Country-specific information on paediatric GH therapy is available from multi-national studies.
MethodsA total of 1294 children in Spain enrolled in the observational Genetics and Neuroendocrinology of Short-stature International Study (GeNeSIS). Adverse events were assessed in all GH-treated patients (n=1267) and effectiveness in those with GH deficiency (GHD, 78%).
ResultsMean age at time of entry to the study was 9.8 years. GH was initiated at a median (Q1–Q3) 0.22 (0.20−0.25)mg/kg/week and administered for 2.8 (1.6–4.4) years. For 262 patients with GHD and 4-year data, mean (95% CI) height velocity was 4.3 (4.1–4.6)cm/year at baseline, 9.0 (8.7–9.4) cm/year at 1-year, and 5.5 (5.2–5.8)cm/year at 4-years. Height standard deviation score (SDS) was −2.48 (−2.58 to −2.38) at baseline and −1.18 (−1.28 to −1.08) at 4 years. Final height SDS minus target height SDS (n=241) was −0.09 (−0.20 to 0.02). In 1143 GH-treated patients with ≥1 year follow-up, 93 (8.1%) reported treatment-emergent adverse events. Serious events were reported for 7 children, with 2 considered GH-related.
ConclusionThese data confirm the benefit of GH replacement therapy on height gain for the patients in Spain. The safety profile was consistent with that already known for GH therapy.
La información específica de cada país sobre el tratamiento pediátrico con hormona de crecimiento (GH) proviene de estudios multinacionales.
MétodosEn España, 1.294 niños participaron en el estudio internacional y observacional sobre genética y neuroendocrinología de la talla baja (GeNeSIS). En los pacientes tratados con GH (n=1.267) se evaluaron los acontecimientos adversos. En aquellos con deficiencia de GH (DGH, 78%) también se evaluó la efectividad.
ResultadosLa media de edad al inicio del estudio fue 9,8 años. La mediana (Q1-Q3) de duración del tratamiento fue 2,8 (1,6-4,4) años y la dosis inicial de GH 0,22 (0,20-0,25) mg/kg/semana. En 262 pacientes con DGH con datos a 4 años, la velocidad media (IC 95%) de crecimiento fue 4,3 (4,1 a 4,6) cm/año al inicio; 9,0 (8,7 a 9,4) cm/año tras un año y 5,5 (5,2 a 5,8) cm/año a los 4 años. La puntuación de desviación estándar (SDS) de talla fue —2,48 (—2,58 a —2,38) al inicio y −1,18 (−1,28 a −1,08) a los 4 años. La SDS de talla final menos la SDS de talla diana (n=241) fue −0,09 (−0,20 a 0,02). De 1.143 pacientes tratados con GH con seguimiento ≥1 año, 93 (8,1%) comunicaron acontecimientos adversos surgidos durante el tratamiento. En 7 niños se comunicaron acontecimientos adversos graves, que en 2 casos se consideraron posiblemente relacionados con GH.
ConclusiónLa terapia de sustitución con GH fue efectiva para el aumento de talla en los pacientes españoles. El perfil de seguridad fue acorde con el ya conocido para el fármaco.
The main goal of treatment with growth hormone (GH) in children and adolescents is to promote normal growth. Since the introduction of recombinant human GH in 1985, final height outcomes have improved in thousands of children with growth-related disorders. The main indication for treatment with GH continues to be GH deficiency (GHD) in children1 or adults.2 However, its use has evolved with time and new indications have been approved. The newly approved indications in the paediatric age group outside of GHD include Turner syndrome, short stature in children born small for gestational age (SGA), Prader-Willi syndrome, chronic renal failure and short stature due to short-stature homeobox-containing gene (SHOX) haploinsufficiency.1,3–10
Many studies have demonstrated that adverse events related to GH therapy are rare, especially at the currently recommended doses,6,7,11–15 although cases of intracranial hypertension, scoliosis and slipped femoral capital epiphysis have been reported in a small number of patients.1,14–16 There has been concern about a possible association of GH therapy with glucose metabolism disorders and the development of neoplasms in patients with risk factors.12,14,17–19
The long-term outcomes and safety of GH therapy have been documented in large international registries, such as the Kabi International Growth Study (KIGS), the National Cooperative Growth Study (NCGS), and the Genetics and Neuroendocrinology of Short Stature International Study (GeNeSIS). It is also important that data specific for each country are gathered to assess the effectiveness and safety of clinical practise and treatment regimens in comparison to the overall data. The aim of this study was to assess the safety and effectiveness of GH therapy in Spanish paediatric patients based on data from GeNeSIS.
