Infection by the novel coronavirus (SARS-CoV-2) initially detected in 2019 in Wuhan, China, is the causative agent of the coronavirus disease 2019 (COVID-19), currently responsible of a global pandemic with significant repercussions in Spain.
Vertical transmission of SARS-CoV-2 remains unknown to date. Different authors have suggested that SARS-CoV-2 may be transmitted in utero, but it is not clear whether transmission occurs by crossing the placenta, through the birth canal or during the immediate postpartum period.1 Isolated case reports, case series2,3 and guidelines developed by experts of different scientific associations4,5 have been published, with significant heterogeneity in the definition of vertical transmission, types of samples used for investigation and clinical manifestations documented in newborn infants.
We present the case of a preterm neonate born to a mother positive for COVID-19 that came to the emergency department after going into labour at 29 + 6 weeks of gestation. 9 days before delivery she had a positive result for SARS-CoV-2 antigen test in respiratory swab, performed on account of close contact with a positive case (household partner). Polymerase chain reaction (PCR) test from nasopharyngeal swab before delivery was positive. The mother was asymptomatic. She had a normal course of pregnancy and attended prenatal care visits, with normal ultrasound and laboratory outcomes. Drugs for tocolysis and fetal lung maturation were administered, but it was not possible to stop preterm labour.
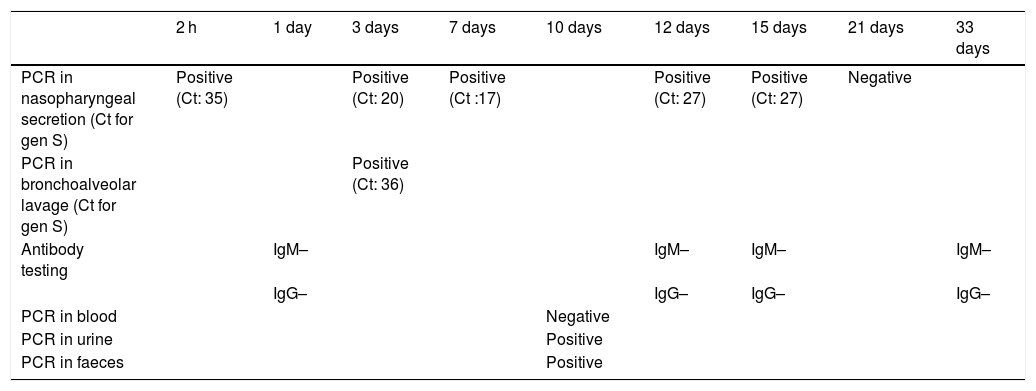
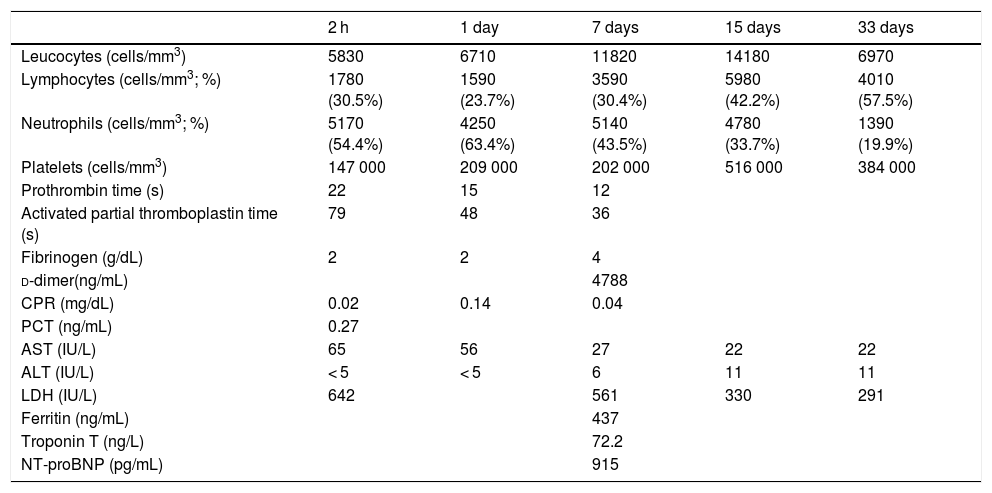
A male boy was born by vaginal delivery at 29 + 6 weeks, (1455 g birth weight) Intrapartum amniorrhexis. Apgar score 6/8. The preterm neonate required intubation at birth due to ineffective breathing, conventional mechanical ventilation and surfactant administration in the first hour of life. Chest radiograph revealed a bilateral reticular interstitial pattern suggesting neonatal respiratory distress syndrome. The neonate was admitted to the NICU into an individual room under contact and droplet isolation measures in the incubator, according to current recommendations.4,5 Respiratory secretion samples obtained at birth and on day 3 were tested positive for SARS-CoV-2 using PCR, with low cycle threshold (Ct) values (Table 1). Blood test results were normal, without lymphopenia or increased inflammatory markers (Table 2). The patient was extubated on day 2 and exhibited improvement, with no symptoms other than apnoea of prematurity. Polymerase chain reaction tests in nasopharyngeal samples at 7, 12 and 15 days remained positive. Extended analytical study at 7th day revealed no evidence of increased systemic inflammatory markers (Table 2) except for d-dimer levels (4788.00 ng/mL). At 10 days, PCR blood test was negative with no detectable viral load in blood even viral shedding in urine and faeces was present. Results of antibody tests in the neonate (IgM and IgG) were persistently negative. The first negative PCR result from nasopharyngeal sample at 21 days allowed discontinuation of isolation measures. There was no evidence of seroconversion at 4 weeks post birth (Table 1).
