An unexpected increase in the incidence of necrotising enterocolitis (NEC) cases was observed in our hospital. Just in case, our feeding policy could be responsible, it was decided to conduct a systematic review and develop a clinical guideline regarding enteral nutrition of very low birth weight infants (VLBW).
ObjectiveTo assess the impact of the new feeding protocol in the incidence of NEC.
MethodA “before” (2011) and “after” (May 2012–April 2013) study was performed on the new feeding protocol. This included initiation of enteral feeding in the absence of haemodynamic problems, a trophic feeding period of 5–7 days, and subsequent increments of 20–30mL/kg/day, of breast milk/donor human milk from the beginning. Probiotics were not administered. Primary outcome: incidence of NEC II 2 Bell's stage. Secondary outcomes: focal intestinal perforation, overall mortality and mortality due to NEC, nosocomial sepsis; weight at 28 days and 36 weeks; % of infants with weight p<10 at discharge; and length of stay.
ResultsOf the 270 VLBW infants, 155 were included in the “before” group, and 115 in the “after” group. NEC significantly decreased (12/155 vs 1/115, p=.008). A decrease in mortality rate was also observed (17.4% vs 7.8%, p=.02). In four cases NEC was part of the sequence of events that led to death in the first cohort, with none in the second. There was no difference in the incidence of focal intestinal perforation or of the other secondary variables analysed.
ConclusionsImplementation of an evidence-based enteral feeding protocol leads to a decrease in incidence of NEC, without increasing hospital stay or the incidence of sepsis.
En nuestro hospital asistimos a un incremento inesperado en la incidencia de enterocolitis necrosante (ECN). Por si nuestra política de alimentación estaba influyendo, se realizó e implementó una guía de práctica clínica (GPC) de alimentación enteral del recién nacido de muy bajo peso al nacimiento (RNMBP).
ObjetivoValorar el impacto del nuevo régimen de alimentación en la incidencia de ECN.
MétodoEstudio antes (2011) y después (mayo del 2012-abril del 2013) de la introducción del nuevo protocolo de alimentación, que incluye: inicio de la alimentación enteral en ausencia de problema hemodinámico; periodo de nutrición trófica de 5-7 días, incrementos posteriores de 20-30ml/kg/día; leche materna/banco desde el inicio. No se utilizaron probióticos. La variable principal a estudio fue la incidencia de ECN ≥ II de Bell. Variables secundarias: perforación focal, mortalidad global y atribuida a ECN, sepsis nosocomial; peso a los 28 días y 36 semanas; % de RN con peso<p10 al alta; estancia hospitalaria.
ResultadosDoscientos setenta RNMBP, 155 antes y 115 después. La ECN descendió significativamente (12/155 vs. 1/115, p=0,008); la mortalidad se redujo (17,4% vs. 7,8%, p=0,02); en 4 casos la ECN formó parte de la secuencia que condujo a la muerte en la primera cohorte; ninguno en la segunda. No hubo diferencias en la incidencia de perforación intestinal focal ni en las otras variables secundarias analizadas.
ConclusionesLa protocolización del régimen de alimentación enteral con la máxima evidencia disponible produce un descenso en incidencia de ECN sin incrementar la estancia hospitalaria o la incidencia de sepsis.
Preterm newborns are born during a critical period of their development. Nutrition is one of the cornerstones in their management, but it is hindered by the immaturity of their metabolism and digestive system, and by the presence of comorbidities. As a result, many of these patients experience delays in their extrauterine growth that frequently compound previous intrauterine growth delays.1 At present, there is a tendency to try to reduce extrauterine growth restriction by means of early and aggressive parenteral nutrition (with nutrients that approximate those that the foetus would receive through the placenta), introducing enteral nutrition as soon as possible.2,3
But this is not an easy task. Neonatologists are often torn between further extending a scheme of parenteral nutrition that ensures nutritional intake but increases the risk of catheter-related sepsis, or hurrying to switch to enteral nutrition that could carry a higher risk of necrotising enterocolitis (NEC).
