis is a gram-negative diplococcus and strictly human pathogen that adheres to the surface of mucosal cells in the nasopharynx, resulting in an asymptomatic carrier status. Nasopharyngeal colonization is a necessary condition for IMD to develop. The carriage prevalence ranges from 4.5% in infants and 23.7% in individuals aged 19 years, thereby decreasing until reaching 7.8% in individuals aged 50 years.3,4 Individuals that carry the pathogen in the nasopharynx can transmit the infection to young children, adolescents and elderly adults, which are the age groups in which the incidence of IMD is highest.
There are 12 serogroups of N. meningitidis, of which 6 (A, B, C, W, Y and X) cause more than 95% of cases of meningococcal disease.5,6 Prevention through vaccination is available for 5 of these serogroups, and 5 types of vaccines are currently available: 2 conjugate monovalent vaccines for groups A and C (MenC conjugate vaccine); one conjugate quadrivalent vaccine (ACWY), and 2 non-conjugated vaccines against group B. Three pentavalent vaccines are currently undergoing trials,7–9 one of which includes group X (ACWYX), of great interest for the African continent, and 2 others that include group B (ABCWY), more applicable to Europe.
In Spain, the incidence of meningococcal disease has decreased significantly in the past 20 years, in part thanks to the introduction of routine vaccination with the MenC conjugate vaccine in 2000 followed by excellent vaccine coverages. Elective vaccination against serogroup B, which has been available since 2015, may also have contributed, as there is evidence of cross-protection against serogroup C.10 Nevertheless, meningococcal disease continues to be serious and associated with a high mortality. Based on data from the national epidemiological surveillance system of Spain,11 in the 2017-2018 period there were 346 confirmed cases, corresponding to an incidence of 0.74 confirmed cases per 100 000 inhabitants and a 76.2% increase relative to the 2013-2014 period (195 cases and incidence of 0.42); in the late 1990s, before the introduction of vaccination against meningococcal C disease, the incidence of IMD peaked at 4 cases per 100 000 inhabitants. However, considering the data by age group, the incidence in the latter period was much higher in children aged less than 1 year (8.65 cases per 100 000 inhabitants). Among the confirmed cases, 41% corresponded to serogroup B, 13.9% to serogroup W, 11.6% to serogroup C and 10.7% to serogroup Y. The rest of the cases corresponded to nontypeable strains (9.2%), serogroups less prevalent in Spain (1.1%), or a serogroup that had not been determined (12.1%).11
Despite the high vaccine coverage rate, cases of IMD caused by serogroup C have been notified in recent years.11–17 We ought to highlight an increase in the number of cases caused by serogroup C in the last documented period, 2017-2018 (40 cases), compared to 2013-2014 (15 cases).11 Nevertheless, from the time it was included in the routine vaccination schedule, vaccination against meningococcal C disease has had a significant impact in controlling the disease.11,12,18
The aim of our study was to analyse the cases of IMD that required admission to a tertiary care hospital from the time the MenC conjugate vaccine was included in the routine immunization schedule of the autonomous community of Catalonia in 2000, analyse the serogroup distribution of the cases, and determine the vaccination status of patients with meningococcal C disease.
Patients and methodsWe conducted a retrospective analysis of the health records of patients aged less than 16 years with a diagnosis of IMD confirmed by culture or gene amplification (polymerase chain reaction [PCR]), admitted to our hospital (a tertiary referral children’s hospital with more than 3000 admissions per year and 41 intensive care beds) between January 2001 and December 2018. We collected data on the microbiological tests performed and epidemiological and clinical variables.
