Intrauterine growth restriction (IUGR) and prematurity have been associated with increased perinatal morbidity and mortality and also with cardiovascular foetal programming. However, there are few studies on the impact of placenta-related IUGR on perinatal outcomes and cardiovascular biomarkers in pre-term infants.
ObjectivesTo determine differences in neonatal morbidity, mortality and cord blood biomarkers of cardiovascular dysfunction between pre-term placenta-related IUGR and non-IUGR new-borns, and to analyse their relationship with the severity of IUGR according to foetal Doppler evaluation.
Material and methodsProspective cohort study: pre-term infants with placenta-related IUGR and matched pre-term infants without IUGR. A Doppler scan was performed, and placenta-IUGR was classified according to severity. Comparative analysis of perinatal outcomes, neonatal morbidity and mortality, and cord blood levels of biomarkers of cardiovascular dysfunction was performed.
ResultsIUGR new-borns present lower weight, length, head circumference, and Apgar score at birth, as well as increased neonatal and cardiovascular dysfunction biomarker levels, compared with pre-term new-borns without IUGR. These differences increase with the severity of IUGR determined by prenatal umbilical artery Doppler scan.
ConclusionsPlacenta-related-IUGR pre-term infants, irrespective of gestational age, present increased neonatal morbidity and mortality that is significantly proportional to the severity of IUGR. Placental impairment and severity also determine levels of cardiovascular dysfunction biomarkers at birth.
La restricción del crecimiento intrauterino (RCIU) y la prematuridad se han asociado con una mayor morbimortalidad perinatal, así como con una reprogramación fetal a nivel cardiovascular. Sin embargo, son escasos los estudios sobre el impacto de la RCIU de causa placentaria en los resultados perinatales y en biomarcadores cardiovasculares de recién nacidos prematuros.
ObjetivosDeterminar las diferencias en morbimortalidad neonatal y biomarcadores de disfunción cardiovascular en sangre de cordón entre prematuros con RCIU de origen placentario y sin RCIU, así como estudiar su relación con la gravedad de la RCIU según el estudio Doppler fetal.
Material y métodosEstudio prospectivo de cohortes: prematuros con RCIU de causa placentaria y prematuros sin RCIU adecuadamente apareados. Clasificación de la gravedad de la RCIU según el Doppler. Análisis comparativo de resultados perinatales, de morbimortalidad neonatal y de niveles en sangre de cordón de biomarcadores de disfunción cardiovascular.
ResultadosLos prematuros con RCIU presentan un menor peso, longitud, perímetro craneal y Apgar al nacimiento, así como un aumento de la morbilidad neonatal y de los niveles de biomarcadores de disfunción cardiovascular, comparado con los prematuros sin RCIU. Estas diferencias aumentan con la gravedad de la RCIU determinada por el estudio hemodinámico Doppler prenatal.
ConclusionesLos prematuros afectados de RCIU de causa placentaria presentan un incremento de la morbimortalidad neonatal independiente de la prematuridad, que aumenta de forma estadísticamente significativa con la gravedad de la RCIU. La afectación placentaria y su gravedad también determinan la alteración de biomarcadores de disfunción cardiovascular al nacimiento.
