Clinical scales are currently the best method to assess pain in the neonate, given the impossibility of self-report in this age group. A study is designed with the aim of determining the current practices as regards the clinical assessment of pain in Spanish Neonatal Units and the factors associated with the use of clinical scales.
MethodsA prospective longitudinal observational study was conducted. A total of 30 Units participated and 468 neonates were included.
ResultsOnly 13 Units (43.3%) had pain assessment protocols. Pain was evaluated with a scale in 78 neonates (16.7%, 95% CI; 13.1–20.1) and the mean number of pain assessments per patient and per day was 2.3 (Standard Deviation; 4.8), with a median of 0.75. Of the total number of 7189 patient-days studied, there was at least one pain assessment in 654 (9.1%). No pain assessment was performed with a clinical scale on any patient in 20 (66.7%) Units. Among those that did, a wide variation was observed in the percentage of patients in whom pain was assessed, as well as in the scales used. The CRIES (C-Crying; R-Requires increased oxygen administration; I-Increased vital signs; E-Expression; S-Sleeplessness) scale was used in most Units. In the multivariate analysis, only invasive mechanical ventilation was associated with receiving a pain assessment with a scale (OR 1.46, P=.042).
DiscussionThe majority of neonates admitted into Intensive Care in Spain do not receive a pain assessment. Many units still do not routinely use clinical scales, and there is a wide variation between those that do use them. These results could serve as a basis for preparing national guidelines as regards pain in the neonate.
Las escalas clínicas son hoy en día el mejor método para evaluar el dolor en el neonato, dada la imposibilidad de autorreporte en este grupo de edad. Se diseñó un estudio con el objetivo de determinar las prácticas actuales en relación con la valoración clínica del dolor en España y los factores asociados al uso de escalas clínicas.
MétodosEl estudio es de tipo observacional, longitudinal y prospectivo. Participaron 30 unidades y se reclutó a 468 neonatos.
ResultadosSolo 13 unidades (43,3%) disponían de protocolos de valoración del dolor. Se evaluó el dolor con una escala en 78 neonatos (16,7%, IC del 95%, 13,1-20,1) y el número medio de valoraciones del dolor por paciente y día de estancia fue de 2,3±4,8, con una mediana de 0,75. Del total de 7.189 días-paciente estudiados, 654 (9,1%) conllevaron al menos una valoración del dolor. Veinte unidades (66,7%) no realizaron evaluación del dolor con una escala clínica en ningún paciente. Entre las que sí lo hicieron, se observó una gran variabilidad en el porcentaje de pacientes en los que se evaluó el dolor y en las escalas utilizadas. La escala CRIES (C-Crying; R-Requires increased oxygen administration; I-Increased vital signs; E-Expression; S-Sleeplessness) fue la que se usó en más unidades. En el análisis multivariante solo la ventilación mecánica invasiva se asoció a recibir valoración del dolor con una escala (OR 1,46, p=0,042).
DiscusiónLa mayoría de los neonatos ingresados en cuidados intensivos en España no recibe una valoración del dolor. Muchas unidades todavía no utilizan rutinariamente las escalas clínicas y entre las que las utilizan existe una gran variabilidad. Estos resultados pueden servir de base para la elaboración de guías nacionales al respecto del dolor en el neonato.
Pain management is a priority in neonatal intensive care. Schematically, proper management of pain in neonatology is based on four key points: (1) the reduction of potentially painful procedures, (2) the use of non-pharmacological measures, (3) the use of analgesic drugs and (4) the use of clinical pain assessment scales.1,2 The self-report, which is the gold standard for pain assessment from school age, is for obvious reasons impossible in the neonate and this limitation has probably influenced the historical undertreatment of pain neonatology.3 In an attempt to objectify the presence or absence of pain and measure its intensity, clinical scales that combine a number of physiological and behavioural parameters have been developed.
While several review articles on this subject have been published recently,3–6 few studies address the frequency and type of pain assessments in neonatal clinical practice. Most of the studies that assess the use of clinical scales are based on surveys that ask about the general approach of neonatal units to pain management.7–9
To our knowledge, there are no data on how pain is assessed in Spanish neonatal intensive care units (NICUs). In the framework of the international European Europain project, we designed a specific study of the Spanish sample to determine the current clinical practices in the assessment of pain based on the proportion of newborns that underwent pain assessments by means of clinical scales, the clinical scales used and the frequency of their use. We also attempted to identify factors associated with the use of clinical scales and the frequency of assessments.
Materials and methodsWe conducted a prospective, longitudinal observational study. A more detailed description of the methods used is available in the recently published study on pharmacological pain management.10 Thirty neonatal units from all over Spain participated in the study (Appendix 1). We included every newborn with a corrected age of up to 44 weeks admitted in the course of one month (November 2012) to any of the participating units. For each newborn, we collected data on demographic variables, the modes of ventilatory support, the use of sedative or analgesic drugs, and performance of pain assessments by means of clinical scales. The duration of data collection per participant was of 28 days or until discharge. We obtained the approval of the Agencia Española de Medicamentos y Productos Sanitarios (Spanish Agency of Medicines and Health Products) and the overseeing clinical research ethics committee.