Microbiological testingWe retrieved the results of culture or PCR in blood or cerebrospinal fluid (CSF) samples. Blood samples were cultured in Bact/Alert® bottles (bioMérieux; Marcy-l’Étoile, France) and CSF samples, following centrifugation, were cultured in conventional solid media and enriched liquid media. All samples were incubated for 5 to 6 days. Culture isolates were identified by biochemical tests or protein fingerprinting (Vitek®2 NH Card or the Vitek MS MALDI-TOF mass spectrometry identification system, respectively, bioMérieux; Marcy-l’Étoile, France). In the interpretation of antimicrobial susceptibility tests, we applied the cut-off points recommended in the guidelines of the European Committee on Antimicrobial Susceptibility Testing and the Clinical and Laboratory Standard Institute. In the molecular analysis of CSF, sample volumes of at least 100 μL were used to perform multiplex real-time PCR for detection of N. meningitidis (ctrA region, capsular transport gene), Haemophilus influenzae type b (bexA, capsulation gene) and Streptococcus pneumoniae (ply, pneumolysin gene) using TaqMan® fluorescent probes in a SmartCycler® thermal cycler (Cepheid, Sunnyvale, USA).19 Serotyping of N. meningitidis was done by slide agglutination tests with specific antisera to groups B and C (Difco™ Neisseria meningitidis Antiserum Group B/Group C; Becton Dickinson, Sparks, MD, USA) or by real-time PCR for detection of groups B, C, W and Y.20,21 We submitted strains that could not be typed in our laboratory to the Neisseria Reference Laboratory of the National Microbiology Centre of the Instituto de Salud Carlos III in Majadahonda (Madrid, Spain), where strain 29E was identified, among others.
Epidemiological variablesWe collected demographic data and information about the MenC conjugate vaccine in patients with disease caused by group C. We retrieved the number of doses of vaccine received and the age at the time of vaccination from the personal vaccination card and the health records of the patients. We established the following vaccination status categories:
- •
Unvaccinated: patient that had not received a single dose of MenC vaccine at the time of diagnosis.
- •
Incomplete vaccination: patient that had received fewer doses than established for their age in the current routine immunization schedule at the time of diagnosis.
- •
Vaccine failure: patient with complete vaccination for age based on the routine immunization schedule at the time of diagnosis that had symptoms of the disease at least 14 days after the last dose of vaccine.
We retrieved data on the presentation of IMD and the results of the functional complement assays performed in the patients for screening of immunodeficiencies (classic haemolytic complement [CH50] and alternative haemolytic complement [AP50]).
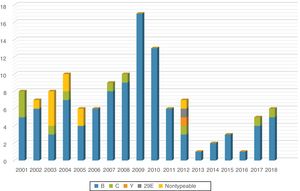
ResultsBetween January 2001 and December 2018, our hospital admitted 128 patients with a clinical diagnosis of IMD (7.1 patients/year) subsequently confirmed by culture, PCR or both in blood or CSF samples. The case distribution was homogeneous during the first years under study (6-10 cases per year), followed by a slight increase in 2009 and 2010 (17 and 13 cases, respectively) and a decreasing trend in the years that followed, with the lowest incidence recorded in 2013 (Fig. 1).
There was a predominance of male patients (79 boys and 49 girls), and the age range of the sample was 1 month to 16 years (median, 2 years; interquartile range, 9 months-5 years). Of the 128 patients, 90 were aged less than 5 years (70.3%) and one fourth were aged less than 1 year (34; 26.6%). When it came to the clinical diagnosis, 68 patients (53.1%) presented with sepsis, 39 (30.5%) with meningitis and 21 (16.4%) with sepsis associated with meningitis. Six patients died (mortality, 4.7%), 5 with infection by group B and 1 with infection by group C, corresponding to a mortality of 4.8% and 10%, respectively; the deceased patient with meningococcal C disease was an unvaccinated male adolescent. Table 1 highlights the main differences in the demographic and clinical characteristics of patients with meningococcal C disease compared to those with disease by other serogroups.
Comparison of the group of patients with invasive meningococcal disease by serogroups other than C and patients with invasive meningococcal disease by serogroup C.
| Serogroup other than C (n = 118) | Serogroup C (n = 10) | |
|---|---|---|
| Age | 1 month-16 years | 1-15 years |
| (median, 1 year; IQR, 7 months-5 years) | (median, 5.5 years; IQR, 1 year-9 years) | |
| <5 years | 87 (73.7%) | 3 (30%) |
| <1 year | 34 (28.8%) | 0 |
| [0,1-3]Sex, n (%) | ||
| Male | 74 (62.7) | 5 (50) |
| Female | 44 (37.3) | 5 (50) |
| [0,1-3] | ||
| [0,1-3]Clinical presentation, n (%) | ||
| Sepsis | 61 (51.7) | 7 (70) |
| Meningitis | 38 (32.2) | 1 (10) |
| Sepsis con meningitis | 19 (16.1) | 2 (20) |
| [0,1-3] | ||
| Mortality (%) | 4.2 | 10 |
IQR, interquartile range.
