A critical analysis is performed on the results of a newborn hearing screening programme in a regional hospital.
PatientsScreening results from 14,247 newborns in our maternity ward from 2002 to 2013.
MethodsTwo step recordings of bilateral otoacoustic emissions (initial and repeat, if failed, at about one month of life). Assessment by clinical brainstem responses.
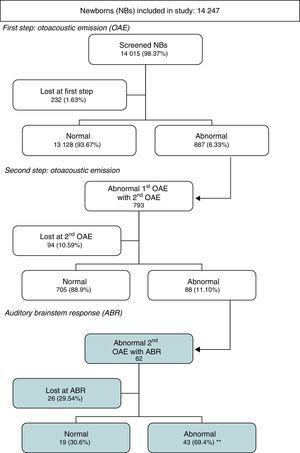
ResultsThe first step was performed on 14,015 newborns (98.3% of the total) reaching the screening objective. The first step pass figures were 93.7%, which implies a good pass rate with a few patients to repeat.
The second step is also good because it has a pass rate of 88.9% of newborns examined (only 0.63% of initial group needed brainstem responses assessment), but 10.6% were lost to follow up, and that is a major problem.
In newborns, scheduled for brainstem responses, the loss to follow-up is worse, with a figure of 29.5%, despite the high accuracy of this test given that 69.4% of those assessed showed hearing loss. This figure represents a 0.31% of the initial group, and is similar to that published for congenital hearing loss. Including patients that were lost to follow up this figure could be greater.
ConclusionNewborn hearing screening is useful but needs stronger control to avoid the follow up loss. In order to achieve this, it is crucial to have a good database and a screening coordinator.
Se evalúa el protocolo de cribado de hipoacusia neonatal en un hospital comarcal.
PacientesAnálisis en 14.247 recién nacidos en la maternidad del centro desde el año 2002 al 2013 inclusive.
MétodosProtocolo con registro de otoemisiones bilaterales (registro inicial y repetición si falla, al mes de vida) y potenciales auditivos (confirmación).
ResultadosSe realizó la prueba antes del alta a 14.015 neonatos (98,3% de la muestra) cumpliendo así los objetivos de cobertura del cribado. En el primer paso resultaron normales el 93,7%, lo que implica una adecuada tasa de paso y no obliga a excesivas repeticiones. La segunda determinación obtiene también buenos resultados, puesto que el 88,9% de los que acuden resultan normales, dejando solo un 0,63% del grupo inicial para valorar con potenciales. Un 10,6% no acude a la cita, lo que constituye el principal problema detectado. En los remitidos a potenciales esta pérdida es mayor, con un 29,5%, a pesar de que la rentabilidad en aquellos pacientes que se exploran con esta técnica es muy alta dado que el 69,4% de ellos presenta unos potenciales alterados. Esta cifra de alterados representa el 0,31% del total de recién nacidos estudiados, cifra similar a la incidencia de sordera congénita. Teniendo en cuenta las pérdidas en seguimiento referidas la incidencia real podría ser mayor.
ConclusionesEl programa es muy útil pero necesita un control estricto del seguimiento para evitar pérdidas de pacientes. Para ello es fundamental tener una base de datos dedicada y un coordinador del programa.
The importance of normal hearing in the psychological and social development of children is unquestionable, especially as it concerns the acquisition of that characteristically human skill, speech. Thus, hearing disorders need to be detected as early as possible. Early detection is not easy, especially in newborns, and until recently it was believed that newborns were not fit to have their hearing assessed by objective methods and that their hearing problems could not be corrected once they were identified.
The newborn hearing loss screening programme of the Autonomous Community of Valencia was first introduced in all public hospitals in late 2001.1 Its purpose is the early diagnosis of congenital hearing loss to allow initiation of treatment at ages 6–12 months, as numerous studies in the literature have evinced that early intervention improves final outcomes,2–5 and developmental delays in children due to delayed diagnosis are thus unjustifiable and have potential legal ramifications.6
At present, there are few data on the results of the initial step and nearly none on the final results of the screening in Spain. Thus, we need to learn how many of the children assessed in the screening programme had abnormal results in the confirmatory test requiring referral for monitoring and/or treatment.
The aim of the study was to assess the process and outcomes of the screening protocol carried out in the maternity unit of our hospital to identify its strengths and weaknesses and determine its yield.