The literature shows a wide intercentre variability in the incidence of NEC and in the approach to the introduction and maintenance of enteral nutrition. This variability has been described between countries, between hospitals and even between providers in a single facility, and can be explained by the high degree of uncertainty that surrounds many of these interventions.4,5
In our hospital, we witnessed a sudden increase in the incidence of NEC between 2010 and 2011. In case this was associated with our approach to nutrition, we decided to develop a clinical guideline on enteral nutrition for very low birth weight (VLBW) infants. This guideline introduced a series of changes that were consolidated in a new nutrition protocol in the unit. The aim of this study is to assess the impact of this protocol in the incidence of NEC in our hospital.
MethodsDesignWe conducted a prospective quasiexperimental pre–post intervention study. The intervention consisted in the set of measures described in the nutrition protocol for VLBW infants in our unit, which we present in Table 1. Our definition of high risk for NEC was not limited to low weight, but also included asphyxia and diagnosis by the obstetrician of intrauterine growth restriction (IUGR) with umbilical artery and/or foetal middle cerebral artery Doppler abnormalities (whether or not postnatal weight was below the 10th percentile). The protocol was introduced in the unit in April 2012, although some of its measures had already been implemented in the preceding months, which is why these months were not included in the study. In the pre-intervention period analysed in the study, there was no written protocol on how to administer enteral nutrition.
Enteral nutrition protocol in the post-intervention cohort.
| Timing of introduction | Haemodynamically stable (no vasoactive drugs and adequate perfusion in the last 24h). No asphyxia If unstable, maintain enteral fasting | ||
| Method | Continuous infusion Bolusa during trophic feeding period and in patients with nIPPV | ||
| Type of milk recommended | Colostrum; banked milk (if not available, assess delaying introduction) | ||
| Trophic feeding period | High risk of NEC 0.3mL/kg/h×3d 0.5mL/kg/h×3d 1mL/kg/h×1d | 750–1000g 0.5mL/kg/h×3d 1mL/kg/h×3d | 1000–1500g 1mL/kg/h×5d |
| Subsequent increases | 10mL/kg every 12h in <1000 (20mL/kg/day) 15mL/kg every 12h in >1000 (30mL/kg/day) | ||
| Maximum volume | 160mL/kg/day | ||
| Duration of trophic feedings | 5–7 days | ||
| Definition of intolerance | Decision tree. Gastric residuals+clinical examination | ||
| Fortifier | Starting at 100mL/kg (100mL, 1g%; 120mL, 2g%; 140mL, 3g%; 160mL, 4g%). Customise based on BUN | ||
| Constipation | Normal saline enema if no bowel movements in 24h | ||
BUN, blood urea nitrogen; NEC, necrotising enterocolitis; nIPPV, nasal intermittent positive pressure ventilation.
High risk of NEC included three conditions: birth weight <750g and/or gestational age <25 weeks; asphyxia; prenatal IUGR+abnormal Doppler ultrasound.
Bolus: when trophic feedings are used, they are administered with a syringe every 3h in the dose corresponding to that period. If the patient is under nIPPV during the incremental-dose period, the milk is administered over one hour with a pump, the pump is then closed in the second hour and is open again during the third hour in each three-hour period.
There were two additional changes in the management of patients with haemodynamically significant patent ductus arteriosus. Thus, during the first period, omeprazole was usually added to treatment with ibuprofen, and enteral feeds were maintained based on digestive tolerance. In comparison, during the second period enteral feeding was withdrawn and omeprazole no longer given. There were no other significant changes in care between the two periods.
Enfamil Fortifier® was the fortifier used during both periods. It was added when the mother brought in her breast milk, and the milk was then separated into bottles in the volume to be administered in each feeding and stored under refrigeration until used. Its administration was initiated at a dose of 1g% when the infant reached an intake of 100mL/kg/day, and was increased by 1g each day until reaching 4g/100mL.
