Para-infectious seizures are afebrile seizures that are associated with mild infections, and occur in children with no pre-existing neurological illness. They are still little known in our environment.
MethodsA multicentre retrospective study was conducted that included patients with normal psychomotor development and had presented with one or more seizures in the context of a mild afebrile infection.
ResultsA total of 38 patients (47% male, 53% female) were included in the study over a period of three years (2012–2015). The mean age was 2.1 years. A previous history of febrile seizures was found in 7.9% of them. Mean number of seizures per patient was 2.2, with 57.9% of them being tonic-clonic seizures. The mean duration of seizures was 3.2min. An EEG was performed during admission in 73.7% of cases. Lumbar punctures were performed in 34.2% of cases. All were normal. Neuroimaging tests were carried out in 36.9% of cases. Brain MRI was the imaging test performed in most cases (21.1%), with no any pathological findings. The most frequent infection found was acute gastroenteritis (68%), followed by upper respiratory tract infection (32%). Almost two-thirds (63.2%) of patients did not require anticonvulsant medication. Rectal diazepam was the most frequently used drug in emergencies. Intravenous medication was required by 28.9% of patients due to repeated seizures. The most frequently used drug in the non-emergency setting was valproic acid. Anticonvulsant treatment was continued after discharge in 16% of patients. Para-infectious seizures was the diagnosis in 76.3% of cases when discharged.
ConclusionsKnowledge of para-infectious seizures, their clinical diagnosis and benign course is crucial, as this would avoid further testing and unnecessary treatments.
Las crisis parainfecciosas son crisis convulsivas afebriles en el contexto de infecciones banales en niños sin afectación neurológica, siendo aún una patología poco conocida en nuestro medio.
MétodosEstudio retrospectivo multicéntrico donde se incluye a pacientes con crisis única o múltiple en el contexto de una infección banal afebril, con desarrollo psicomotor normal.
ResultadosSe recogió a 38 pacientes (47% varones, 53% mujeres) en un periodo de 3 años (2012-2015) con edad media de 2,1 años. El 7,9% presentaba antecedentes de crisis febriles. La media de crisis por paciente fue de 2,2, siendo el 57,9% crisis tónico-clónicas generalizadas, con una duración media de 3,2 min. Se realizó electroencefalograma durante su ingreso al 73,7%. Se efectuó punción lumbar en un 34,2% (todas normales) y prueba de neuroimangen en el 36,9%, siendo la más realizada la RM craneal en el 21,1%, sin hallazgos patológicos. El proceso infeccioso más frecuente (68%) fue tener gastroenteritis aguda seguida de la infección respiratoria de vías altas (32%). El 63,2% no precisó medicación anticomicial. En urgencias el fármaco más usado fue el diazepam rectal. Posteriormente, debido a la agrupación de crisis, un 28,9% de los casos precisó administración de fármacos por vía intravenosa (el más usado fue el ácido valproico), manteniéndose en el 16% tratamiento antiepiléptico al alta. El 76,3% de los pacientes fue diagnosticado al alta de crisis parainfecciosas.
ConclusionesEs fundamental el conocimiento de las crisis parainfecciosas, su diagnóstico clínico y evolución benigna, ya que su identificación evita la realización de pruebas complementarias y tratamientos innecesarios.
Parainfectious seizures are afebrile convulsive seizures associated with mild infectious diseases, such as acute gastroenteritis (AGE) without electrolyte imbalance or signs of dehydration, or upper respiratory tract infections (URTIs).1–5 They were first described in 1982 by Morooka in Japan.6 Since then, they have been the subject of numerous studies and case series, especially in the Asian region.3,4,6–8 However, they are still little known in Spain.1,2,5,9,10 This lack of knowledge leads to their underdiagnosis and to performance of a large number of unnecessary diagnostic tests to determine their aetiology and make a diagnosis and prognosis.1–10 The aims of this study were to determine the incidence of parainfectious seizures in hospitals of Castilla y León and analysing the main characteristics of these seizures, their treatment, and their natural history.
Patients and methodsWe conducted a retrospective descriptive study by collecting data for all children aged less than 14 years admitted to six hospitals in the autonomous community of Castilla y León over a period of three years (January 2012 to January 2015) with a diagnosis of convulsive seizure. The inclusion criteria were: single or multiple afebrile seizure (maximum temperature, 37.9°C) associated with mild disease such as upper respiratory tract infection or AGE without electrolyte imbalances or clinical signs of dehydration; normal psychomotor development prior to admission, and diagnostic tests with unremarkable results. We excluded all patients with fever before, after or during the seizure, a prior diagnosis of epilepsy or a history of psychomotor delay (Table 1).