We performed a descriptive analysis of the characteristics of the participating units and the patients included in the study. We have expressed quantitative variables as mean and standard deviation, median, interquartile range (IQR) and/or range. We summarised qualitative variables as frequency distributions and percentages.
We analysed variables associated to the performance of pain assessments using a clinical scale (Yes or No) and the frequency of pain assessments by patient and day of hospitalisation in the newborns that had been assessed with a scale. In the bivariate analysis, we used the chi square test or Fisher's exact test as applicable. We used the Mann–Whitney U test for quantitative variables.
Subsequently, we developed a logistic regression model to determine which variables were independently associated to pain assessment. We built the model using forward selection, introducing all variables with P-values of less than 0.1 in the bivariate analysis. We performed the statistical analysis with the SPSS software version 19.0 for Windows. All tests were two-tailed. We set the level of statistical significance at P<.05.
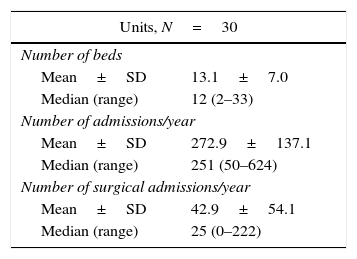
ResultsOf the 30 neonatal units that participated in the study, 20 (66.6%) had local guidelines for pain management, and 13 (43.3%) local guidelines for pain assessment. Six units (20%) had a designated medical pain leader and four (13.3%) a nursing pain leader. Thirteen units (43.3%) had pain management teams. Table 1 presents the care-related characteristics (number of beds and of admissions) of participating units. The mean percentage of inclusion in the study for all units was very high (94.79%), and the number of patients included per unit correlated directly to the number of beds (correlation coefficient, 0.77) and the number of admissions per year (correlation coefficient, 0.87).
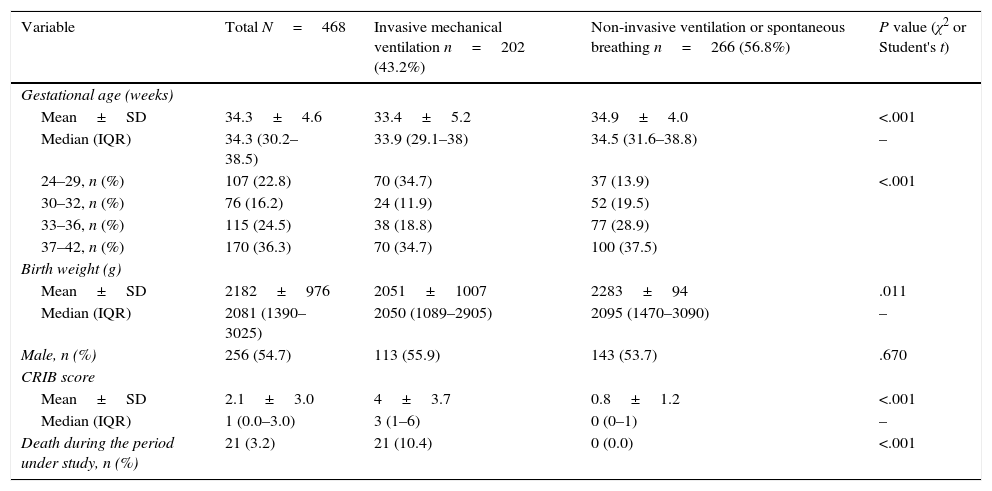
The study included a total of 468 newborns. Table 2 summarises their main characteristics. Overall, 198 newborns received some type of sedative or analgesic drug during the period under study (42.3%; 95% CI, 37.7–46.8) and 155 newborns (33.1%; 95% CI, 28.7–37.5) received potent sedative or analgesic drugs (opioids, benzodiazepines, ketamine or propofol).
Characteristics of the neonates included in the study, overall and by the highest level of respiratory support received during the period under study.
| Variable | Total N=468 | Invasive mechanical ventilation n=202 (43.2%) | Non-invasive ventilation or spontaneous breathing n=266 (56.8%) | P value (χ2 or Student's t) |
|---|---|---|---|---|
| Gestational age (weeks) | ||||
| Mean±SD | 34.3±4.6 | 33.4±5.2 | 34.9±4.0 | <.001 |
| Median (IQR) | 34.3 (30.2–38.5) | 33.9 (29.1–38) | 34.5 (31.6–38.8) | – |
| 24–29, n (%) | 107 (22.8) | 70 (34.7) | 37 (13.9) | <.001 |
| 30–32, n (%) | 76 (16.2) | 24 (11.9) | 52 (19.5) | |
| 33–36, n (%) | 115 (24.5) | 38 (18.8) | 77 (28.9) | |
| 37–42, n (%) | 170 (36.3) | 70 (34.7) | 100 (37.5) | |
| Birth weight (g) | ||||
| Mean±SD | 2182±976 | 2051±1007 | 2283±94 | .011 |
| Median (IQR) | 2081 (1390–3025) | 2050 (1089–2905) | 2095 (1470–3090) | – |
| Male, n (%) | 256 (54.7) | 113 (55.9) | 143 (53.7) | .670 |
| CRIB score | ||||
| Mean±SD | 2.1±3.0 | 4±3.7 | 0.8±1.2 | <.001 |
| Median (IQR) | 1 (0.0–3.0) | 3 (1–6) | 0 (0–1) | – |
| Death during the period under study, n (%) | 21 (3.2) | 21 (10.4) | 0 (0.0) | <.001 |
CRIB is a neonatal disease severity scoring system that consists of six items assessed in the first 12hours of life. The score ranges from 0 to 23, with higher values indicating increased severity.