Prematurity and intrauterine growth restriction (IUGR), together with congenital malformations, infections and anoxia, are the most important problems in foetal medicine, and are the main causes of perinatal morbidity and mortality. Incidence of severe IUGR is estimated at 3%–5% of pregnancies.1
Several studies have reported increased perinatal and neonatal morbidity and mortality in newborns with IUGR or low birth weight, together with an increase in cardiovascular morbidity in adulthood, a phenomenon known as foetal programming of adult disease.2–23 The neonatal morbidity and mortality described in these patients include complications related to prematurity (respiratory distress, bronchopulmonary dysplasia, necrotising enterocolitis, sepsis, low 5-minute Apgar test score, altered thermoregulation, retinopathy of prematurity [ROP], intraventricular haemorrhage, periventricular leukomalacia and neonatal death), and haematological and metabolic complications (thrombocytopaenia, coagulopathy, leukopaenia, initial hypoglycaemia, subsequent hyperglycaemia, altered cord blood lipids, cholestasis and jaundice).2–23 Increased neonatal morbidity and mortality and the foetal programming phenomena may be due to the foetal hypoxia-ischaemia presented by these IUGR patients. Hypoxia-ischaemia gives rise to haemodynamic adaptation, a phenomenon in which the brain, heart and adrenals are spared in detriment to the maturity of other organs.11,14,16,24 However, some studies seem to demonstrate that this haemodynamic redistribution does not completely spare priority organs from hypoxia-ischaemia and nutrient deficiency.25–32 Cardiovascular abnormalities have been described in IUGR patients using both echocardiography and measurement of cord blood cardiovascular dysfunction markers, specifically, increased levels of B-type natriuretic peptide (BNP).25–27 These alterations could be related to the mechanisms underlying foetal programming of cardiovascular disease in adulthood.
Very few studies have investigated the relationship between neonatal morbidity and mortality and severity of IUGR. Damodaram et al., reported an increase in risk of intrauterine and neonatal death and intraventricular haemorrhage in IUGR foetuses and newborns with both umbilical artery and venous Doppler abnormalities compared with those presenting only umbilical artery abnormalities.3
The main problem in the literature is that most studies on neonatal morbidity and mortality related to IUGR are conducted in “low birth weight for gestational age” newborns, without clearly identifying placenta-related IUGR patients, which are the ones with hypoxia-ischaemia phenomena. Another problem is that most of these studies are retrospective and include a poorly selected control cohort of adequate birth weight newborns (frequently without excluding foetuses with congenital anomalies, aneuploidies or chorioamnionitis)4,8–13 Finally, very few authors have studied early plasma markers of cardiovascular dysfunction in IUGR patients, or the impact of IUGR severity on both perinatal outcomes and the extent to which levels of cardiovascular dysfunction markers are increased.
ObjectivesThe objectives of our study were the following: to determine differences in neonatal morbidity, mortality and cord blood levels of metabolic-cardiovascular markers in 2 well-defined cohorts of premature infants with and without IUGR; and to study the relationship between these variables and the severity of IUGR assessed by foetal Doppler scan.
Material and methodsProspective cohort study: a cohort of pre-term placenta-related IUGR infants and a gestational age- and gender-matched cohort without IUGR. The IUGR cohort was formed of preterm infants with a clinical prenatal diagnosis of placenta-related IUGR based on standard weight charts and foetal Doppler scan33,34 (estimated foetal weight [EFW] below the 3rd percentile and/or within the 3rd–10th percentile with abnormal Doppler findings), born and monitored at the Hospital Vall d’Hebron between 2007 and 2010. The non-IUGR cohort was formed of preterm infants without IUGR (EFW and birth weight above the 10th percentile but below the 90th percentile according to standard foetal weight charts,33 with normal Doppler findings and no pre-eclampsia), matched with the IUGR cohort and born at the same hospital between 2007 and 2010.
Exclusion criteria for both cohorts were the following: parents’ refusal to participate; diseases affecting growth potential (embryopathies, congenital infections, chromosomopathies, genetic diseases, foetal malformations), certain maternal pathologies (type 1 diabetes mellitus, neuropathy, drug addiction, human immunodeficiency virus), chorioamnionitis, monochorionic multiple pregnancies, pregnancies not monitored at the Vall d’Hebron hospital or with no Doppler scan.
Maternal, obstetric, Doppler scan, perinatal and neonatal morbidity and mortality data were collected from all study patients (129 preterm infants: 72 with IUGR and 57 without IUGR). Severe neonatal morbidity was defined as the presence of 1 or more of the following complications: severe respiratory distress (defined as the need for intubation), intraventricular haemorrhage grade 3 or 4, patent ductus arteriosus (treated), kidney failure, necrotising enterocolitis, intestinal perforation, sepsis of vertical transmission, nosocomial sepsis, laser-treated retinopathy of prematurity (ROP), bronchopulmonary dysplasia, periventricular leukomalacia, administration of postnatal corticosteroids, administration of inotropic drugs, and/or death.