Groups under studyGroup I (pre-intervention): born in year 2011.
Group II (post-intervention): born between May 1, 2012 and April 30, 2013.
Study sampleWe included all newborns with birth weights of less than 1500g that received care in our unit, including those born in other facilities that were transferred within one week from birth. We excluded newborns that had been referred exclusively to undergo a specific diagnostic test or treatment and subsequently transferred back to the original facility.
Variables under studyPrimary outcome: incidence of NEC (Bell stage II or higher).
Secondary outcomes: focal intestinal perforation, overall mortality and mortality attributed to NEC (cases in which NEC was included in the sequence of events that led to death), nosocomial sepsis, weight at 28 days and 36 weeks of corrected age, percentage of newborns with a weight below the 10th percentile at the time of hospital discharge, and length of stay in days (excluding newborns that died or were transferred).
The definitions of all the variables were consistent with the definitions used in the SEN 1500 database.6 Furthermore, for the primary outcome of the study, NEC, and for patients diagnosed with focal intestinal perforation, the clinical history and imaging tests for all cases were reviewed by peers.
Analysis of resultsFor quantitative variables, we first tested the normality of the distribution by means of the Kolmogorov–Smirnov test. Based on its results, we used a parametric test (Student's t) for variables with normal distributions, and otherwise a nonparametric test (Mann–Whitney U).
We summarised length of stay as median and interquartile range to minimise the influence of outliers.
For qualitative variables, we compared percentages for independent samples by means of the chi square test. When the proportion of any result was less than 5%, we used Fisher's test. We defined statistical significance as an alpha level of less than 0.05.
We performed the analysis with the EPIDAT statistical software (version 4.1).
ResultsThere were more patients in the first cohort than in the second (155 vs 115). Table 2 summarises the baseline characteristics of both groups. We did not find statistically significant differences in any of the analysed variables.
Demographic, perinatal and neonatal characteristics of the patients included in the study.
| Before (n=155) | After (n=115) | Significance | |
|---|---|---|---|
| Gestational age, days | 204 (19.47) | 206 (19) | N.S. |
| (weeks+days) | (29+1) | (29+3) | |
| Birth weight, g | 1097 (287) | 1148 (273) | N.S. |
| PEG | 59 (37%) | 44 (38%) | N.S. |
| Male | 79 (51%) | 57 (49.6%) | N.S. |
| Caesarean delivery | 125 (80%) | 89 (77.3%) | N.S. |
| Multiple birth | 48 (31%) | 32 (28%) | N.S. |
| Twins | 32 | 29 | N.S. |
| Triplets | 16 | 3 | N.S. |
| Prenatal corticosteroids | |||
| Full course | 100 (64.5%) | 84 (73%) | N.S. |
| Partial course | 33 (21.3%) | 17 (14.8%) | N.S. |
| None | 18 (11.6%) | 10 (8.7%) | N.S. |
| Not documented | 4 (2.6%) | 4 (3.5%) | N.S. |
| Apgar 5min | |||
| <4 | 1 (0.6%) | 1 (0.8%) | N.S. |
| 4–6 | 20 (12.9%) | 11 (9.6%) | N.S. |
| >6 | 134 (86.5%) | 103 (89.6%) | N.S. |
| CRIB | 2.98 (3.44) n=152 | 2.56 (3) n=100 | N.S. |
| Major malformations | 7 (4.5%) | 4 (3.5%) | N.S. |
| Hypotension in 1st week | 39 (24.4%) | 20 (17.4%) | N.S. |
| Corticosteroids in 1st week | 2 (1.2%) | 0 (0%) | N.S. |
| Prenatal antibiotics | 59 (36.9%) | 48 (41.7%) | N.S. |
N.S.: not significant.
Qualitative variables are expressed as absolute frequency and percentage (in parentheses). Quantitative variables are expressed as mean±standard deviation (in parentheses).