SD, standard deviation; IQR, interquartile range.
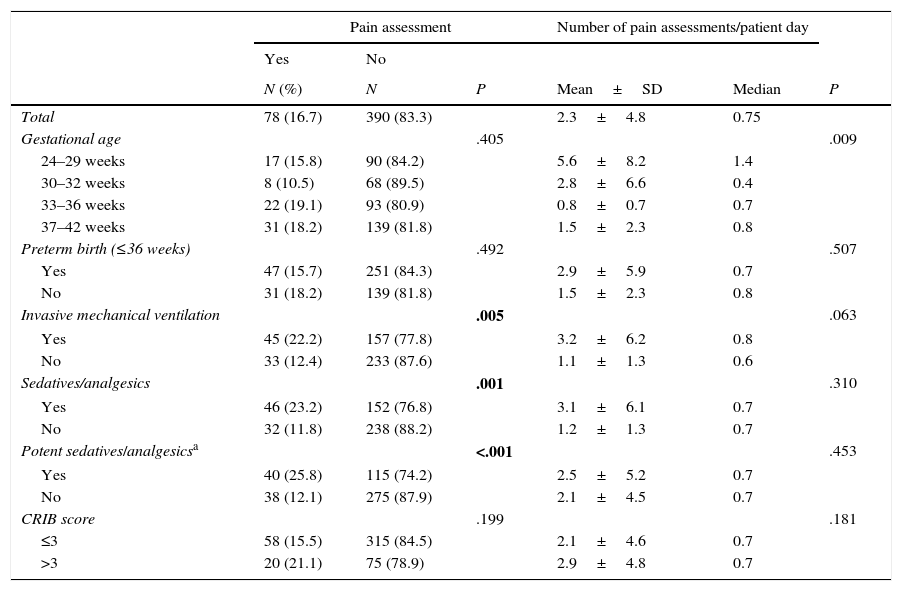
Pain was assessed with a clinical scale in 78 of the 468 neonates (16.7%; 95% CI, 13.1–20.1). In these 78 newborns, the mean number of pain assessments by patient in the period under study was 42.2±105.4, with a median of 10.5 (IQR, 5–30.5), and the mean number of pain assessments by patient day was 2.3±4.8, with a median of 0.75 (IQR, 0.4–1.7). Only 45 (22.2%) of the 202 newborns that received invasive mechanical ventilation (MV) and 40 (25.8%) of the 155 newborns that received potent sedative or analgesic drugs underwent some type of pain assessment.
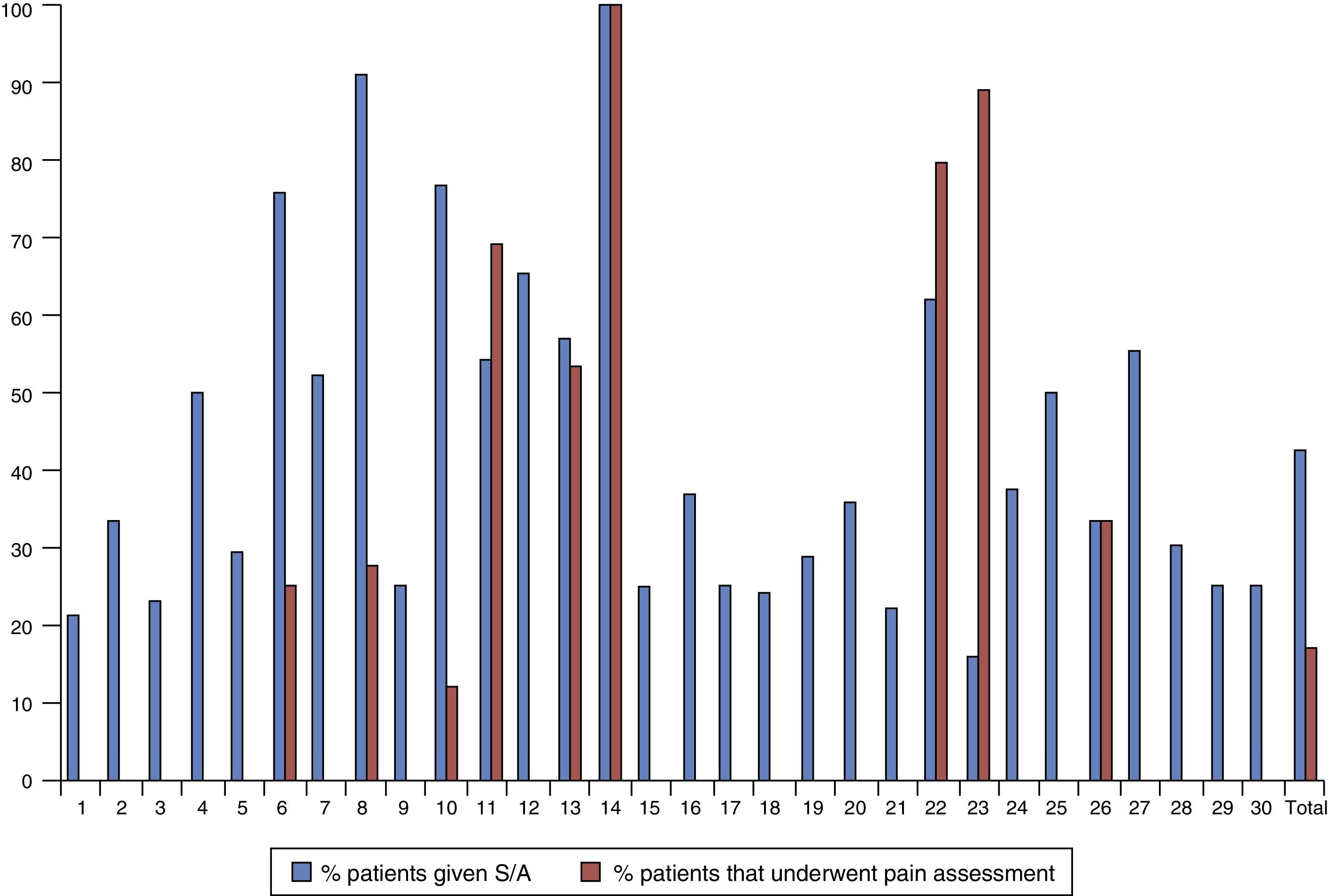
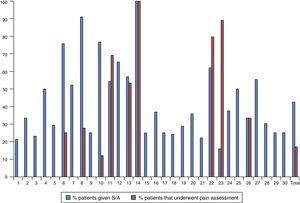
Fig. 1 represents the percentage of patients that underwent pain assessment and were given sedative or analgesic drugs in each of the 30 units that participated in the study, showing a marked variability between them. Twenty units (66.7%) did