Severity of IUGR was classified on the basis of the Doppler findings, and defined as 1 or more of the following findings at the end of pregnancy: absent or reversed end-diastolic umbilical artery flow and/or abnormal ductus venosus flow due to increased ductus venosus pulsatility index or absent/reversed diastolic flow.
Whenever possible, and after obtaining the mother's informed consent (see Appendix), cord blood was collected and tested for lipid markers (cholesterol and triglyceride) and cardiovascular dysfunction markers (N-terminal pro b-type natriuretic peptide [NT-proBNP], cardiac troponin T [cTnT] and heart-type fatty acid binding protein [hFABP]). All data were entered into a separate form for each patient, and subsequently uploaded to an Excel database designed for the study. Statistical analysis was performed on STATA v. 11, with the help of the Biomedical Research Methodology Support Unit. All variables were compared between cohorts, and according to severity classification in the IUGR cohort.
ResultsMaterno-foetal characteristics of the cohortsTable 1 shows that both cohorts were comparable in terms of gestational age and gender. There were statistically significant differences between the cohorts in all variables relating to maternal and obstetric history of IUGR and/or pre-eclampsia, and in some maternal and obstetric variables from the current pregnancy related to prematurity (higher rate of multiple pregnancies, assisted-reproduction, premature and prolonged rupture of membranes and threat of premature delivery in the non-IUGR cohort) and IUGR (higher rate of high blood pressure during pregnancy, chronic high blood pressure, pre-eclampsia, Hellp syndrome, caesarean delivery and prenatal administration of magnesium sulphate in the IUGR cohort).
Perinatal and maternal-obstetric characteristics of the two cohorts.
| Non-IUGR (n=57) | IUGR (n=72) | p-value | |
|---|---|---|---|
| Gestational age (weeks) | 32.2 (30.6–34)c | 31.3 (29.05–34.1)c | NSa |
| Gender (male) | 29/57 (50.88%) | 39/72 (54.17%) | NSb |
| Previous obstetric history: gHTN, PE, IUGR and/or LWGA | 1/57 (1.75%) | 12/72 (16.67%) | 0.005b |
| Chronic maternal pathology: DM2, cHTN and/or obesity | 0/57 (0%) | 5/72 (6.94%) | 0.042b |
| Maternal thrombophilia | 2/57 (3.51%) | 8/72 (11.11%) | <0.001b |
| Maternal age | 33 (30–36)c | 32 (28.5–36)c | NSa |
| Initial maternal BMI | 21.74 (21.1–23.8)c | 22.58 (20.4–25.9)c | NSa |
| Caucasian ethnicity | 23/25 (92%) | 41/48 (85.42%) | NSb |
| Tobacco consumption during pregnancy | 5/57 (8.77%) | 13/72 (18.06%) | NSb |
| Multiple pregnancy | 31/57 (54.39%) | 7/72 (9.72%) | <0.001b |
| Assisted reproduction (IVF or AI) | 12/57 (21.05%) | 5/72 (6.95%) | 0.016b |
| PRM | 11/57 (19.3%) | 1/72 (1.39%) | 0.001b |
| PL | 28/57 (49.12%) | 5/72 (6.95%) | <0.001b |
| Prenatal administration of corticosteroids | 45/57 (78.95%) | 58/72 (80.56%) | NSb |
| Caesarean delivery | 28/57 (49.13%) | 66/72 (91.66%) | <0.001b |
| g/cHTN, PE, Hellp | 0/57 (0%) | 38/72 (52.78%) | <0.001b |
| Mg sulphate | 0/57 (0%) | 27/72 (61.36%) | <0.001b |
TPL: threatened pre-term labour; LWGA: low weight for gestational age; DM2: type 2 diabetes mellitus; IVF: “in vitro” fertilisation; Hellp: Hellp syndrome; cHTN: chronic hypertension; g/cHTN: gestational/chronic hypertension; gHTN: gestational hypertension; AI: artificial insemination; BMI: body mass index; Mg: magnesium; PE: pre-eclampsia; IUGR: intrauterine growth restriction; PRM: premature rupture of membranes; PL: preterm labour.