Table 3 shows the results for the clinical variables under study. We found that the second cohort had a lower incidence of NEC at Bell stage II or higher (0.9% vs 7.7%) and a lower mortality (7.8% vs 17.4%), and observed no difference in the incidence of focal intestinal perforation or nosocomial sepsis. While the percentage of patent ductus arteriosus was similar in both periods, in the first period there was a tendency towards a more frequent use of ibuprofen and a significantly higher frequency of surgical closure.
Main clinical variables under study, n (%).
| Before, n=155 | After, n=117 | Significance | |
|---|---|---|---|
| PDA | 68 (42–5%) | 41 (35–6%) | N.S. |
| Ibuprofen | 59 (38%) | 32 (27–8%) | N.S. |
| Surgical closure | 15 (9–7%) | 4 (3–5%) | P=.04 |
| Focal intestinal perforation | 3 (1–8%) | 3 (2–6%) | N.S. |
| NEC≥II | 12 (7–7%) | 1 (0–9%) | p=0–008 |
| NEC with surgery | 7 | 1 | N.S. |
| Mortality | 27 (17–4%) | 9 (7–8%) | p=0–02 |
| Deaths attributable to NEC | 4 | 0 | N.S. |
| Nosocomial sepsisa | 64 (41–3%) | 45 (39–1%) | N.S. |
| Coagulase-negative Staph | 50 (31–9%) | 41 (35–7%) | N.S. |
| Other bacteria | 18 (11–2%) | 13 (11–3%) | N.S. |
| Fungi | 3 (1–9) | 0 (0%) | N.S. |
| Gram-negative spp. | 17 (10–6%) | 7 (6–1%) | N.S. |
NEC, necrotising enterocolitis; N.S., not significant; PDA, haemodynamically significant patent ductus arteriosus (confirmed by echocardiography on the basis of the LA/Ao ratio AI/Ao, ductal size and reverse flow in the descending aorta).
In order to compare the incidence of each type of sepsis between the two cohorts, we show the percentage of each in relation to the cohort sample size. A patient may develop sepsis due to more than one microbe, which is why the sum of the frequencies for every type exceeds the number of infants with nosocomial sepsis.
Table 4 summarises some of the data pertaining to weight and the need for parenteral nutrition at 28 days and 36 weeks’ corrected age, as well as length of stay. Due to discharges that occurred before 28 days and 36 weeks (due to death, transfer or to discharge from hospital before that corrected age) the sample size for these variables is smaller. None of the patients that were transferred or discharged from hospital were on parenteral nutrition. We found no significant differences in weight gain at 28 days or 36 weeks, or in length of stay.
Weight and parenteral nutrition at 28 days and 36 weeks of corrected age; length of stay.
| Before, n=155 | After, n=115 | Significance | |
|---|---|---|---|
| 28 days post birth | n=110a | n=92a | |
| Peso, g (media±DS) | 1449 (328.8) | 1527.7 (345.5) | N.S. |
| N. parenteral | 15/110 (13.6%) | 14/92 (15.2%) | N.S. |
| Daily weight gain | 12.1g | 12.8 | N.S. |
| 36 weeks’ corrected age | n=84a | n=78a | |
| Weight, g (mean±SD) | 1730 (276.3) | 1796.9 (297.8) | N.S. |
| Weight at 36 weeks <10th %ile | 76/84 (90%) | 68/78 (87%) | N.S. |
| Length of stay | n=112a | n=105a | |
| Median and interquartile range | 44 days (35–66) | 48 days (32–61) | N.S. |
N.S., not significant.
The only case of NEC in the post-intervention group developed shortly after the introduction of the protocol and in a patient in whom the protocol was not followed. The patient had been born at 32 weeks’ gestation with a weight of 1430g and a history of IUGR and abnormal Doppler findings, but at birth he appeared to be in very good health, so enteral nutrition was initiated within hours from birth to avoid insertion of a catheter, and formula was administered because banked breast milk was not available at the time. Symptoms of NEC developed on day four.