The results of culture were positive in 92 patients and negative in 36 (28.1%). Molecular testing by PCR analysis of blood or CSF samples was performed in 95 patients and yielded positive results in 86 (90.5%). Of the patients that had positive results of PCR tests, 41.9% had negative culture results. Of all isolated strains, 120 were subjected to antibiotic susceptibility testing: 105 were susceptible to penicillin (87.5%), 12 exhibited intermediate penicillin resistance and 3 were resistant to penicillin but susceptible to third generation cephalosporins.
Serotyping was performed in 125 of the 128 cases (97.6%). There was a high proportion of group B strains, detected in 103 cases (82.4%); 10 were group C cases (8%), mostly clustered in the early period under study, and 2 corresponded to serogroups Y and 29E. Only 10 cases (8%) were nontypeable, whereas the serogroup could not be established in the remaining 3 (Fig. 1).
Functional complement assays were performed in 86 patients and detected deficiencies in 3 (3.5%): 2 patients with serogroups with a low prevalence in Spain (serogroups Y and 29E) and 1 patient with infection by an unknown serogroup. All 3 patients had C5 deficiency, and had additional risk factors for immunodeficiency (consanguinity, positive family history…).
Table 2 summarises the vaccination history of the 10 patients with invasive meningococcal C disease. Four of the patients were unvaccinated, 3 patients aged 2, 5 and 10 years had an incomplete vaccination status (the first 2 had only received 2 doses before age 1 year, and the patient aged 10 years did not receive the catch-up dose recommended in the updated routine vaccination schedule). The 3 remaining patients, aged 1, 9 and 7 years, were fully vaccinated based on their age and the current routine immunization schedule, having received 3 doses (1 patient, 3 doses in the first year of life, and the other 2 patients in a 2 + 1 schedule).
Vaccination history of patients with invasive meningococcal disease caused by serogroup C.
| Case | Year of diagnosis | Age at time of diagnosis | Doses of vaccine | Vaccination status |
|---|---|---|---|---|
| 1 | 2001 | 6 years | 0 | Unvaccinated |
| 2 | 2001 | 15 months | 0 | Unvaccinated |
| 3 | 2001 | 6 years | 0 | Unvaccinated |
| 4 | 2003 | 15 years | 0 | Unvaccinated |
| 5 | 2007 | 2 years | 2 in 1st year | Incomplete vaccination |
| 6 | 2008 | 5 years | 2 in 1st year | Incomplete vaccination |
| 7 | 2012 | 10 years | 3 in 1st year | Incomplete vaccination |
| 8 | 2004 | 13 months | 3 in 1st year | Vaccine failure |
| 9 | 2017 | 9 years | 3 (2 + 1 schedule)a | Vaccine failure |
| 10 | 2018 | 7 years | 3 (2 + 1 schedule)a | Vaccine failure |
Invasive meningococcal disease is a global health problem that is endemic in most countries and can also occur in the context of outbreaks. It most frequently manifests in the form of meningitis (in 50% to 70% of cases),22 although in our case series the most frequent presentation was sepsis, in 53.1% of the total, while meningitis was observed in 30.5% and both sepsis and meningitis in 16.4%. The mortality in cases of meningococcal C disease was double that of cases of meningococcal B disease (10% vs 4.8%). As described in the previous literature, the patients at highest risk were the youngest children: 70% of cases occurred in children under 5 years and 38% of children in this group were aged less than 1 year; patients with group C disease were older, and only 30% were aged less than 5 years. In light of this, the Advisory Committee on Vaccines of the Asociación Española de Pediatría (Spanish Association of Paediatrics) (CAV-AEP) recommends routine vaccination against serogroup B, which is the most prevalent (67.6%), with an incidence of 5.85 confirmed cases per 100 000 in infants aged less than 12 months.11
The development of molecular techniques constituted a breakthrough in the microbiological diagnosis of IMD, as these methods are more sensitive, specific and quick and their results are not influenced by the administration of antibiotics before the sample is obtained.23–25 The use of these techniques made it possible to confirm the diagnosis in 28.1% of the patients, who had negative culture results probably due to initiation of antibiotherapy before testing. Nevertheless, culture remains the gold standard of diagnosis, in addition to allowing performance of antimicrobial susceptibility testing and serotyping of isolated strains, which is very important for the purpose of epidemiological surveillance.