MethodsStudy and population characteristicsGeNeSIS was an open-label, multinational, prospective observational research programme the purpose of which was to assess the long-term safety and effectiveness of GH therapy (Humatrope®, Eli Lilly and Company, Indianapolis, USA) in children with short stature. Patients were diagnosed and managed according to standard paediatric endocrine practice. The programme was conducted in accordance with the ethical guidelines of the Declaration of Helsinki, received institutional board approval, and met all applicable regulatory requirements in the participating countries. The parents or legal guardians of each patient provided written consent for data collection, processing and publication prior to enrolment.
The study included patients undergoing or that were going to start treatment with GH for growth promotion. Patients with a previous history of neoplasm or a SHOX deficiency disorder were only included if they had never received GH therapy before. Patients with closed epiphyses were not eligible for entry, although those whose epiphyses closed during the study were allowed to continue participating in the study. In Spain, 56 centres with paediatric endocrinology units participated in the study.
Our study analysed the data for Spanish prepubertal and pubertal children included in GeNeSIS from its beginning in 1999 to September 2012. All decisions involved in the diagnosis and management of the patients were made by their attending physicians, and the data are presented as documented by the investigators. We assessed the auxological parameters of a subset of this cohort of Spanish children diagnosed with GHD that had not received treatment with GH prior to enrolling in the study and for whom data for four years’ followup was available. We also evaluated the final height (when patients were nearing adulthood) of another subset treated with GH during the study, independent of whether or not they had received GH therapy prior to enrolment.
Study measuresThe following variables were assessed at the time of enrolment: chronological age, target height (mean height of father and mother±6.5 depending on the patient's sex), weight, body mass index, bone age, and peak GH value in the GH stimulation tests. The initial GH dose for each patient was documented. At enrolment time and also once a year during followup, the patient's height, growth velocity (GV), bone age and pubertal status (Tanner stage) were assessed. For patients that reached their final heights, the final height and the difference between final height and target height were recorded. We calculated standard deviation scores (SDS) based on the 2000 US National Center for Health Statistics standards.20
We assessed safety based on the adverse events and serious adverse events (SAEs) experienced by the Spanish patients treated with GH reported by the investigators. We defined serious adverse events as those resulting in death, hospitalisation, permanent or significant disability, that were life threatening or otherwise considered significant by the investigator. We classified adverse events according to the definitions of the Medical Dictionary for Regulatory Activities (MedDRA) version 11.0. The researcher determined the severity of each event and its relation to GH therapy. Furthermore, we evaluated adverse events developing during treatment (AEDDT), defined as new events or pre-existing events, the intensity of which increased after initiation of GH treatment, in all patients treated with GH (whether or not they had received GH prior to enrolment) that attended at least one follow-up visit. We paid especial attention to specific neoplastic events (included in the MedDRA system organ class “neoplasms benign, malignant and unspecified [incl cysts and polyps]”) and glucose metabolism disorders.
Statistical analysisWe have expressed the data as means and standard deviations (SDs) or medians and interquartile ranges (IQRs) for continuous variables, and as absolute frequencies and percentages for discrete variables. The estimated means are presented with the corresponding 95% confidence intervals (CIs). We performed the statistical analysis by means of the Statistical Analysis System software version 9.1(SAS®; SAS Institute Inc, Cary, NC, USA).