not assess pain with a clinical scale in any patients. The ten units that assessed pain in at least one patient did so in variable percentages that ranged between 4.3% and 100% of the newborns in that unit included in the study.
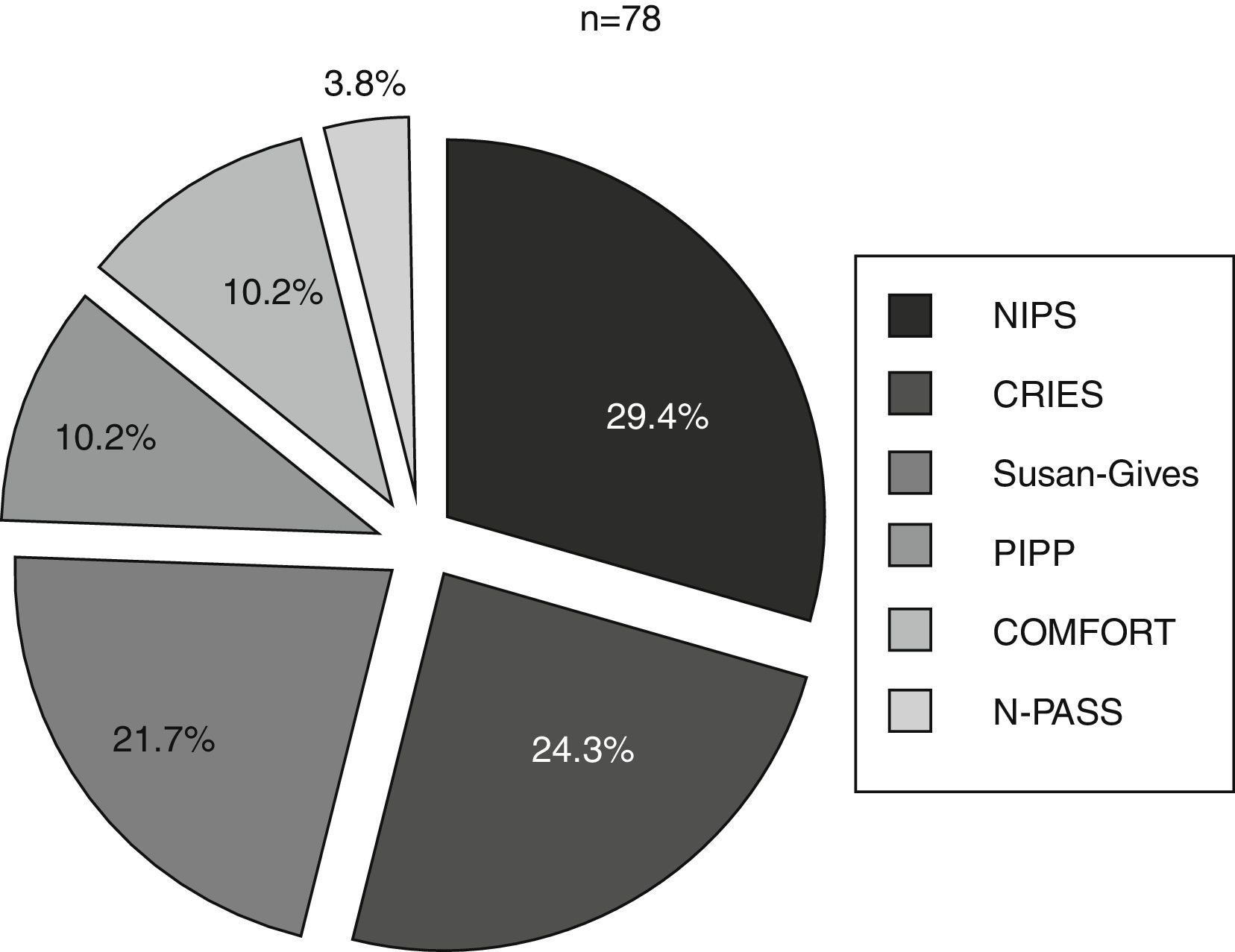
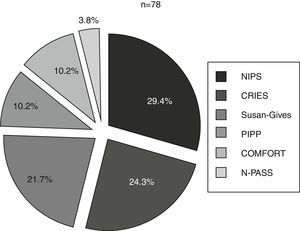
Six different clinical scales were used in these 10 units. The scales used most often were the NIPS, the CRIES and the Susan-Givens scale. Fig. 2 shows the percentage of the use of each scale for the group of newborns that underwent some type of pain assessment. By unit, the CRIES scale was used in four units, the PIPP, Susan-Givens and COMFORT scales were used in two units, and the NIPS and N-PASS in one unit.
Proportion of newborns (%) in whom each of the scales was used of the total newborns that underwent a pain assessment. CRIES: Crying, Requires Oxygen, Saturation, Increased Vital Signs, Expression and Sleeplessness; NIPS: Neonatal Infant Pain Score; N-PASS: Neonatal Pain, Agitation and Sedation Scale; PIPP: Premature Infant Pain Profile.