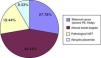
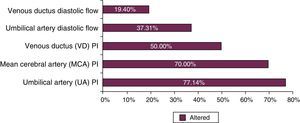
In the IUGR cohort, about 56% of patients were classified as severe, based on Doppler findings. Figure 1 shows end-of-pregnancy foetal Doppler findings in the IUGR cohort, showing very severe haemodynamic alterations due to end-diastolic ductus venosus abnormalities in 19.4% of patients.
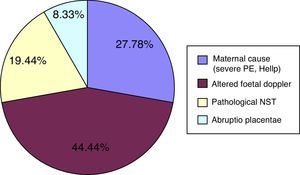
Figure 2 shows that delivery was most frequently indicated in the IUGR cohort due to foetal-related causes (abnormal Doppler findings or pathological foetal non-stress test [NST]), followed by maternal causes (severe pre-eclampsia or Hellp syndrome), and placental causes (placenta abruptio). In the non-IUGR cohort, preterm delivery was most frequently due to unstoppable labour (59.6%), followed by abnormal findings in the other foetus in multiple pregnancies (12.28%), and triple pregnancy (8.77%). The remaining causes occurred in ≤5% of cases, and included placenta previa, pathological NST, risk of uterine rupture, and placenta abruptio.
Differences in neonatal morbidity and mortality and cord blood cardiovascular and metabolic dysfunction marker levels between preterm infants with and without IUGRTable 2 shows the differences in neonatal anthropometrics, morbidity and mortality between study cohorts. The IUGR cohort presented a statistically significantly less standard deviation in weight, length and head circumference at birth. In this cohort, corrected gestational age was higher (longer hospital stay), weight at discharge was lower, and length at discharge was smaller.
Neonatal morbidity and mortality in the cohorts.
| Non-IUGR (n=57) | IUGR (n=72) | p-value | |
|---|---|---|---|
| SD of weight at birth | −0.25 (−0.68; 0.29)c | −2.24 (−2.62; −1.75)c | <0.001a |
| SD of height at birth | −0.20 (−0.69; 0.56)c | −2.54 (−3.61; −1.69)c | <0.001a |
| SD of OFC at birth | 0.01 (−0.64; 0.44)c | −1.50 (−1.84; −1.07)c | <0.001a |
| Cord pH (artery) | 7.30 (7.22–7.32)c | 7.28 (7.21–7.3)c | NSa |
| Cord pH (vein) | 7.35 (7.3–7.41)c | 7.33 (7.29–7.35)c | NSa |
| pH at admission | 7.28 (7.25–7.32)c | 7.30 (7.22–7.36)c | NSa |
| 1-minute Apgar | 8 (7–9)c | 7 (5.5–8)c | 0.030a |
| 5-minute Apgar | 9 (8–10)c | 9 (8–10)c | NSa |
| RDS | 36/57 (63.13%) | 41/72 (56.94%) | NSb |
| Severe RDS | 9/57 (15.79%) | 23/72 (31.94%) | 0.0349b |
| IVH | 4/57 (7.02%) | 12/72 (16.67%) | NSb |
| Grades 3–4 IVH | 0/57 (0%) | 3/72 (4.17%) | NSb |
| Treated PDA | 8/57 (14.04%) | 12/72 (16.22%) | NSb |
| Kidney failure | 3/57 (5.26%) | 5/72 (7.04%) | NSb |
| NEC | 2/57 (3.51%) | 5/72 (6.94%) | NSb |
| Intestinal perforation | 0/57 (0%) | 1/72 (1.82%) | NSb |
| Vertical sepsis | 1/57 (1.75%) | 6/72 (8.33%) | NSb |
| Nosocomial sepsis | 7/57 (12.28%) | 28/72 (38.89%) | 0.001b |
| Hypoglycaemia | 10/57 (17.54%) | 41/72 (56.94%) | <0.001b |