ResultsDistribution and characteristics of the patientsSpain participated in the GeNeSIS study with 1294 children (44% female). At the time of enrolment, 85% of the patients had never been treated with GH and 14% were already receiving it. We excluded 27 patients from the analysis because they did not receive GH therapy or due to insufficient data. Table 1 summarises the diagnoses for which GH therapy was prescribed in the 1267 patients with complete data. Most of the children had GHD (78.1%), with most cases corresponding to the classical idiopathic form of GDH (65.3% of the total treated with GH). The second most frequent category was SGA (10.9%), followed by SHOX deficiency (5.8%), of which most cases corresponded to girls with Turner syndrome.
Diagnoses leading to treatment of short stature with growth hormone (GH) in 1267 children.
| Diagnosis | n (% of total) | % of superior level |
|---|---|---|
| GH deficiency | 989 (78.1) | |
| Idiopathic | 875 (69.1) | (88.5) |
| Classical | 827 (65.3) | (94.5) |
| Neurosecretory dysfunction | 44 (3.5) | (5.0) |
| Organic | 114 (9.0) | (11.5) |
| Acquired | 28 (2.2) | (24.6) |
| Intracranial tumoura | 20 (1.6) | (71.4) |
| Other | 8 (0.6) | (28.6) |
| Congenital | 86 (6.8) | (75.4) |
| Abnormal development of pituitary glandb | 72 (5.7) | (83.7) |
| Clinical syndromesc | 7 (0.6) | (8.1) |
| Genetic abnormality | 1 (0.1) | (1.2) |
| Other CNS malformations | 3 (0.2) | (3.5) |
| Small for gestational age | 138 (10.9) | |
| Unknown cause | 132 (10.4) | (95.7) |
| Known caused | 5 (0.4) | (3.6) |
| SHOX deficiency disorders | 73 (5.8) | |
| Turner syndrome | 52 (4.1) | (71.2) |
| Léri-Weill dyschondrosteosis | 15 (1.2) | (20.5) |
| Other | 6 (0.5) | (8.2) |
| Other causes of short stature or decreased linear growth | 29 (2.3) | |
| Genetic abnormality | 8 (0.6) | (27.6) |
| Othere | 21 (1.7) | (72.4) |
| Other defects of the GH axis | 22 (1.7) | |
| Bioinactive GH | 20 (1.6) | (90.9) |
| Other | 2 (0.2) | (9.1) |
| Idiopathic short stature | 12 (0.9) | |
| Skeletal dysplasia | 4 (0.3) |
Due to the observational nature of the study, information was not always provided on diagnostic subcategories.
Craniopharyngioma (n=6), medulloblastoma (n=5), germinoma (n=3), astrocytoma (n=1), ependymoma (n=1), pituitary adenoma (n=1), other unspecified (n=3).
Ectopic posterior pituitary (n=32), pituitary hypoplasia (n=21), pituitary aplasia (n=6), pituitary stalk interruption (n=5), septo-optic dysplasia (n=5), enlarged pituitary gland (n=1).
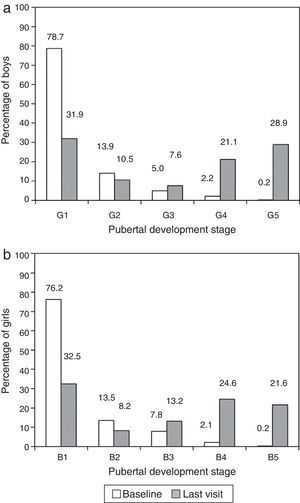
The mean age at the time of entry in the GeNeSIS study was 9.8 years (95% CI, 9.7–10.0 years). The age interval that contained the highest proportion of patients was the 10–15 years group (45.2%), followed by the 5–10 years group (38.0%), both with similar proportions of both sexes. There was a slightly higher proportion of girls (16.5%) compared to boys (11.4%) in the group under five years, while there was a higher proportion of males (2.4%) compared to females (0.7%) in the group 15 years and older. Most patients were prepubertal (Tanner stage 1, Fig. 1) when they started treatment. At the time of the last visit, 57.6% of males and 59.3% of females were pubertal (Tanner stage ≥3).