Inclusion and exclusion criteria in the study.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Children with convulsive seizures associated with mild infectious diseases, such as AGE or an URTI | Children with signs of meningitis, encephalitis or acute encephalopathy associated to infection (changes in consciousness in the presence or absence of focal neurologic signs) |
| Single or multiple convulsive seizures | Children with disorders of psychomotor development or a history of epilepsy |
| Maximum axillary temperature of 37.9°C | Axillary temperature equal to or greater than 38°C |
The variables we analysed in the sample under study were sex, family and personal history of disease, type of infection, seizure semiology, diagnostic tests and their results, antiepileptic drugs (AEDs) used (first-line drugs at the emergency department and drugs used during admission and for maintenance after discharge), and subsequent outcome during followup at paediatric neurology clinics.
ResultsThe study included 38 patients aged between 3 months and 8 years (mean±standard deviation, 2.1±1.8 years); 47% were male and 53% female. Only 9% of patients had a past personal history of typical febrile seizures. As for family history, 10.5% of the patients had first-degree relatives with epilepsy, and only 2.6% had a first-degree relative with a history of febrile seizures during childhood.
The time that elapsed between the onset of infectious disease and the development of convulsive seizures was nearly two days on average (mean, 43.8h), and ranged between 2h and 7 days.
The mean number of seizures was 2.2±1.8, and this number ranged between a single seizure to a cluster of 10 episodes. The duration of seizures ranged between less than one minute and twenty minutes (mean, 3.24min). The most frequent type of seizure was generalized tonic-clonic seizures (58%), followed by tonic seizures (18%).
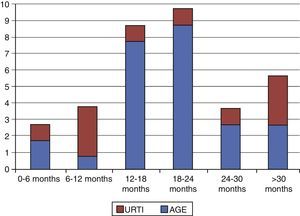
As for intercurrent infection, out of the 38 patients, 32% had upper respiratory tract infections and 68% AGE. Acute gastroenteritis was the most frequent aetiology in all age groups save for infants aged 6 months to 1 year (Fig. 1), in whom URTIs were more frequent. Out of the 26 stool cultures and analyses performed, Salmonella was isolated in one, Campylobacter in one, adenovirus in one, and rotavirus, which was the most frequent aetiologic agent in our sample, in five. Only five nasopharyngeal aspirate specimens from patients with URTIs were analysed, of which one tested positive for influenza type A and one other for rhinovirus.
Blood samples were collected for a complete blood count (CBC) and metabolic panel on arrival to the emergency department in 97% of children, and the results were unremarkable in 76% of them. An electroencephalogram (EEG) was performed during admission in 76% of the patients, detecting slow activity in 14% and irritative activity in 20%, with normal activity in all others (60%). Thirteen children (34%) underwent lumbar punctures for analysis, the results of which were normal. Neuroimaging tests were performed in 37% of the children, the most frequent being cranial MRI (21% of the total), and the results were normal in all.
When it came to treatment, 63.2% of the patients did not need AED therapy, and only 15.8% required more than one AED. Twenty-one percent required AED therapy to control their seizures in the emergency department, where benzodiazepines (diazepam or midazolam) were used in all cases, with rectal diazepam being used most frequently.
Subsequently, 28.9% of admitted patients required AED therapy for repetitive seizures, and the most frequently used drug was valproic acid, followed by levetiracetam and phenytoin. At discharge, only six patients (16%) remained on oral AED therapy, half of them with valproic acid and half with levetiracetam, with one patient on combined therapy with both.
All patients were re-evaluated in a paediatric neurology clinic, and more than 90% had favourable outcomes. Two of the patients went on to develop epilepsy and had secondarily generalized partial seizures. Another patient developed symptoms of autism.
We analysed the potential differences between two subgroups corresponding to the type of infection: AGE or URTI (Table 2). In patients that had AGE, the time elapsed from onset to the development of seizures was of 50.41±48.24h, and in patients with an URTI, it was 27.55±51.35h; the difference between these two aetiologies was statistically significant (P=.037). The number of seizures was greater in children with AGE (mean±standard deviation, 2.44±1.91) compared to children with URTIs (mean±standard deviation, 1.45±0.69), and the duration of seizures was shorter in children with AGE compared to children with URTIs (mean±standard deviation, 2.52±1.83 for AGE and 4.91±5.72 for URTIs), although the difference was not statistically significant.
Comparison of parainfectious crisis in children with AGE and URTIs.
| AGE | URTI | |
|---|---|---|
| Age (years)a | 2.06 (1.81) | 2.35 (2.15) |
| Time elapsed to development of seizure (hours)a | 50.41 (48.24) | 27.55 (51.35) |
| Number of seizuresa | 2.44 (1.91) | 1.45 (0.69) |
| Duration of seizures (minutes)a | 2.52 (1.83) | 4.91 (5.72) |
| AED therapy, yes/no (percentage) | 33.3/66.7 | 45.5/54.5 |
AED, antiepileptic drug; AGE, acute gastroenteritis; URTI, upper respiratory tract infection.