| Hyperglycaemia | 1/57 (1.75%) | 14/72 (19.44%) | 0.002b |
| Insulin treatment | 1/57 (1.75%) | 9/72 (12.5%) | 0.023b |
| Leukopaenia | 3/57 (5.26%) | 35/72 (48.61%) | <0.001b |
| Thrombocytopaenia | 6/57 (10.53%) | 41/72 (56.94%) | <0.001b |
| Coagulopathy | 1/57 (1.75%) | 7/72 (9.72%) | NSb |
| Phototherapy | 31/57 (54.39%) | 54/72 (75%) | 0.014b |
| Cholestasis | 0/57 (0%) | 4/72 (5.56%) | NSb |
| Inotropic drugs | 2/57 (3.51%) | 3/72 (4.17%) | NSb |
| Postnatal corticosteroids | 0/57 (0%) | 2/72 (2.78%) | NSb |
| ROP | 0/57 (0%) | 2/72 (2.78%) – laser 12/72 (16.67%) – yes | 0.004b |
| BPD | 0/57 (0%) | 17/72 (23.94%) | <0.001b |
| PVLM | 3/57 (5.26%) | 5/72 (6.94%) | NSb |
| Mortality | 1/57 (1.75%) | 3/72 (4.17%) | NSb |
| CGA at discharge (weeks) | 36.14 (35.6–37)c | 38.14 (37–40.2)c | <0.001b |
| Weight at discharge (grams) | 2270 (2150–2590)c | 2060 (1895–2260)c | <0.001b |
| Height at discharge (cm) | 45.5 (44–47)c | 44 (41–45)c | <0.001b |
| OFC at discharge (cm) | 32 (31–33)c | 32 (31.5–33.5)c | NSb |
| Severe morbidity | 18/57 (31.58%) | 42/72 (58.33%) | 0.002b |
OFC: occipitofrontal circumference; SD: standard deviation; BPD: bronchopulmonary dysplasia; RDS: respiratory distress syndrome; NEC: necrotising enterocolitis; IVH: intraventricular haemorrhage; PVLM: periventricular leukomalacia; PDA: persistent ductus arteriosus; ROP: retinopathy of prematurity; CGA: corrected gestational age.
Other statistically significant differences in the IUGR cohort included higher incidence of severe neonatal morbidity, lower 5-minute Apgar test score, together with a higher incidence of the following complications: severe respiratory distress, nosocomial sepsis, hypoglycaemia, hyperglycaemia, need for insulin therapy, leukopaenia, thrombocytopaenia, jaundice, need for phototherapy, ROP, and bronchopulmonary dysplasia (Table 2).
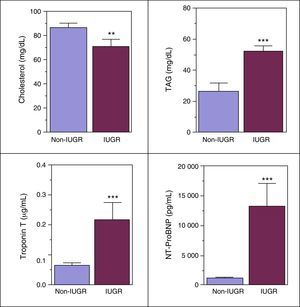
Figure 3 shows that patients in the IUGR cohort presented significantly higher levels of triglycerides, cTnT and NT-proBNP markers, and significantly lower levels of total cholesterol in cord blood.
Correlation of neonatal morbidity, mortality and levels of cardiovascular dysfunction biomarkers in cord blood with intrauterine growth restriction severityTable 3 shows the statistically significant differences in the incidence of certain neonatal morbidities between severe and non-severe IUGR patients. Preterm infants with severe IUGR presented increased incidence of: severe morbidity, respiratory distress syndrome, bronchopulmonary dysplasia, patent ductus arteriosus, kidney failure, nosocomial sepsis, hyperglycaemia, need for insulin, leukopaenia, thrombocytopaenia, jaundice, and need for phototherapy. As shown in Table 4, greater abnormality in each end-of-pregnancy foetal Doppler parameter was associated with a statistically significant increase in the incidence of different adverse neonatal outcomes.