The time during which the 468 newborns were under study amounted to 7189 patient days, of which 654 (9.1%) included at least one pain assessment. Pain assessments were performed in 235 out of the 1000 (23.5%) patient days when newborns received potent sedative or analgesic drugs and in 419 out of the 6189 (6.8%) patient days when newborns did not receive potent sedative or analgesic drugs (P<.001). Pain assessments were performed in 262 out of the 1292 (20.3%) patient days when newborns received MV and 392 of the 5897 (6.6%) patient days when they did not receive MV (P<.001).
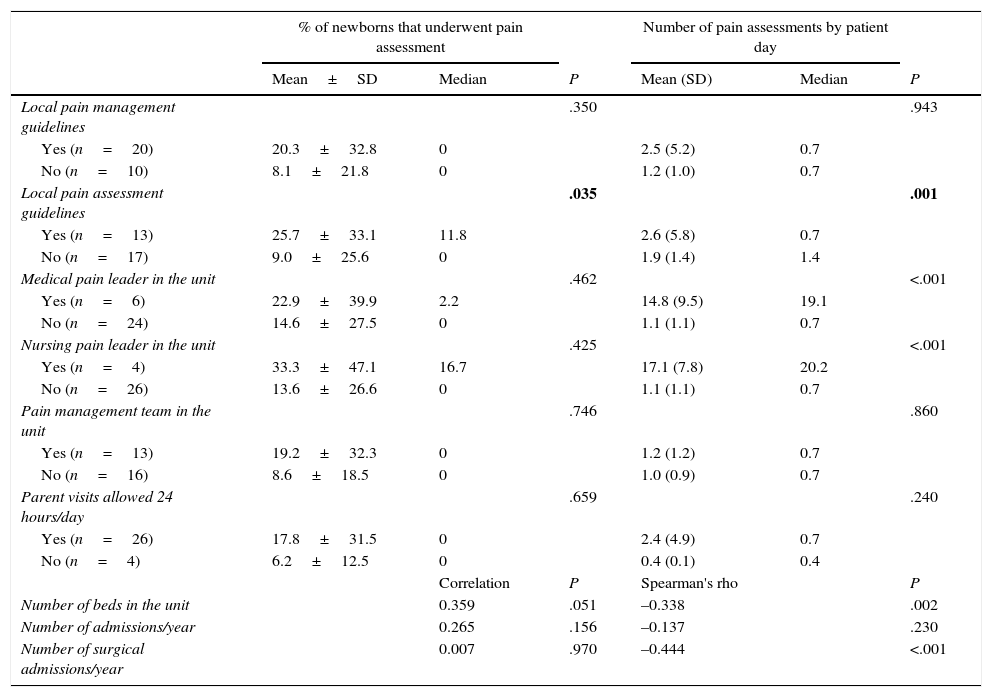
Tables 3 and 4 show the associations between variables concerning the characteristics of newborns or units and the use of clinical scales for pain assessment (Yes or No) and the number of assessments per patient day in newborns that were assessed by such scales.