Since the MenC conjugate vaccine was included in the routine immunization schedule, there have been 3 vaccination schedules. Initially, in 1999, the Interterritorial Council of the National Health System (known as the CISNS) approved the recommendation of including the MenC conjugate vaccine in the official immunization schedule,13 which was introduced in the schedules of every autonomous community of Spain in 2000 with 3 doses at ages 2, 4 and 6 meses (and in the 2001 immunization schedules published by the CISNS and the CAV-AEP). In January 2006, the vaccination schedule changed to primary vaccination with 2 doses given in the first year of life and a booster dose in the second year of life (as featured in the 2005 schedule of the CAV-AEV and the 2007 schedule of the CISNS), as evidence showed that vaccine effectiveness dwindled with time and that the antibody titre decreased more rapidly with vaccination in the first year of life14 (only 30% of children that received primary vaccination continued to be protected at age 5 years). The percentage of immunised children increases with the age at vaccination to up to 70% at 10 years post-vaccination in individuals given 1 dose of vaccine at age 16 years.15 For this reason, a third schedule was introduced in 2014 in which one of the primary vaccination doses is eliminated, and a second booster dose added in adolescence (as established in the immunization schedules published by the CISNS in 2013 and the CAV-AEV in 2014).13,15
These 3 schedules are an example of a dynamic vaccination strategy based on the epidemiology of the disease and the monitoring of vaccine effectiveness over time. This dynamic approach to vaccination continues, and this year a new change is being introduced: in this instance, the third dose of monovalent MenC vaccine is being replaced by a dose of quadrivalent MenACWY vaccine (2019 schedule of the CISNS) due to the observed epidemiological changes in the prevalence of meningococcal serogroups.
Following the introduction of the MenC conjugate vaccine in the routine immunization schedule, the incidence of meningococcal C disease decreased considerably,11,12,18 although there have been cases of vaccine failure—primary vaccine failure when the patient does not produce a sufficient concentration of antibodies after vaccination, and secondary vaccine failure when the patient can initially mount an adequate antibody response but the response wanes over time, which is the predominant type of failure in IMD.14 When it comes to the persistence of antibodies, several studies have generally evinced decreasing titres as time passes after vaccination, especially if primary vaccination took place in the first two years of life.14 The protective immune response following the primary series in the first year of life wanes quickly, so a booster dose is needed in the second year of life. Primary vaccination in the second year of life also confers protection that wanes within a few years and is insufficient in the long term, while vaccination from age 5 years achieves antibody titres closer to those achieved by naturally acquired active immunity, which also increase with the age at the time of vaccination.25–29
Studies conducted in Spain have demonstrated that vaccine effectiveness decreases after 1 year postvaccination, especially in individuals vaccinated in the first year of life, and that age at the time of primary vaccination and the time elapsed from the last dose are 2 of the most important factors at play in vaccine failure.14,30
Of the 10 patients with IMD caused by serogroup C in our study, 4 had not received any doses of vaccine and 3 had incomplete vaccination for their ages based on the current immunization schedule. The 3 remaining patients were cases of vaccine failure, as their vaccinations were up to date based on their age and the official immunization schedule. In 2 of these children, aged 9 and 7 years (cases 9 and 10), more than 5 years had elapsed since the last dose had been administered, which was probably the reason for the low levels of circulating antibodies (secondary vaccine failure). The third child (case 8), aged 13 months, had received the 3 doses of the primary vaccination series established by the official immunization schedule at the time, so this case could be attributed to primary vaccine failure.
Ongoing epidemiologic surveillance, even in diseases with a decreasing incidence, as is the case of IMD caused by serogroup C, is very important for the purpose of assessing vaccine effectiveness as years go by and to implement new interventions or adapt vaccination strategies, as was done in 2000. The introduction of a booster dose in the second year of life, first, and subsequently of a second booster dose in adolescence was motivated by studies demonstrating the waning of immunity with increasing time elapsed after primary vaccination.
Conflicts of interestFAML has received fees from GSK and Pfizer as a consultant and to be a speaker in scientific conferences. The rest of the authors have no conflicts of interest to declare in relation to this study.
Please cite this article as: Rius N, Lung M, Fernández-San José C, Iglesias T, Esperalba J, Moraga-Llop FA, et al. Enfermedad meningocócica invasiva por serogrupo C en la era posvacunal y fallos vacunales. An Pediatr (Barc). 2020;93:396–402.
Previous presentation: Data from this study were presented at the X National Congress of the Asociación Española de Vacunología, October 3-5, 2019, Oviedo, Spain.