Table 5 presents the most relevant characteristics of the NEC cases in the first cohort. The incidence of NEC was higher in the pre-intervention period in infants with lower weights, but also in other infants with higher weights and a history of IUGR. Enteral nutrition had been initiated early, usually with artificial formula, sometimes concurrently with inotropic therapy, and enteral feeds were often increased with barely a period of trophic feeding. Ductus was present in a high percentage, albeit one expected for the gestational age of the patients, and many were given ibuprofen and omeprazole. None of the patients were given hydrocortisone or any other steroid after birth. Few of the patients had been exposed to prenatal antibiotics, and most had received antibiotherapy postnatally.
Major characteristics of the NEC cases in the first cohort.
| Weightb | 6/12 <750g 4/12 750–999g 2/12 1000–1500 | |
| Risk stratification based on the criteria of the new protocol | 8/12 high risk 3/12 intermediate risk 1/12 low risk | Of the high-risk infants, 6 were high risk due to weight <750g and 2 to IUGR (one with abnormal Doppler; another a twin; weights 940 and 1320g) |
| Haemodynamically unstable when enteral nutrition was initiated | 2/12 | Two patients started enteral nutrition while still receiving inotropic agents |
| Antenatal IUGR diagnosis | 2/12 | Both with abnormal umbilical artery Doppler findings |
| Introduction of enteral nutrition with artificial formula | 10/12 | |
| Haemodynamically significant ductus arteriosus | 8/12 | Seven given ibuprofen |
| Omeprazole | 7/11a | Five received it during treatment of patent ductus arteriosus |
| Postnatal steroids (such as hydrocortisone) | 0/12 | |
| Day enteral nutrition introduced | Median: day 2 | Day 1 in 5 cases Day 2 in 4 cases Day 3 in 2 cases and day 6 in one case |
| Duration of trophic feedings in days (feeds<24mL/kg/day) | Median: 1 daya | 0 days in 2 cases 1 day in 6 cases 2 days in 2 cases 3 days in 1 case |
| Antenatal antibiotic exposure | 3/11a | In all 3 cases, due to chorioamnionitis |
| Antibiotics in 1st week of life | 10/12 | |
| Age at onset of NEC Median (range) | 9 (3–30) |
The introduction of the new nutrition protocol based on the evidence of best practices found in the literature was associated with a considerable reduction in the incidence of NEC in our hospital and in the percentage of infants that required surgery for this reason. At the same time, we found a reduction in mortality that exceeded the reduction that could be directly attributed to the reduction in NEC, for which we could not fully account. Although enteral nutrition was introduced later, we did not observe an increase in the incidence of nosocomial infection, a reduction in weight gain or an increase in lengths of stay.
The reduced incidence of NEC in relation to a change in nutritional practices is not a new phenomenon. A review of pre–post intervention studies showed an overall reduction of 87% in the incidence of NEC.7 Several subsequent studies have reported reductions of great clinical relevance.8–10
It is difficult to discern which of the modifications (one or several) in each of these studies led to the reduction in NEC, and the impact that each of them would have in isolation. The nutrition protocols used in these studies are not completely homogeneous, but they are all consistent in the cautious introduction of enteral nutrition, especially in children at higher risk of NEC, and in the preferential use of breast milk.