(a) Stage of pubertal development of boys during the enrolment and final visits of the GeNeSIS study in Spain. (b) Stage of pubertal development of girls during the enrolment and final visits of the GeNeSIS study in Spain. Pubertal development was assessed based on breast development (B) and genital development (G) applying the Tanner scale.
The median dose of GH at the time of entry was 0.22mg/kg/week (IQR, 0.20–0.25); after one year, it was maintained at 0.23 (IQR, 0.21–0.25) mg/kg/week, with no evident changes observed during treatment. The median initial dose was within the same range for all the diagnoses, except for Turner syndrome (median, 0.32; IQR, 0.29–0.35mg/kg/week). The median duration of followup was 2.4 years (IQR, 1.0–4.0); and followup lasted more than four years in 326 patients (25.7%). The maximum duration of followup was 10.5 years. Since 14% of patients were already being treated with GH at the time of enrolment, the median total duration of treatment exceeded 2.8 years (IQR, 1.6–4.4 years). The records indicated that 348 patients discontinued treatment. Aside from having reached their final heights or achieved a predefined height, growth velocity or bone age, the reported reasons for discontinuation were: decision by physician in 35 patients, decision by patient/parents in 32, ineffectiveness in 15, and adverse events in 2 patients.
Effectiveness in patients with growth hormone deficiencyWe analysed the auxological data of 262 patients with GHD (167 male and 95 female) that had not been treated with GH prior to enrolment in the study and underwent treatment with GH for at least four years. Table 2 presents their baseline characteristics.
Baseline characteristics of patients with growth hormone (GH) deficiency that had not received treatment with GH before enrolment in the study and that received treatment for at least 4 years, and a cohort with or without prior treatment with GH at time of entry and with final height data available.
| Baseline characteristics | Group not previously treated with GH and with followup data for 4 yearsN=262a | Group previously or not previously treated with GH with final height dataN=253b | ||||
|---|---|---|---|---|---|---|
| n | Mean±SD | 95% CI | n | Mean±SD | 95% CI | |
| Age (years) | 262 | 9.8±3.1 | 9.4 to 10.2 | 253 | 11.7±2.5 | 11.4 to 12.0 |
| Height SDS | 262 | −2.48±0.80 | −2.58 to −2.38 | 253 | −2.43±0.67 | −2.51 to −2.35 |
| Growth velocity (cm/year) | 201 | 4.3±1.6 | 4.1 to 4.6 | 202 | 4.4±1.6 | 4.2 to 4.7 |
| Growth velocity SDS | 199 | −1.26±1.52 | −1.48 to −1.05 | 199 | −0.95±1.26 | −1.13 to −0.77 |
| Weight (inkg) | 260 | 26.7±9.5 | 25.6 to 27.9 | 249 | 33.2±10.4 | 31.9 to 34.5 |
| BMI SDS | 259 | −0.30±1.56 | −0.49 to −0.11 | 249 | 0.03±1.41 | −0.15 to 0.20 |
| Bone age SDS | 191 | −2.55±1.21 | −2.72 to −2.37 | 197 | −2.08±1.16 | −2.25 to −1.92 |
| Target height SDS | 250 | −0.97±0.73 | −1.07 to −0.88 | 241 | −1.12±0.75 | −1.22 to −1.03 |
| Height SDS – target height SDS | 250 | −1.51±0.96 | −1.63 to −1.39 | 241 | −1.33±0.84 | −1.43 to −1.22 |
| Peak GH level (μg/L) | 261 | 6.90c | 4.30–9.05d | 252 | 7.00c | 4.60 to 9.20d |
| GH dose (mg/kg/week) | 255 | 0.22±0.04 | 0.21 to 0.22 | 248 | 0.21±0.04 | 0.20 to 0.21 |
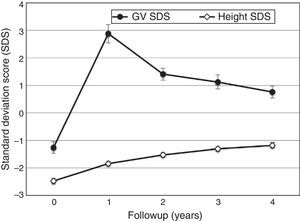
The GV increased from a mean of 4.3cm/year (95% CI, 4.1–4.6) at the beginning of followup to 9.0cm/year (95% CI, 8.7–9.4) by the end of the first year; reaching a value of 5.5cm/year (95% CI, 5.2–5.8) in the fourth year. The mean GV SDS (Fig. 2) increased from a value below the population mean at the beginning of followup (−1.3; 95% CI, −1.5 to −1.1) to a value above the population mean in the first year (2.9; 95% CI, 2.6–3.2), remaining high through year four (0.8; 95% CI, 0.5–1.0). The mean increase in height SDS was 0.64 (95% CI, 0.58–0.70) in the first year, and remained positive through year four (0.15; 95% CI, 0.11–0.19) (Fig. 2). The difference between the final height SDS and the target height SDS increased compared to the baseline (−1.51; 95% CI, −1.63 to −1.39) during the first year (−0.89; 95% CI, −0.99 to −0.78) and through year four (−0.21; 95% CI, −0.32 to −0.10).