Materials and methodsMaterialsInclusion criteria: newborns aged 24–72h delivered in the maternity ward of the Hospital Francesc de Borja in Gandía (Valencia) between 2002 and 2013 inclusive.
Exclusion criteria: newborns in whom otoacoustic emissions could not be recorded within the established age range.
MethodsWe obtained and analysed the results of the first otoacoustic emission (OAE) screening. We used these data to identify the newborns that had abnormal results in the first test and assessed whether they had been tested for a second time and the results of the second test. Out of these, we selected the newborns with abnormal results in the second OAE test that were referred for auditory brainstem response (ABR) testing and analysed the results of this step.
Screening protocol: the protocol comprises three steps. In the first step, all newborns are assessed by bilateral OAE recordings as late as possible before discharge from the maternity ward (approximately 48h post birth). This test is always performed by floor nurses working any of the shifts based on current availability and case loads. It is performed without sedation while the newborn is at rest in a room in the unit or even inside the crib in the room. Newborns with normal results are discharged, and those with abnormal results referred to the outpatient clinic for re-screening at an approximate age of 1 month. In the outpatient clinic, testing is performed by a dedicated nurse. Infants that pass the screening are discharged, and those that fail it are referred to the Department of Neurophysiology to undergo an ABR evaluation under sedation. If the results of this evaluation are abnormal, the infant is referred to an ear, nose and throat (ENT) specialist for monitoring and treatment, if applicable.
MethodsEvoked otoacoustic emission (OAE) testing: OAEs were recorded with an ECHOCHECK OAE Screener®, which incorporated the ILO 88 system (Otodynamics Ltd.), attached to a neonatal ILO ECP® probe (Otodynamics Ltd.). This is an automated device. The device delivers a nonlinear click of 1ms of duration and an intensity of 84±3dB sound pressure level at a frequency of 80 cycles per second. A normal result was defined as an OAE-to-noise floor difference of at least 6dB. Only bilateral normal results were considered nonpathological. After providing information and obtaining verbal consent, the test was performed in a room in the maternity ward with a low level of background noise and in the presence of the mother. The test was usually performed after feeding, when the newborn was likely to be calm.
ABR testing: ABR evaluations were performed in the Department of Neurophysiology. All patients were assessed with a Viking IV-D electromyography and evoked potential response unit (Nicolet®, Natus Medical Inc.).
Patients came for the test as deprived of sleep as possible and were given 0.3mL per kilogram of body weight of a chloral hydrate solution (100mg/mL) to induce sleep. Electrodes were placed on the patient according to the international 10–20 placement system and headphones fitted to deliver the stimuli.
A series of trials was performed in each ear with alternating click stimuli at 27.7Hz and at intensity levels of 80, 60, 50, 40 and 30dB, with contralateral masking noise at −30dB for each series. Two trials were performed for each level.
The recording settings were: high-pass filter at 3kHz, low-pass filter at 150Hz, line filter at 50Hz. An average of 1500 stimuli were delivered in each trial (2 per level).
We measured the latencies and interpeak intervals for waves I, III and V at the 80dB level, documenting the wave V latency at all other levels. We defined the auditory threshold of each ear as the lowest intensity at which wave V could be measured.
The Department of Neurophysiology interpreted the results and produced a final report. Auditory thresholds were considered abnormal when they exceeded 30dB hearing level.
Data analysis: we entered the data in a Microsoft Access® database. We performed the statistical analysis in a Microsoft Excel datasheet® and with the SPSS 20.0® (IBM).
Variables under study- –
Result of the first and second OAE test (normal/abnormal).
- –
Result of the ABR evaluation (normal/abnormal), and for newborns with abnormal results, distribution of the auditory thresholds measured in dB.
The number of newborns that met the criteria for inclusion was 14,247.
Tables 1–7 and Fig. 1 present the results of the study.
The first screening (Tables 1–2) was performed in the maternity ward for 98.3% of the newborns, and only 1.7% of newborns were not screened there due to various reasons, such as the child not being fit for it, malfunctioning of the probe or device, heavy workloads, etc., and were scheduled for screening at the outpatient clinic (data not included).
The second screening at the outpatient clinic (Table 3) should have been performed in 887 infants, but only 793 were brought in to have it done. Of those that underwent the screening, 88.9% had normal results and 11.1% (0.6% of the initial sample) abnormal results. The latter were referred for ABR evaluation (n=88).