Neonatal morbidity and mortality according to IUGR severity (n=69).
| Severe IUGR (n=30) | Non-severe IUGR (n=39) | p-value | |
|---|---|---|---|
| RDS | 94.87% (37/39) | 83.33% (25/30) | <0.001a |
| PDA | 35.9% (14/39) | 3.33% (1/30) | 0.001a |
| Kidney failure | 12.82% (5/39) | 0% (0/30) | 0.042a |
| Nosocomial sepsis | 48.72% (19/39) | 23.33% (7/30) | 0.031a |
| Hyperglycaemia | 30.77% (12/39) | 0% (0/30) | 0.001a |
| Insulin treatment | 20.51% (8/39) | 0% (0/30) | 0.008a |
| Leukopaenia | 66.67% (26/39) | 30% (9/30) | 0.003a |
| Thrombocytopaenia | 71.79% (28/39) | 36.67% (11/30) | 0.004a |
| Phototherapy | 84.62% (33/39) | 60% (18/30) | 0.021a |
| BPD | 33.33% (13/39) | 10% (3/30) | 0.023a |
| Severe morbidity | 74.36% (29/39) | 36.67% (11/30) | 0.020a |
BPD: bronchopulmonary dysplasia; PDA: persistent ductus arteriosus; RDS: respiratory distress syndrome.
Differences in neonatal morbidity and mortality according to end of pregnancy Doppler scan.
| Umbilical artery doppler | ||||
|---|---|---|---|---|
| Diastolic flow | Present | Absent | Reversed | p-value |
| Severe morbidity | 20/44 (45.45%) | 9/12 (75%) | 10/13 (76.92%) | 0.048a |
| RDS | 17/44 (38.64%) | 10/12 (83.33%) | 12/13 (92.31%) | <0.001a |
| IVH | 3/44 (6.82%) | 3/12 (25%) | 5/13 (38.46%) | 0.015a |
| Grade 3–4 IVH | 0/44 (0%) | 0/12 (0%) | 3/13 (23.08%) | 0.001a |
| Kidney failure | 1/44 (2.27%) | 0/12 (0%) | 3/13 (23.08%) | 0.012a |
| Vertical sepsis | 1/44 (2.27%) | 2/12 (16.67%) | 3/13 (23.08%) | 0.036a |
| BPD | 5/44 (11.36%) | 8/12 (66.67%) | 4/13 (30.77%) | <0.001a |
| Hypoglycaemia | 21/44 (47.73%) | 8/12 (66.67%) | 11/13 (84.62%) | 0.048a |
| Hyperglycaemia | 4/44 (9.09%) | 4/12 (33.33%) | 5/13 (38.46%) | 0.022a |
| Leukopaenia | 15/44 (34.09%) | 8/12 (66.67%) | 10/13 (76.92%) | 0.009a |
| Thrombocytopaenia | 18/44 (40.91%) | 9/12 (75%) | 11/13 (84.62%) | 0.006a |
| Coagulopathy | 2/44 (4.55%) | 1/12 (8.33%) | 4/13 (30.77%) | 0.022a |
| Ductus venosus Doppler | ||||
|---|---|---|---|---|
| Diastolic flow | Present | Absent | Reversed | p-value |
| Severe morbidity | 28/56 (50%) | 7/8 (87.5%) | 5/5 (100%) | 0.019a |
| RDS | 28/56 (50%) | 7/8 (87.5%) | 5/5 (100%) | 0.019a |
| Kidney failure | 1/56 (1.79%) | 1/8 (12.5%) | 3/5 (60%) | <0.001a |
| Vertical sepsis | 3/56 (5.36%) | 1/8 (12.5%) | 2/5 (40%) | 0.029a |
| Hyperglycaemia | 9/56 (16.07%) | 1/8 (12.5%) | 3/5 (60%) | 0.049a |
| Insulin treatment | 5/56 (8.93%) | 1/8 (12.5%) | 3/5 (60%) | 0.005a |
| Coagulopathy | 3/56 (5.36%) | 2/8 (25%) | 2/5 (40%) | 0.016a |
| Postnatal corticosteroids | 0/56 (0%) | 1/8 (12.5%) | 1/5 (20%) | 0.009a |
| Pulsatility index | Normal | Increased | p-value |
|---|---|---|---|
| Severe morbidity | 14/35 (40%) | 25/34 (73.53%) | 0.013a |
| RDS | 15/35 (42.86%) | 24/34 (70.59%) | 0.046a |
| Severe RDS | 5/15 (33.33%) | 17/24 (70.83%) | 0.039a |
| NEC | 1/35 (2.86%) | 3/34 (8.82%) | 0.001a |