We also assessed the impact of GH treatment in the final height of 253 patients with GHD (117 male and 136 female) that were or were not being treated with GH at the time of enrolment. Table 2 shows the baseline characteristics and Table 3 the final height data for these patients. The final height SDS in these patients was close to their target height SDS, with a mean deficit of 0.09 (SD, 0.85).
Final height data for Spanish children with growth hormone (GH) deficiency (N=253) that had or had not received treatment with GH prior to entry in the study.
| Parameter | n | Mean±SD (95% CI) |
|---|---|---|
| Age final height was reached (years) | 253 | 16.4±1.7 (16.2 to 16.6) |
| Duration of GH treatment (years) | 246 | 4.3±2.1 (4.0 to 4.6) |
| Latest GV before reaching final height (cm/year) | 243 | 2.5±1.9 (2.3 to 2.8) |
| Final height SDS | 253 | −1.21±0.89 (−1.31 to −1.10) |
| Increase in final height SDS | 253 | 1.22±0.89 (1.11 to 1.33) |
| Final height SDS – target height SDS | 241 | −0.09±0.85 (−0.20 to 0.02) |
The safety analysis included 1284 children (704 male, 573 female, 7 of unspecified sex) that were treated with GH during the study. The mean duration of treatment was 2.5 years (IQR, 1.0–4.1). Of the 1143 patients that had attended at least one follow-up visit, a total of 121 AEDDTs were reported in 93 children (8.1%) (Table 4); the investigators considered that 25 AEDDTs in 23 children (2.0%) were probably related to GH use. Specific neoplastic AEDDTs were reported in 7 patients and considered unrelated to treatment with GH with the exception of a case of melanocytic nevus diagnosed in a patient aged 8.4 years and treated for 0.3 years, an event that was not considered serious.
Serious adverse events recorded for the total population of patients treated with growth hormone and adverse events that developed during treatment.
| Patients with adverse events, n (%) | Number of adverse events, N | |
|---|---|---|
| Total participants included in safety analysis, N=1284 | ||
| Patients with ≥1 SAE | 7 (0.5) | 9 |
| Related to GH | 2 (0.2) | 2 |
| Choroidal neoplasm | 1 (0.1) | 1 |
| Epiphysiolysis | 1 (0.1) | 1 |
| Unrelated to GH | 5 (0.4) | 7 |
| Appendicitis | 1 (0.1) | 1 |
| Epiphysiolysis | 1 (0.1) | 1 |
| Kyphoscoliosis | 1 (0.1) | 1 |
| Paraplegia | 1 (0.1) | 1 |
| Shunt obstructiona | 1 (0.1) | 1 |
| Bone marrow neoplasm | 1 (0.1) | 1 |
| Upper extremity fracture | 1 (0.1) | 1 |
| Followup of patients treated with GH, N=1143 | ||
| Patients with ≥1 AEDDT | 93 (8.1) | 121 |
| Related to GH | 23 (2.0) | 25 |
| Arthralgia | 3 (0.3) | 3 |
| Scoliosis | 3 (0.3) | 3 |
| Gynecomastia | 2 (0.2) | 2 |
| Benign intracranial hypertension | 1 (0.1) | 1 |
| Abnormal bone development | 1 (0.1) | 1 |
| Choroidal neoplasm | 1 (0.1) | 1 |
| Epiphysiolysis | 1 (0.1) | 1 |
| Fatigue | 1 (0.1) | 1 |
| Headache | 1 (0.1) | 1 |
| Hypoglycaemia | 1 (0.1) | 1 |
| Hypothyroidism | 1 (0.1) | 1 |
| Haematoma at site of injection | 1 (0.1) | 1 |
| Elevated IGF-I | 1 (0.1) | 1 |
| Knee deformity | 1 (0.1) | 1 |
| Kyphosis | 1 (0.1) | 1 |