Results of second otoacoustic emission test at the outpatient clinic.
| Otoacoustic emission test at outpatient clinic | ||||
|---|---|---|---|---|
| Frequency | Percentage of total newborns n=14,015 | Percentage of referred newborns n=887 | Percentage of tested newborns n=793 | |
| Abnormal | 88 | 0.63 | 9.92 | 11.1 |
| Missing | 94 | 0.67 | 10.60 | – |
| Normal | 705 | 5.03 | 79.48 | 88.9 |
| Total | 14,015 | 100.0 | 100.0 | |
In 0.1% of the total of newborns initially included in the study, the screening was not performed because the parents denied consent, although in recent years the first screening has been performed in 100% of newborns (Table 4), and the analysis of the patients lost to followup showed that 10.6% of newborns were lost at the second screening, although we observed a decreasing tendency as years passed, with the rate of loss to followup reduced to 4% in the last few years.
Patients missed by screening and lost to followup by year.
| Year | % | % | % |
|---|---|---|---|
| Lost at 1st OAE | Lost at 2nd OAE | Lost at ABR | |
| 2002 | 0.1 | 28.1 | 0 |
| 2003 | 0.3 | 23 | 10 |
| 2004 | 0.2 | 23.1 | 0 |
| 2005 | 0.1 | 8.6 | 20 |
| 2006 | 0.2 | 15.7 | 20 |
| 2007 | 0.1 | 25.5 | 0 |
| 2008 | 0.1 | 5.1 | 17.6 |
| 2009 | 0 | 6.4 | 0 |
| 2010 | 0 | 8.4 | 23.1 |
| 2011 | 0 | 8.4 | 33.3 |
| 2012 | 0 | 6.6 | 54.5 |
| 2013 | 0 | 4.3 | 50 |
Of the infants referred for ABR testing that were actually evaluated (Table 5), 69.4% had abnormal results and 30.6% normal results, that is, two thirds had hearing loss. The auditory threshold was determined in 39 of the infants with abnormal results (Tables 6 and 7), and the most frequent threshold was 50dB (30.8%), followed by 80dB (25.6%). The rate of loss to followup at the ABR step varied widely by year (Table 4), with an average rate of 29.5% (Table 5).
Results of patients referred for auditory brainstem response testing (ABR).
| Auditory brainstem response (ABR) | ||||
|---|---|---|---|---|
| Frequency | Percentage of total newborns n=14,015 | Percentage of referred newborns n=88 | Percentage of tested newborns n=62 | |
| Normal | 19 | 0.14 | 21.6 | 30.65 |
| Abnormal | 43 | 0.31 | 48.9 | 69.35 |
| Missing | 26 | 0.19 | 29.5 | |
| 88 | 100.0 | 100.0 | ||
Distribution of children with abnormal auditory brainstem responses by hearing loss threshold (n=39).
| ABR, dB | Frequency | Percentage assessed | Cumulative percentage |
|---|---|---|---|
| 40 | 3 | 7.7 | 7.7 |
| 50 | 12 | 30.8 | 38.5 |
| 60 | 7 | 17.9 | 56.4 |
| 70 | 4 | 10.3 | 66.7 |
| 80 | 10 | 25.6 | 92.3 |
| 85 | 1 | 2.6 | 94.9 |
| 90 | 1 | 2.6 | 97.4 |
| 95 | 1 | 2.6 | 100.0 |
| Total | 39 | 100.0 | |
Hearing loss is an entity that meets all the criteria for screening, and at present safe methods are available for its diagnosis. Our protocol used the otoacoustic emission method because it is the cheapest, quickest and easiest to implement,7,8 and has an adequate sensitivity and specificity.2,9
The screening process is integrated in everyday clinical practice, and therefore is carried out by all the nursing staff working on the floor. Previous studies have demonstrated that hearing screening does not required a dedicated staff.10,11
Results of otoacoustic emission testingThe coverage rate of 98.3% (Table 1) amply meets the 95% of the target population that must be screened for the programme to be considered successful.9 Of the children that were assessed (Table 2), 93.7% had normal results, leaving only 6.3% to be referred for a second test, a more than acceptable figure that approaches the referral rates achieved by protocols based on automated ABR measurements (4%).7 In any case, this volume of retesting can easily be assumed by one or two experienced staff in the outpatient clinic. Retesting results in much better screening, reducing the number of children referred for ABR testing and to the ENT specialist.