Parainfectious seizures or convulsive seizures associated with mild infectious diseases, such as AGE or URTI, have not been described as such in the classification of epilepsy and epileptic syndromes of the International League against Epilepsy, although it has been suggested that they be included under the heading of special syndromes, as has been done with febrile convulsions.11 They are disease processes that have been studied little in Spain, but thoroughly analysed in Asia.3,4,6–8 To date, most of the case series studied in Spain have found an association between parainfectious seizures and AGE.1,2,10,12–16 These studies, such as the one conducted by Lacasa Maseri et al.,1 included patients with febrile seizures as well as patients with afebrile seizures. However, our study only included patients with afebrile seizures.
Our sample included a total of 38 patients, so it is the largest sample of its kind in Spain up to date.1,2,9,13–16 The series of Lara Herguedas et al.9 found that parainfectious seizures developed in the context of AGE in 67.6% of their sample and of URTIs in 32.4%, figures that are practically identical to those found in our study, of 68% and 32%, respectively. As reported in most of the literature, rotavirus was the aetiological agent identified most frequently in our study.1–10,12–17
Some of the published studies only included patients aged between 6 months and 5 years.3,12 Our study, however, did not exclude patients aged less than 6 months, and we observed that the outcomes in these patients were similar to those reported in the literature.1,2,10,12–16 The median age of patients in our study at the time of seizure onset was 25 months, which is similar to the findings of other authors in Spain.1,2,9,13–16
It is common for seizures to cluster during a single course of infection, but this is not a necessary criterion for diagnosis.1–10,12–16 In fact, we found that 44.7% of our patients had a single seizure. When it came to seizure semiology, the seizures observed most frequently were tonic-clonic (58%), followed by tonic seizures (18%), and most had a short duration. These results are consistent with those of studies conducted in both Europe and Asia.3,4,9,12–16
When it came to the characteristics of the seizures based on the type of infection, we found a statistically significant difference in the time elapsed from the onset of infection to development of seizures, which was longer in patients with AGE. Also, patients with AGE had repeated seizures more frequently and shorter seizures compared to patients with URTIs (these differences were not statistically significant). These results are consistent with those reported in previous studies.5,9
The diagnostic tests performed in the patients included blood testing with a CBC and metabolic panel in 97%, with unremarkable results in most. Lumbar punctures were performed in 34% of the patients and neuroimaging tests in 31% (most frequently an MRI, which was ordered in 21% of the patients), mostly for the purpose of ruling out infections of the central nervous system (meningitis and encephalitis) and structural lesions that could cause provoked or symptomatic seizures. These data are similar to those reported in the study by Lara Herguedas et al.,9 in which the results of all tests were normal. This evinces the large number of tests that are conducted in Spain and do not contribute any relevant information in the assessment of parainfectious seizures.
When it comes to the management of seizures, there is a generalized trend in the literature in reporting seizures that are refractory to treatment whose control requires two or more drugs.3,4,7,10,12–14 We did not observe this in our study, as 63.2% of the patients did not need AED therapy and only 15.8% required more than one drug.
Of all the patients with a diagnosis of parainfectious disease reviewed in our study, only 76.3% were discharged with this diagnosis, while the rest received it during followup at a specialty clinic. All patients were followed up at a paediatric neurology clinic, and most had favourable outcomes. Neurologic symptoms were only observed in three patients, two of whom had partial epilepsy and one symptoms of autism. This amounts to 8% of the patients and is consistent with the results reported in the literature,3,8,9,14,18 which show that most patients have normal outcomes after developing parainfectious seizures. In Spain, Lara Herguedas et al. also observed the later development of partial epilepsy in one child and language delay in another in their series of 34 patients.9 The seizures of these patients had not differed in any way from the parainfectious seizures of the rest of the patients. We did not find any variable associated with a poor prognosis.
In conclusion, it is essential for us to be aware of the existence of parainfectious seizures to be able to make a clinical diagnosis, preventing the unnecessary use of diagnostic tests and treatments, and to offer an accurate prognosis as regards its benign outcome and low rate of recurrence.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Conejo Moreno D, Rodríguez Fernández C, Ruíz Ayúcar de la Vega I, Ortiz Madinaveitia S, Hedrera Fernández A, Maldonado Ruiz E, et al. Crisis parainfecciosas: estudio retrospectivo multicéntrico. An Pediatr (Barc). 2016;85:300–304.