| Lipoatrophy | 1 (0.1) | 1 |
| Melanocytic nevus | 1 (0.1) | 1 |
| Osteochondrosis | 1 (0.1) | 1 |
| Papilloedema | 1 (0.1) | 1 |
| Vascular surgery | 1 (0.1) | 1 |
| Unrelated to GH/relationship unknown | 70 (6.1) | 96 |
| Specific neoplastic eventsb | 7 (0.6) | 7 |
| Melanocytic nevus (benign) | 3 (0.3) | 3 |
| Choroidal neoplasm (benign) | 1 (0.1) | 1 |
| Neoplasmc | 1 (0.1) | 1 |
| Cutaneous papilloma (benign) | 1 (0.1) | 1 |
| Bone marrow neoplasm | 1 (0.1) | 1 |
This also includes AEDDTs that were considered specific neoplastic events related to treatment with GH, based on the judgement of the investigators.
SAE: serious adverse event; AEDDT, adverse event developing during treatment; GH, growth hormone.
At least one SAE was reported in seven patients (0.6%) (Table 4). The investigators considered that two were at least probably related to treatment with GH: (1) one neoplastic vascular malformation in the left-eye choroid that could be fully resected in a girl aged 13.5 years with idiopathic isolated GHD that had been treated with GH for 0.9 years; and (2) an epiphysiolysis in a boy aged 14.6 years with multiple pituitary hormone deficiencies, including GHD, with a diagnosis of ectopic posterior pituitary.
Treatment was discontinued due to adverse events in two patients: (1) a boy aged 11.9 years with GHD secondary to medulloblastoma in whom a bone marrow neoplasm and obstruction of a ventriculoperitoneal shunt were suspected, and treatment discontinued after 1.6 years; both events were classified as serious and unrelated to GH treatment; and (2) male patient aged 16.5 years with idiopathic GHD in whom treatment with GH was discontinued after 3.4 years upon receiving a diagnosis of insulin resistance believed to be unrelated to treatment.
DiscussionThis study provides long-term data on the use of GH in a large cohort of Spanish children enrolled in the GeNeSIS observational study. The patients received GH for the treatment of short stature of different aetiologies at least 2 SDs below the mean. Our study demonstrated that GH therapy was effective in improving height deficits in patients with GHD, consistent with the outcomes of international studies, and did not find significant safety problems during the followup.
In our sample, 78% of the patients received a diagnosis of GHD, compared to 64% for the total participants in the GeNeSIS study. The second most frequent diagnosis in Spain was SGA, found in 11% of Spanish patients compared to 6% of the total sample. SHOX deficiency disorders, including Turner syndrome, were reported in 6% of the patients, compared to 12% in the total GeNeSIS participants. There is great variability among studies on the prevalence of SHOX deficiency disorders, excluding Turner syndrome, which is partly due to the different methods used for its detection and for patient selection21,22; moreover, molecular diagnostic methods were not widely available to Spanish investigators at the time of the study. Consequently, our results suggest that SHOX haploinsufficiency may be underdiagnosed in Spain.