Pain assessment by means of clinical scales by unit characteristics. Bivariate analysis.
| % of newborns that underwent pain assessment | Number of pain assessments by patient day | |||||
|---|---|---|---|---|---|---|
| Mean±SD | Median | P | Mean (SD) | Median | P | |
| Local pain management guidelines | .350 | .943 | ||||
| Yes (n=20) | 20.3±32.8 | 0 | 2.5 (5.2) | 0.7 | ||
| No (n=10) | 8.1±21.8 | 0 | 1.2 (1.0) | 0.7 | ||
| Local pain assessment guidelines | .035 | .001 | ||||
| Yes (n=13) | 25.7±33.1 | 11.8 | 2.6 (5.8) | 0.7 | ||
| No (n=17) | 9.0±25.6 | 0 | 1.9 (1.4) | 1.4 | ||
| Medical pain leader in the unit | .462 | <.001 | ||||
| Yes (n=6) | 22.9±39.9 | 2.2 | 14.8 (9.5) | 19.1 | ||
| No (n=24) | 14.6±27.5 | 0 | 1.1 (1.1) | 0.7 | ||
| Nursing pain leader in the unit | .425 | <.001 | ||||
| Yes (n=4) | 33.3±47.1 | 16.7 | 17.1 (7.8) | 20.2 | ||
| No (n=26) | 13.6±26.6 | 0 | 1.1 (1.1) | 0.7 | ||
| Pain management team in the unit | .746 | .860 | ||||
| Yes (n=13) | 19.2±32.3 | 0 | 1.2 (1.2) | 0.7 | ||
| No (n=16) | 8.6±18.5 | 0 | 1.0 (0.9) | 0.7 | ||
| Parent visits allowed 24 hours/day | .659 | .240 | ||||
| Yes (n=26) | 17.8±31.5 | 0 | 2.4 (4.9) | 0.7 | ||
| No (n=4) | 6.2±12.5 | 0 | 0.4 (0.1) | 0.4 | ||
| Correlation | P | Spearman's rho | P | |||
| Number of beds in the unit | 0.359 | .051 | –0.338 | .002 | ||
| Number of admissions/year | 0.265 | .156 | –0.137 | .230 | ||
| Number of surgical admissions/year | 0.007 | .970 | –0.444 | <.001 | ||
SD: standard deviation.
P values of less than .05 are displayed in boldface.
Patient variables associated with the assessment of pain by means of clinical scales. Bivariate analysis.
| Pain assessment | Number of pain assessments/patient day | |||||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| N (%) | N | P | Mean±SD | Median | P | |
| Total | 78 (16.7) | 390 (83.3) | 2.3±4.8 | 0.75 | ||
| Gestational age | .405 | .009 | ||||
| 24–29 weeks | 17 (15.8) | 90 (84.2) | 5.6±8.2 | 1.4 | ||
| 30–32 weeks | 8 (10.5) | 68 (89.5) | 2.8±6.6 | 0.4 | ||
| 33–36 weeks | 22 (19.1) | 93 (80.9) | 0.8±0.7 | 0.7 | ||
| 37–42 weeks | 31 (18.2) | 139 (81.8) | 1.5±2.3 | 0.8 | ||
| Preterm birth (≤36 weeks) | .492 | .507 | ||||
| Yes | 47 (15.7) | 251 (84.3) | 2.9±5.9 | 0.7 | ||
| No | 31 (18.2) | 139 (81.8) | 1.5±2.3 | 0.8 | ||
| Invasive mechanical ventilation | .005 | .063 | ||||
| Yes | 45 (22.2) | 157 (77.8) | 3.2±6.2 | 0.8 | ||
| No | 33 (12.4) | 233 (87.6) | 1.1±1.3 | 0.6 | ||
| Sedatives/analgesics | .001 | .310 | ||||
| Yes | 46 (23.2) | 152 (76.8) | 3.1±6.1 | 0.7 | ||
| No | 32 (11.8) | 238 (88.2) | 1.2±1.3 | 0.7 | ||
| Potent sedatives/analgesicsa | <.001 | .453 | ||||
| Yes | 40 (25.8) | 115 (74.2) | 2.5±5.2 | 0.7 | ||
| No | 38 (12.1) | 275 (87.9) | 2.1±4.5 | 0.7 | ||
| CRIB score | .199 | .181 | ||||
| ≤3 | 58 (15.5) | 315 (84.5) | 2.1±4.6 | 0.7 | ||
| >3 | 20 (21.1) | 75 (78.9) | 2.9±4.8 | 0.7 | ||
CRIB is a neonatal disease severity scoring system that consists of six items assessed in the first 12hours of life. The score ranges from 0 to 23, with higher values indicating increased severity.
SD, standard deviation
Furthermore, we fitted a logistic regression model for the variable concerning the use of clinical scales for pain assessment (Yes or No). When we adjusted for the administration of sedative or analgesic drugs, the existence of local guidelines for pain assessment and the number of beds in the unit, we found that only MV was significantly associated with patients undergoing at least one pain assessment with a clinical scale (P=.042; OR, 1.46; 95% CI, 1.01–2.11).