SARS-CoV-2 test results.
| 2 h | 1 day | 3 days | 7 days | 10 days | 12 days | 15 days | 21 days | 33 days | |
|---|---|---|---|---|---|---|---|---|---|
| PCR in nasopharyngeal secretion (Ct for gen S) | Positive (Ct: 35) | Positive (Ct: 20) | Positive (Ct :17) | Positive (Ct: 27) | Positive (Ct: 27) | Negative | |||
| PCR in bronchoalveolar lavage (Ct for gen S) | Positive (Ct: 36) | ||||||||
| Antibody testing | IgM– | IgM– | IgM– | IgM– | |||||
| IgG– | IgG– | IgG– | IgG– | ||||||
| PCR in blood | Negative | ||||||||
| PCR in urine | Positive | ||||||||
| PCR in faeces | Positive |
Ct, cycle threshold; PCR, polymerase chain reaction.
Laboratory variables.
| 2 h | 1 day | 7 days | 15 days | 33 days | |
|---|---|---|---|---|---|
| Leucocytes (cells/mm3) | 5830 | 6710 | 11820 | 14180 | 6970 |
| Lymphocytes (cells/mm3; %) | 1780 (30.5%) | 1590 (23.7%) | 3590 (30.4%) | 5980 (42.2%) | 4010 (57.5%) |
| Neutrophils (cells/mm3; %) | 5170 (54.4%) | 4250 (63.4%) | 5140 (43.5%) | 4780 (33.7%) | 1390 (19.9%) |
| Platelets (cells/mm3) | 147 000 | 209 000 | 202 000 | 516 000 | 384 000 |
| Prothrombin time (s) | 22 | 15 | 12 | ||
| Activated partial thromboplastin time (s) | 79 | 48 | 36 | ||
| Fibrinogen (g/dL) | 2 | 2 | 4 | ||
| d-dimer(ng/mL) | 4788 | ||||
| CPR (mg/dL) | 0.02 | 0.14 | 0.04 | ||
| PCT (ng/mL) | 0.27 | ||||
| AST (IU/L) | 65 | 56 | 27 | 22 | 22 |
| ALT (IU/L) | < 5 | < 5 | 6 | 11 | 11 |
| LDH (IU/L) | 642 | 561 | 330 | 291 | |
| Ferritin (ng/mL) | 437 | ||||
| Troponin T (ng/L) | 72.2 | ||||
| NT-proBNP (pg/mL) | 915 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPR, C-reactive protein; LDH, lactate dehydrogenase; NT-proBNP, N-terminal pro-hormone brain natriuretic peptide; PCT, procalcitonin.
During delivery, the mother wore a FFP2 mask. Skin-to-skin contact and delayed cord clamping were avoided. The neonate was fed with donor human milk initially, followed by artificial formula. Mother and child remained separated until the mother’s quarantine could be lifted (positive IgG test), and the first mother-child visit took place 5 days after birth. However, tests were not performed for detection of the virus in the placenta, umbilical cord blood or amniotic fluid, so no definitive results could verify vertical transmission of SARS-CoV-2.
In this article, we present the case of a neonate born before term to a mother with COVID-19 whose symptoms were most likely due to prematurity. The persistence of positive PCR results with low Ct values in the infant and the early timing of the first positive result suggest early infection. Although it is possible that the infant was infected in utero, this case is not sufficient to prove the possibility of vertical transmission.
The vertical transmission of SARS-CoV-2 is not well known. Although there have been reports of detection of the virus in the placenta, amniotic fluid, umbilical cord blood and human milk,6 most of the SARS-CoV-2 tests conducted in neonates born to infected mothers are negative,3 so data is inconclusive to prove vertical infection.
It is reasonable to conclude that vertical transmission of SARS-CoV-2 in infants born to mothers that are positive for the virus is possible and requires further research. Contact and droplet isolation measures should be implemented and maintained through the hospital stay until the PCR tests in respiratory secretion samples become negative, as seroconversion is rare in neonates, especially those born preterm.
Please cite this article as: Márquez Isidro EM, García García MJ, Solo de Zaldívar Tristancho M, Romero Peguero R. SARS-CoV-2 y prematuridad. ¿Existe evidencia de transmisión vertical? An Pediatr (Barc). 2021;95:375–377.