| Leukopaenia | 11/35 (31.43%) | 22/34 (64.71%) | 0.013a |
| Thrombocytopaenia | 14/35 (40%) | 24/34 (70.59%) | 0.025a |
| Cholestasis | 1/35 (2.86%) | 2/34 (5.88%) | <0.001a |
BPD: bronchopulmonary dysplasia; RDS: respiratory distress syndrome; NEC: necrotising enterocolitis; IVH: intraventricular haemorrhage.
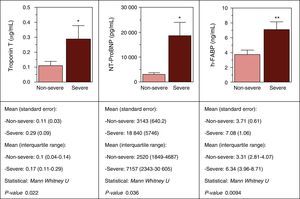
Figure 4 shows the correlation between levels of cord blood cardiovascular biomarkers and severity of IUGR. Note the significantly elevated levels of cTnT, NT-proBNP and h-FABP in the most severe IUGR patients compared with the rest.
DiscussionIn this study, morbidity, mortality and corrected gestational age at discharge (hospital stay) were higher, and somatometry was lower among placenta-related IUGR infants with respect to non-IUGR infants.
Most studies published to date report higher neonatal morbidity and mortality among IUGR infants.2–14 Specifically, the IUGR cohort shows higher incidence of severe morbidity and certain morbidities closely related with prematurity: lower 1-minute Apgar score3,9 and higher incidence of nosocomial sepsis,3,11,14 respiratory complications2–4,8–10 and ROP.2,4 However, in contrast with other authors,3,4,9,10 no statistically significant differences were observed in neonatal mortality or the rate of necrotising enterocolitis.2–4,8,9,11 Evidence of intraventricular haemorrhage and periventricular leukomalacia is contradictory in the literature. In this study, no differences were found in this regard between IUGR and non-IUGR patients, a finding that is consistent with some earlier studies.3,8,15 Several studies coincide with our findings of a higher incidence of neonatal metabolic and haematological complications among IUGR patients, including hypoglycaemia, hyperglycaemia, need for insulin, leukopaenia, thrombocytopaenia and jaundice treated with phototherapy.2,14,16–18
However, as outlined in the introduction, most studies so far have been retrospective, with a poorly defined IUGR group. This is one of the main strengths of this study, which is prospective and in which cases were limited to placenta-related IUGR patients. In addition, both cohorts were carefully defined using restrictive criteria, and were gestational age- and gender-matched. The maternal and obstetric differences between the cohorts were inherent to the pathology associated with each (IUGR and other causes of prematurity), and are consistent with the literature. As such, they should not be a limitation for this study.1,5,35–37 Moreover, the most common cause of medically indicated delivery in the IUGR cohort (foetal cause: altered foetal Doppler scan and/or altered NST) are also consistent with the literature.7,36,37
Nevertheless, this study is limited by the final sample size, which was to a large extent due to the restrictive criteria used for the selection of the two cohorts.