The results of the second screening at the outpatient clinic (Table 3) were abnormal in 11.1% of the patients (0.6% of the total sample). This figure is far below the maximum referral rate of 4% of the initial group recommended by the CODEPEH9 as a quality indicator. We ought to note that the incidence of hearing loss reported in large case series ranges between 0.2 and 0.6%.12–14
Thus, based on our results we infer that a large percentage of the newborns initially referred to the outpatient clinic will have normal test results (89%). This can be fully explained by the improvement in OAEs over time. The yield of retesting is high, even after short periods of time, but it is optimal starting at 21 days, which supports rescreening approximately one month after the initial test.15
It could be argued that postponing the first screening to 15–30 days post birth, when the response is more intense, would prevent healthy children failing the first test, but such an approach would increase the risk of losing patients to followup, which is one of the main problems of screening programmes.
Our study shows a decreasing trend in the loss to followup over the years (Table 4), with the rate being as low as 4% in the last few years thanks to the purchase of a second OAE device after breakdowns of the first one in previous years had led to losses of 15–25%, and to increased efforts in persuading parents to have the screening done, even by phone. In the early years, losses to follow-up were as high as 23% due to the immaturity of the programme. These figures may be striking, but they are not a first in this type of protocols, as demonstrated by a review by Akinpelu et al.15 of 558 articles on hearing screening protocols based on OAEs that found rates of loss to followup ranging between 0% (in small studies) and 11% (in larger studies). Metzger-Müller et al.,16 who conducted a study in Switzerland, also reported that 13% of patients were lost to followup at the second test, which shows that this is a global problem that also affects developed countries. The roots of the problem lie in several areas involving deficiencies in the health system's capacity, insufficient awareness and knowledge of the programme by health providers, gaps in information and in communication with the families, and gaps in coordination. Ameliorating this problem would require setting up an appropriate database for followup and appointing a coordinator to oversee the entire process.
Results of patients referred for auditory brainstem response testing (Tables 5–7)The percentage of patients that had abnormal results (69.4%) amounted to 0.31% of all the newborns included in the initial sample, which was consistent with rates reported in similar studies of 0.36%.17 Our result is similar to the actual incidence of hearing loss reported in the literature (0.1–0.6%),1 demonstrating the high yield of this test (Table 5), which confirmed hearing loss in two thirds of the assessed children. Of all patients with hearing loss, 56.4% had mild to moderate hearing loss (<60dB) and the rest severe hearing loss, the incidence of which was high.
A serious drawback of ABR testing is that parents often refuse to consent to the test, with 29.5% not showing up for the appointment (Table 5). Although this percentage varies widely by year (Table 4), given that there are few patients in this group and each influences the percentage heavily, we observed an increasing trend in the loss to followup that has resisted improvement, despite phone calls being made to insist on its importance. In this regard, it would be convenient to reassess the sedation of infants with chloral hydrate, which makes parents fearful of potential complications. Other studies conducted in developed countries have also reported this problem, with rates of loss to followup of up to 42.6% in Belgium18 or 45.1% in the United States,19 a dire circumstance considering that these children are at very high risk of having hearing loss.
About the protocol usedSeveral studies have analysed the cost-benefit of screening, and all concluded that screening of any type is beneficial. As for the protocols used, the most recent studies6 have reported that two-step protocols (OAE followed by ABR) are more cost-efficient in the general population. Other studies7,8 advocate for the initial evaluation of all children by means of ABR because it is associated with a higher pass rate, but such an approach depends too greatly on the correct performance of OAE testing; furthermore, our pass rates were very similar to those that have been achieved with ABR.
A key factor in achieving high pass rates is the number of hours elapsed since birth,15,20 with pass rates optimised starting from 48h. This factor has a greater impact on OAEs, which are more sensitive to disorders of the middle ear, than on ABRs.10
In high-risk populations, single-step screening by means of ABR as recommended by the CODEPEH9 would be more efficient. In our hospital, children at high risk are always referred to an ENT specialist for evaluation, even if their results are normal.
The cost of the equipment and consumables used in ABR testing is much higher, and the time spent in the evaluation is also longer, ranging between twice7,8 and five times10,20 that spent in OAEs, a factor to take into account in the management of large populations.