Our protocol introduced several changes compared to previous practice, and these changes were based on the literature review we had conducted for the purpose of developing a clinical practice guideline on enteral nutrition in preterm infants.11 We ought to note that probiotics were not given to patients in any of the two cohorts compared in the study, so the beneficial effects of the protocol can only be attributed to the way in which nutrition was delivered and the type of milk consumed. A subsequent update of the clinical practice guideline does include the use of probiotics, but their use was introduced after the two cohorts included in this study. The criteria applied to the literature review and its outcomes (evidence and grade of recommendations) can be found in this guideline.12,13
Introduction of enteral nutritionEarly introduction of enteral nutrition seems to be beneficial.14 However, the timing of its introduction does not seem to influence the incidence of NEC, at least in preterm infants that are haemodynamically stable. Based on our protocol, feeding was usually initiated in the first or second day, but was not introduced if the patient showed signs of haemodynamic instability. In this regard, we ought to note that almost all trials that compared enteral fasting with initiation of enteral feeding (even if it consisted of trophic feeding) specifically excluded haemodynamically unstable infants, so it is not known whether it is or is not safe in these patients. It is likely that in our pre-intervention cohort, trophic feeding was initiated in some infants that were still haemodynamically unstable, a practice that we now consider inappropriate. We believe that under these circumstances, there is blood flow redistribution at the skin level (evinced by poor peripheral perfusion) but also in other regions, such as the bowel (which would promote the development of NEC).
Trophic feeding periodThe administration of small volumes of milk (≤20–24mL/kg), without advances or with advances that remain within this limit for a period of five to seven days, is as safe as maintaining preterm newborns under enteral fasting for this duration, and shortens the time elapsed to full enteral feeding.15 On the other hand, daily advances from the beginning or advancing too quickly could foster the development of NEC.16,17 Before the introduction of the protocol, enteral feeds were advanced from the beginning or soon after introduction based on feeding tolerance in order to discontinue parenteral nutrition early and reduce the risk of catheter-related sepsis. Conversely, in the post-intervention period, trophic feeds were maintained for a period of five to seven days. The pre-post intervention studies mentioned above consistently report a trophic feeding period, of variable but protracted duration, prior to advancement of enteral feedings.9,10
Advancement periodAfter a period of trophic feeding, increments of 30–35mL/kg/day do not increase the risk of NEC compared with volumes of 20mL/kg/day18 and shorten the time to establishment of full enteral feeding. In the pre-intervention period, we advanced volumes by 20mL/kg/day, while in the post-intervention period we maintained the 20mL volume in patients with birth weights of less than 1000g, but raised it to 30mL/kg/day in patients with greater weights, and this was not associated with an increased incidence of NEC. Another difference is that in the second period, the increases were made at two different times during the day, which made us re-evaluate feeding tolerance before each increase. It is possible that this approach allowed us to detect problems earlier than before.
Absence of bowel movementsConstipation has been proposed as a possible risk factor for NEC because it can promote bacterial overgrowth in paretic bowel loops. Pietz et al. published a large case series with a very low incidence of NEC (0.8%) and emphasised how important they found this factor; they used glycerine if bowel movements were absent for 24h.8 In our study, we gave patients normal saline enemas. The only change in this regard was that in the post-intervention period, the enemas were routinely prescribed if the patient did not have any bowel movements in a 24-h period, whereas their use was rather discretionary in the first period.
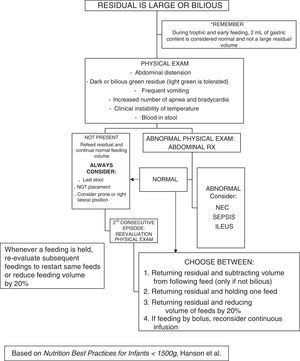
Feeding intoleranceMeasurement of gastric residual volume has been suggested as a means to monitor feeding tolerance, but its routine use is the subject of debate.19 This practice was only occasional in the pre-intervention period, while in the post-intervention period it was proposed as an assessment method during the trophic feeding or advancement periods, but its actual implementation was left to the judgement of the physician in charge. In general, residuals were not checked in the absence of abdominal distension. Our decision tree (Appendix A) was based on the algorithm published by Hanson et al.20 It is possible that suspicion of NEC before the patient develops pneumatosis intestinalis could prevent its progression to more advanced stages if enteral feeding is discontinued and the patient is given antibiotics, as is proposed for Bell I stage.