DiscussionTo our knowledge, this is the first prospective multicentre study on the assessment of pain in Spanish NICUs. These findings complement the data on sedation and analgesia recently published in this journal.10,11 The combination of both works offers a global perspective on pain management in Spanish NICUs. This study showed that clinical scales for pain assessment are underused as well as a significant variability in their use between units. Only 16.7% of the 468 newborns admitted to intensive care were assessed for pain at least once with a clinical scale. Only 23.5% of patient days that included the administration of sedative or analgesic drugs and 20.3% of patient days with MV included a pain assessment. It is also worth noting that 20 out of the 30 participating units did not assess pain by means of clinical scales in any of the patients, and that there were significant differences in the number of assessments done and the clinical scales used between the units that did.
Comparing our results with those of other studies poses a challenge, as very few other studies have collected prospective data for actual patients “at the cribside” on the use of pain assessment scales, as we have done. In a study conducted in the Netherlands that focused on procedure-related pain, Roofthooft et al. described their experience following the introduction of a series of measures for improving pain management that succeeded in achieving performance of pain assessments in nearly all patients (96.6%) during the period under study.12
The literature on the subject consists mostly of survey-based studies of units. For instance, in a survey of 16 French NICUs in 2002, 60% reported using scales for the assessment of acute pain, and 53% for the assessment of prolonged pain.7 This study listed the following reasons given for not using scales (in decreasing order): lack of knowledge, not considering pain a priority, the belief that scales are not valid measures of pain, and lack of time. These reasons probably apply to the day-to-day functioning of any unit. In a survey of 90 Italian neonatal units, only 19% reported using scales to assess pain or treatment with analgesic drugs during MV.9 In an Australian survey conducted in 2005, only 6% of the 105 participating units reported using pain assessment scales,8 and six years later the percentage had only increased to 11%.13 That study, like ours, found significant differences across regions in the country. In Spain, a survey of paediatric intensive care units conducted in 2011 already showed that more than half the units did not routinely use scales to monitor treatment with sedative or analgesic drugs.14
It is worth noting that six different scales were used in the 10 units that assessed pain with clinical scales (Fig. 2). In a survey of 370 neonatal units in Austria, Germany and Switzerland, only 32 units assessed pain by means of scales and they used up to 19 different scales.15 Pain assessment by means of scales is widely recommended by different scientific associations and consensus guidelines,1 but the fact that multiple scales have been published and validated does not entail that the ideal means to assess pain has already been determined.3 The assessment must correspond to the type of pain, distinguishing between short-lived procedure-related acute pain and prolonged pain caused by ongoing noxious stimuli or tissue damage. However, most scales are developed to assess acute pain.5,16 Among the scales used by the units in our study, the NIPS and PIPP are used to assess procedure-related acute pain, the CRIES, postoperative pain, and the COMFORT and N-PASS, prolonged pain in ventilated infants. To our knowledge, there are no published validation studies for the Susan-Givens scale.
One of the reasons that may have contributed to the infrequent use of scales in Spanish NICUs is that at present no single clinical scale is recommended as the gold standard superior to all others.4,6 Furthermore, the integration of scales in clinical practice is difficult and requires training.17 The use of scales also adds to the workload, and medical records often show that it is not associated to specific medical interventions, as there are few pain management protocols based on clinical scales18 and some of the proposed strategies are difficult to implement.3 Deindl et al. have described their experience in two Austrian units in which the implementation of a pain management protocol based on the N-PASS scale led to an increase in the use of opioid drugs with no associated changes in the duration of MV or length of stay.18 In the past decade, the concept that pain is the fifth vital sign and as such ought to be documented in medical records has been disseminated. However, assessments that lead to a diagnosis of pain but are merely documented in clinical records and not used to adjust treatment accordingly are not helpful, and may even be unethical. There are some published recommendations on how to adjust analgesic treatment in response to pain assessments19 and, for instance, it has been recommended that the decision to administer opioids to ventilated newborns be made selectively based on clinical measurements of pain.20 We are aware that the fact that pain assessment scales are not used in some units does not imply that pain is not being managed or assessed in a non-standardised way in them. Scales attempt to provide a standardised and uniform approach to pain assessment, and therefore a degree of objectivity.
As for the factors associated with the use of clinical scales for pain assessment, the bivariate analysis on the variable use of scales (Yes/No) showed statistically significant associations with the use of MV, the use of sedative/analgesic drugs, the use of potent sedative/analgesic drugs, and the existence of local guidelines for pain assessment, although the only variable that maintained significance in the multivariate analysis was MV. The bivariate analysis showed that their use was negatively correlated to the number of beds in the unit and the number of surgical admissions, which may reflect a deleterious impact of heavy workloads on the use of clinical scales. Only the existence of local pain assessment guidelines was positively correlated to the use of assessment scales and the number of assessments per patient day. This is consistent with the findings of previous studies9,15 and reaffirms the importance of pain management protocols. In fact, many sources recommend that units develop their own standards for pain assessment and that assessment be associated with specific therapeutic interventions.2,3,9 It would be logical to assume that the presence of national guidelines would have similar benefits and that the standardisation of recommendations would facilitate the comparison of results and the assessment of the impact of changes in sedation and analgesia protocols.