Dedicated vs. non-dedicated staffIn our study, all nurses in the department performed OAE tests at the times that were most convenient due to low levels of background noise or lesser workloads. A possible drawback of using non-dedicated staff is that it may lead to a greater number of referrals in the first step due to errors in the performance of the test, but the available data shows that this increase only amounts to 6%. This approach prevents patient losses due to holidays or staff absences.9
Other factors to considerFrom a logistics standpoint, it would be convenient for at least two devices to be available to ensure correct performance of the tests in the event of device malfunctioning.
Another factor that has proven helpful is the presence of a programme coordinator responsible for keeping track of the programme, addressing problems related to the programme, training and supporting nurses, recovering patients lost to followup and providing constructive feedback to all involved parties.19
Furthermore, it is essential that parents be provided extensive information prior to testing, emphasising the importance of screening and its impact, and reducing the anxiety that may be elicited by testing and potential abnormal results.
Limitations of the studyThe greatest limitation we found in this study was the loss of patients to followup throughout the screening process. As we noted above, this problem has been described in many other studies, including some conducted in developed countries such as Switzerland,16 United States19 or Belgium,18 but nevertheless, based on the data obtained in our study and considering the frequency of abnormal results in each screening step, we estimate that the number of patients with abnormal results that the programme failed to identify was 15 in the first step, 10 in the second step, and up to 18 in the group referred for ABR evaluation with hearing loss warranting referral to an ENT specialist.
The infants lost to followup are children at risk, and thus their number should be minimised to the extent possible, and they may constitute a bias in the incidence rates reported in this article. These children are the weak point of this type of programmes, and therefore studies of larger scope should be conducted on these populations that are lost to followup, which are very hard to engage, to learn the true global incidence of hearing loss, which is presumably higher.
On the brighter side, we observed that the rate of loss to followup has been decreasing since the time the programme was first introduced. In fact, when we analysed the data for the last three years of the programme, the coverage at the maternity ward had risen to 100%, and the loss to followup of patients scheduled for a repeat test at the outpatient clinic had decreased to 4%, a percentage below the recommended target of 5%.9
Other limitations of the studyAlthough the sensitivity and specificity are similar and of around 95–100% for both OAE and ABR, we must keep in mind that OAEs assess cochlear functioning and not the auditory neural pathway. Thus, both techniques are useful but they are not the same, and can complement one another.21,22
The sample under study was obtained from the population of patients of a public hospital and did not include patients receiving care at private hospitals. This is a limitation, as the incidence of hearing loss may not be the same in both populations given the impact of socioeconomic and environmental factors on hearing loss, which has been described in various studies.23
ConclusionsOur analysis shows that the first step meets the screening objectives, with 98.4% of newborns undergoing assessment and most of them having normal results (94%).
In the second step, a month after the first screening, 89% of infants had normal results, which demonstrates the usefulness of this screening tool (only 0.6% of the initial group was referred for ABR testing). However, 10.6% of infants did not attend the scheduled visit at the outpatient clinic, so it is possible that the screening failed to identify some children with hearing loss. The loss to followup was even higher among the patients referred for ABR evaluation, with an unacceptable rate of 29.5%. The diagnostic yield in children that undergo this assessment is very high, as 69% of them had abnormal evoked potentials and required followup by an ENT specialist.
Patients with abnormal ABR results amounted to 0.31% of the total of infants under study, a percentage that was similar to the expected incidence of hearing loss.
In order to reduce the rate of loss to followup—which is the main weakness of the screening programme—it is essential that a computerised database be developed to keep records of the evaluated newborns, a screening coordinator appointed, and a minimum of two devices available for testing.
Based on the data obtained in this study, it is fair to conclude that the programme is useful but needs improvement in the weak areas discussed above.
FundingThis study was partially funded by a grant of the Fundación Bancaixa (Beca Impulsa 2012).
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank the entire staff of the Department of Paediatrics, Maternity and Neurophysiology of the Hospital Francesc de Borja, Gandía.
Please cite this article as: Sequi Canet JM, Sala Langa MJ, Collar del Castillo JI. Análisis crítico de una década de cribado neonatal de hipoacusia en un hospital comarcal. An Pediatr (Barc). 2016;85:189–196.