Type of milkBased on the existing literature,21–23 this protocol encouraged the use of breast milk from the beginning of enteral feeding. Maternal colostrum was used preferentially, and if it was not available, banked donor milk. In the pre-intervention period, “breast milk or artificial formula” was usually prescribed, and this often led to initiation of enteral feeding with formula, so that bacterial colonisation of the intestine was probably not ideal. The availability of banked donor milk allowed initiation of enteral nutrition with fresh colostrum or donor milk in nearly all patients. We believe that the benefits derived from breast milk are greatly enhanced if it is used from the beginning; in fact, in the years preceding the study, the rate of nutrition with the infant's own mother's milk at the time of discharge had been increasing in our hospital, reaching 74% by 2011 (44.5% for exclusive feeding with breast milk), but this increase had not been associated with a reduction in the incidence of NEC.
StandardisationA recent study on enteral feeding practices in preterm infants showed considerable variability between countries, hospitals and neonatologists working in the same hospital.4 It is possible that this variability accounts at least partly for the also considerable variability found in the NEC incidence in different hospitals.7 It is possible that the reduction of intracentre variability resulting from the implementation of a standardised protocol could have an impact in and of itself.8,9,24
OmeprazoleIn the first period, it was customary practice to add omeprazole for gastric protection from initiation of treatment with ibuprofen. The use of ranitidine had already been discontinued due to its association with an increased incidence of sepsis and death in VLBW infants.25 Since we did not observe any benefits associated with this practice, and studies were being published that pointed at similar risks associated with the use of omeprazole, we decided to restrict its use to infants with active gastrointestinal bleeding, discontinuing treatment as soon as bleeding resolved.
FortifierSupplementation with a fortifier is associated with an improvement in short-term growth in infants fed with breast milk; it has not been found any increase in the incidence of NEC.26 The fortifier was administered in a similar way during the two periods under study, so we exclude the possibility of it having influenced the outcomes.
LimitationsThe diagnostic criteria for NEC and focal intestinal perforation were the same during the entire period under study, and all cases were documented prospectively for submission to the SEN-1500 database. It is possible that the definition of NEC cases in the second period was more stringent than in the first period, and that some cases of focal intestinal perforation were diagnosed as NEC in the first period. To avoid this potential bias, we retrospectively reviewed the diagnosed cases of NEC and focal intestinal perforation, re-evaluating the imaging tests and the histological examinations in surgical cases, and included only those cases that were confirmed. The reduction in the incidence of cases with radiographic evidence of pneumatosis intestinalis (Bell II stage) and, concurrently, of forms of NEC that required surgery, confirmed the actual reduction in the incidence of disease.
ConclusionsClinical enteral nutrition practices in preterm infants have an impact on the risk of NEC.
The implementation of an enteral nutrition protocol for VLBW infants and infants born at less than 32 weeks’ gestation based on the best available evidence and detailing the way in which enteral nutrition should be administered led to a reduction in the incidence of NEC without associated increases in the incidence of nosocomial sepsis or the length of stay.
FundingThis study was not supported by any outside funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study would not have been possible without the close cooperation with the Milk Bank of the Hospital Virgen de las Nieves of Granada and, above all, with our donor mothers, to whom we are profoundly grateful.
- -
Measure before each feed (once or twice daily, especially during periods of trophic feeding and dose increments, and in infants in whom tolerance is uncertain). A large gastric residual volume is defined as ≥50% of the previous feeding volume (or the volume fed in the three previous hours in case of continuous infusion)*.
- -
If residual has a normal appearance and a volume of ≤50%, the full feed will be added and administered again.
Please cite this article as: Sánchez-Tamayo T, Espinosa Fernández MG, Affumicato L, González López M, Fernández Romero V, Moreno Algarra MC, et al. Reducción de la enterocolitis necrosante tras la introducción de un protocolo de alimentación enteral basado en la evidencia en recién nacidos de muy bajo peso. An Pediatr (Barc). 2016;85:291–299.