There are limitations to this study. First of all, the results may not be generalisable to the entire population because some Spanish neonatal units did not participate. However, most of the units asked to participate agreed to do so, and since the study included units from all over Spain and a large number of patients selected by consecutive sampling, we believe that it offers a faithful representation of the status quo. Secondly, we cannot rule out the possibility that participation in the study resulted in a change in practices, as the staff were aware of being observed. We attempted to minimise this issue by establishing relatively long periods of recruitment (one month) and followup (28 days), guaranteeing the rotation of health care staff. Thirdly, the study design did not allow us to gather data on pain scale scores or the clinical impact of these assessments on the newborns. We considered that including the collection of these data in the study would have placed a significant burden on the staff or the researchers and could have resulted in an important reduction in the number of participating units or the completeness of the collected data.
ConclusionsThis study demonstrates that most of the newborns admitted to Spanish NICUs do not undergo pain assessment by means of a clinical scale, and that there are still many units that do not use clinical scales routinely. Furthermore, we observed a wide variability in pain assessment between Spanish NICUs. We believe that these findings highlight the need to develop national clinical practice guidelines for neonatal pain management.
FundingThis study has received funding from the European Commission's Seventh Framework Programme (grant 223767) as part of the NeoOpioid project.
Conflicts of interestThe authors have no conflicts of interest to declare
Helena Viana, Paloma Lopez Ortego (Hospital Infantil Universitario La Paz, Madrid). Pilar Saenz Gonzalez, Raquel Escrig (Hospital Universitari i Politecnic La Fe, Valencia). Eva Bargalló Ailagas, Concepcio Carles (Hospital Universitari Josep Trueta, Girona). Laura San Feliciano, Ana Belén Mateo (Hospital Universitario de Salamanca). Inés Esteban Diéz, Rosa González Crespo (Hospital San Pedro de Logroño). Ersilia González Carrasco, Isabel de la Nogal Tagarro (Hospital Universitario Severo Ochoa, Leganés). María Dolores Elorza Fernández, Nerea Benito Guerra (Hospital Universitario de Donostia). Salud Luna Lagares, Pedro Jiménez Parrilla (Hospital Virgen Macarena, Sevilla). Francesc Botet, Anna Ciurana, Rebeca Tarjuelo (Hospital Clínic de Barcelona). María José García Borau, Nuria Ibáñez (Hospital de la Santa Creu i Sant Pau, Barcelona). Gloria Diáñez Vega (Hospital Universitario Puerta del Mar, Cadiz). María Arriaga Redondo (Hospital General Universitario Gregorio Marañón, Madrid). Gloria Herranz, Virginia de la Fuente Iglesias (Hospital Clínico San Carlos, Madrid). Amaya Pérez Ocón, Sagrario Santiago Aguinaga (Complejo Hospitalario de Navarra). Belén Martín Parra, Antonia Valero Cardona (Hospital General de Castellón). María Dolores Sánchez-Redondo, Antonio Arroyos Plana (Hospital Virgen de la Salud, Toledo). Zenaida Galve Pradel, Nuria Clavero Montañés (Hospital Universitario Miguel Servet, Zaragoza). María Jesús Ripalda Crespo, Raquel Nogales Juárez (Hospital Universitario Príncipe de Asturias, Alcalá de Henares). Aintzane Euba Lopez, Sonia Fernández de Retana (Hospital Universitario de Álava). Mar Reyné Vergeli, Raquel Vidal (Hospital Sant Joan de Déu, Barcelona). Jose Luis Fernandez-Trisac, María Taboada (Complexo Hospitalario Universitario de A Coruña). Aurora Montoro Expósito, Fátima Camba Longueira (Hospital Vall d¿Hebron, Barcelona). Gonzalo Solís Sánchez (Hospital Universitario Central de Asturias, Oviedo). María Purificación Ventura Faci, Marivi Mallen (Hospital Clínico Universitario Lozano Blesa, Zaragoza). Pilar Crespo Suárez, Elvira de Sola (Complexo Hospitalario de Pontevedra). Caridad Tapia Collados (Hospital General de Alicante). Isabel de las Cuevas, Beatriz Martín (Hospital Universitario Marqués de Valdecilla, Santander). María Luz Couce Pico, Alejandro Pérez Muñuzuri, Salomé Quintáns (Complexo Hospitalario Universitario de Santiago). Ana Melgar Bonis, Eugenia Bodas (Hospital Universitario 12 de Octubre, Madrid). Ana Concheiro Guisan, Begoña Pérez Costas (Complexo Hospitalario Universitario de Vigo).
Please cite this article as: Avila-Alvarez A, Carbajal R, Courtois E, Pertega-Diaz S, Anand KJS, Muñiz-Garcia J, et al. Valoración clínica del dolor en unidades de cuidados intensivos neonatales españolas. An Pediatr (Barc). 2016;85:181–188.