Another important contribution made by this study is the finding of differences in metabolic and cardiovascular dysfunction biomarkers levels in cord blood between IUGR and non-IUGR infants. These abnormalities could be related to mechanisms underlying foetal programming of cardiovascular disease in adulthood. Although little has been done to investigate this phenomenon, the cord blood lipid profile found in IUGR infants in this study is consistent with the findings of other authors, namely, a higher proportion of triglyceride and lower cholesterol level.16,17 Moreover, changes in the cardiovascular dysfunction biomarkers cTnT and NT-proBNP in new-borns with IUGR in this study were also consistent with those described by other authors. In particular, patients with IUGR presented higher levels of natriuretic peptides and cTnT compared to newborns without IUGR.38–40 Confirmation of increased levels of these markers is important, as it supports the hypothesis of early cardiovascular pathology in IUGR infants being associated with foetal programming of cardiovascular disease in adulthood. Further studies are needed to investigate the relationship between these blood cardiovascular biomarkers abnormalities and the long-term evolution of IUGR patients.
Another important finding of this study has been the direct correlation between neonatal morbidity, mortality and cord blood cardiovascular dysfunction biomarkers and the severity of Doppler scan haemodynamic abnormalities in IUGR patients.
Unfavourable perinatal outcomes and the presence of cardiovascular dysfunction biomarkers in severe IUGR patients may be due to more severe hypoxia-ischaemia in this population, shown by greater abnormalities in Doppler scan. More severe hypoxia-ischaemia would lead to greater foetal organ damage and dysfunction, thus increasing the risk of associated morbidities in both the neonatal period and in later life.2,14,16–18 Despite the paucity of studies on this phenomenon, Damodaram et al. have described an increased risk of certain morbidities among IUGR patients with both arterial and venous Doppler abnormalities.3
With regard to cord blood biomarkers, our findings echo those of the only other study to explore the association between cord blood cardiovascular dysfunction biomarkers and severity of IUGR determined by Doppler findings.38 In this case, apart from detecting higher levels of NT-proBNP, an increase in the cardiovascular dysfunction biomarkers cTnT and h-FABP in cord blood was also observed in the more severe IUGR cases. Despite the small sample size, this important finding contributes new information that could reveal the benefit of these biomarkers in detecting not only the severity of IUGR and the perinatal consequences of this pathology, but in predicting neonatal or adult cardiovascular dysfunction in these patients.
In conclusion, the main contributions of this study are the following: confirmation of increased neonatal morbidity and mortality in preterm infants with placenta-related IUGR; determination of abnormal cord blood levels of lipid and cardiovascular dysfunction biomarkers in these patients; and a direct correlation between greater risk of neonatal morbidity, mortality and abnormal cord blood biomarkers and severity of IUGR determined by Doppler findings. This study, therefore, confirms the benefit of arterial and venous Doppler scan in the management and stratification of IUGR patients, and highlights the need for prospective studies with larger cohorts with an appropriate characterization of prenatal placental abnormalities and their correlation with short- and long-term morbidity and mortality.
Funding- –
Study conducted within the framework of the project FIS PI07/1095 (2007–2010), financed by the Health Research Fund, titled “Pregnancy as a stress situation for the development of cardiovascular disease: assessment of haemodynamic and biochemical risk markers for atherosclerosis in mothers and foetuses with pre-eclampsia and/or intrauterine growth delay” (principal investigator: Elisa Llurba Olivé).
- –
Scholarship granted before doctoral studies and after the internal medicine residency (IMR) by VHIR (Institut de Recerca Vall d’Hebron) to Júlia Candel Pau (2011–2014).
Please cite this article as: Candel Pau J, Castillo Salinas F, Perapoch López J, Carrascosa Lezcano A, Sánchez García O, Llurba Olivé E. Resultados perinatales y disfunción cardiovascular en prematuros con restricción del crecimiento intrauterino en relación con la gravedad de la insuficiencia placentaria. An Pediatr (Barc). 2016;85:170–180.