The age at initiation of GH therapy ranged between 10 and 15 years in 45% of the patients. The mean age of the patients that had not received treatment with GH before the beginning of followup was 9.8 years, older than the age reported in other studies on GHD.23–25 However, more than 75% were prepubertal when treatment was initiated, and more than 90% were in Tanner stage 2 or under. Research has demonstrated that GH treatment is more effective at earlier ages and in the prepubertal stage.3,24–26
We analysed the effectiveness of GH in patients with GHD, especially in those that had not been treated with GH before entry in the study. The mean GV SDS more than doubled in the first year of treatment, and subsequently declined again, as expected,23,24 although the mean remained consistently above the baseline through the four-year followup. The GV and height gain outcomes were consistent with those reported in other studies.3,23–26 Height SDS showed a gradual increase, from a mean of −2.5 before initiation of treatment to a mean of −1.2 in year four. The final height SDS of patients with GHD treated with GH was −1.21 (95% CI, −1.31 to −1.10), within the normal range for height in the population. The difference between the final height SDS and the target height SDS was −0.09, consistent with the outcomes observed in other studies.27–29
The number of adverse events in patients reported in the context of follow-up visits was consistent with other published data. The types of reported adverse events were consistent with the known safety profile for GH treatment.12,14,30 Only two SAEs were considered by the investigators to be GH-related: a vascular malformation in the left-eye choroid treated with surgery, and one episode of epiphysiolysis. Treatment with GH was discontinued in two patients due to SAEs; in one, due to malfunction of a ventriculoperitoneal shunt and a bone marrow neoplasm, neither of which were considered to be related to GH; in the other, due to the development of insulin resistance, which was also considered to be unrelated to the treatment.
One of the limitations of the study concerns its open-label, observational design in which the reported outcomes were based on the judgement of the investigators. However, this also means that the results are representative of everyday clinical practice. Due to its non-interventional design, the investigators themselves determined on a case-to-case basis whether patients met the diagnostic criteria for the diagnosis of GHD and for GH neurosecretory dysfunction. On the other hand, all cases of patients for whom GH is prescribed in Spain are evaluated by committees of experts that verify that the diagnostic criteria have been met prior to treatment initiation. Nevertheless, since there is no centralised system to assess all cases, there may be variability in the application of these criteria. Furthermore, we must keep in mind that even now there is still uncertainty surrounding the best approach to the diagnosis of GHD,3 which seems particularly relevant for idiopathic isolated GHD. Another limitation was that the data used as reference for calculating SDS were not specific for each country. For the sake of coherence and to facilitate the analysis of the results for the entire sample of the study, the references used to calculate the SDS values for height, GV and BMI for the patients from the thirty different countries that participated in the GeNeSIS programme were based on data from the United States and the United Kingdom.20,31,32 Spanish growth studies may employ different methodologies and thus obtain different results compared to international studies, although the final adult height in the United States and the United Kingdom are similar to that of Spain, so we did not expect to find significant differences in this study.33
In conclusion, the results on this sample of Spanish patients that participated in the GeNeSIS study, the largest cohort studied in Spain in the past decade, are consistent with those of other clinical trials and international registries. Although the specific set of diagnoses that lead to treatment of short stature with GH may vary from one country to another, this study confirmed the effectiveness and safety of GH replacement therapy in the Spanish cohort. In children with GHD, treatment with GH succeeded in gradually increasing height through a 4-year follow-up period, achieving final adult heights that were near the projected target heights.
FundingThe GeNeSIS study is funded by Eli Lilly and Company.
Conflicts of interestCLT and MOI have no conflicts of interest to declare. ECC, LAVS and LEGP are full-time employees of Eli Lilly and Company.
GeNeSIS is a study sponsored by Eli Lilly and Company. We want to thank all participating patients and their families, as well as all the Spanish researchers in the GeNeSIS study in charge of their care. We are also thankful to the study team for their work in the project, and Dr Peter Bates of the Cambridge Medical Writing Services (United Kingdom) for his help in the preparation of the manuscript.
Please cite this article as: Luzuriaga Tomás C, Oyarzabal Irigoyen M, Caveda Cepas E, Vázquez Salvi LA, García-Pérez LE, el grupo de investigadores españoles del estudio GeNeSIS. Seguridad y efectividad del tratamiento con hormona de crecimiento: estudio GeNeSIS en España. An Pediatr (Barc). 2016;84:139–147.