Recurrent meningitis is a rare disease. Anatomical abnormalities and immunodeficiency states are predisposing factors. Four cases, in which immunodeficiency was excluded, are presented. The causal microorganism led to the detection of the anatomical defect responsible for the recurrences.
Patients and methodsRetrospective review of 4 cases with clinical diagnosis of recurrent bacterial meningitis.
ResultsCase 1: a thirty-month-old boy, with unilateral hearing loss, was diagnosed with Mondini abnormality by magnetic resonance imaging (MRI) after 2 episodes of Haemophilus influenzae meningitis. Surgical repair was after third recurrence. Case 2: a fourteen-year-old girl was diagnosed by MRI with cribriform plate defect after 3 episodes of meningitis due to Streptococcus pneumoniae. Ventriculoperitoneal shunt was placed. Case 3: a girl with meningitis due to Staphylococcus aureus at 2 and 7 months. MRI shows occipital dermal sinus requiring excision. Complication with cerebellar abscesses because of a coexisting dermoid cyst. Case 4: a child with meningitis due to Streptococcus bovis at 9 days and Enterococcus faecium, Klebsiella pneumoniae and Escherichia coli at 7 months, with positive cultures to Citrobacter freundii and E. faecium later on. Spinal MRI led to the diagnosis of Currarino syndrome with CSF fistula, which was surgically repaired. The 4 patients had undergone image studies reported as normal during the first episodes.
ConclusionsIn patients with recurrent meningitis the possibility of an anatomical defect should be considered. The isolated microorganism should help to locate it. It is essential to know the normal flora of the different anatomical sites. The definitive treatment is usually surgical.
La meningitis recurrente es una patología infrecuente. Los factores predisponentes son alteraciones anatómicas o situaciones de inmunodeficiencia. Presentamos 4 casos en los que, excluida una inmunodeficiencia, el microorganismo responsable orientó al defecto anatómico causante de las recurrencias.
Pacientes y métodosRevisión retrospectiva de 4 casos clínicos con diagnóstico de meningitis bacteriana recurrente.
ResultadosCaso 1: niño de 30 meses con hipoacusia unilateral, diagnosticado por resonancia magnética (RM) de malformación de Mondini tras 2 episodios de meningitis por Haemophilus influenzae. Reparación quirúrgica tras tercera recurrencia. Caso 2: niña de 14 años diagnosticada por RM de defecto de lámina cribiforme posterior a 3 episodios de meningitis por Streptococcus pneumoniae. Se coloca válvula de derivación ventrículo-peritoneal. Caso 3: niña con meningitis por Staphylococcus aureus a los 2 y 7 meses. La RM muestra seno dérmico occipital que requiere exéresis. Complicación con abscesos cerebelosos por coexistencia de quiste dermoide. Caso 4: niño con meningitis por Streptococcus bovis a los 9 días y por Enterococcus faecium, Klebsiella pneumoniae y Escherichia coli a los 7 meses, con crecimiento de Citrobacter freundii y E. faecium posteriormente. RM compatible con síndrome de Currarino. Incluye fístula rectal de LCR, que se repara quirúrgicamente. A los 4 pacientes se les habían realizado pruebas de imagen durante los primeros episodios de meningitis, informadas como normales.
ConclusionesEn los pacientes con meningitis recurrentes se debe valorar la posibilidad de un defecto anatómico; el microorganismo aislado debe ayudar a localizarlo. Es imprescindible conocer la flora normal de los potenciales focos. El tratamiento definitivo es habitualmente quirúrgico.
Bacterial meningitis is a severe and life-threatening infection. It is associated with a high rate of complications and neurologic sequelae despite advances in antibiotic therapy and intensive care measures. Recurrence occurs in approximately 1–4.8% of acute bacterial meningitis cases, and is defined as the re-emergence of signs and symptoms of meningitis at least 3 weeks after a sterile culture of the cerebrospinal fluid (CSF) if the pathogen is the same, or a new episode caused by a different pathogen.1
Different predisposing factors for recurrent meningitis have been identified, such as humoral and cellular immunodeficiencies and congenital or acquired structural defects that result in an anatomical communication between the subarachnoid space and the skin, the middle ear or the paranasal sinuses2–4 (Table 1).
Main causes of recurrent bacterial meningitis.
| Congenital anatomical defects | Primary or acquired immunodeficiencies | ||
|---|---|---|---|
| Meningocele (cranial or lumbosacral)/meningoencephalocele | HIV infection | ||
| Ethmoid bone | Complement defects | ||
| Skull base defects | Petrous portion of temporal bone | IRAK-4 deficiency | |
| Sphenoid bone | Asplenia | ||
| Inner ear abnormalities (Mondini dysplasia) | IgG subclass deficiency | ||
| Neurenteric cyst | Humoral immunodeficiencies | Common variable immunodeficiency | |
| Dermal sinus and dermoid/epidermoid cyst (from nasal root to conus medullaris) | Agammaglobulinaemia | ||
| Acquired anatomical abnormalities | |||
HIV, human immunodeficiency virus; IgG, immunoglobulin G; IRAK-4, interleukin-1 receptor-associated kinase 4.
The approach to diagnosis must be guided by the medical history, the findings of the physical examination and microbiological test results.5 In many instances, the isolated pathogen gives a clue to the source of meningitis.
We present four cases of paediatric patients with recurrent bacterial meningitis due to congenital anatomical defects in which the causative microorganisms guided the diagnosis (Table 2).
Main characteristics of the 4 presented cases.
| Sex | Episode number | Age | Microorganism | Anatomical defect | Definitive treatment | Outcome | |
|---|---|---|---|---|---|---|---|
| Case 1 | Male | 1 | 20 months | Nontypeable non-beta-lactamase-producing H. influenzae | Mondini dysplasia | Perilymph fistula repair by separation of the middle and inner ear and closure of the mastoid cavity | No neurologic sequelaeNo new episodes of meningitis |
| 2 | 30 months | Non-type B beta-lactamase producing Haemophilus spp. | |||||
| 3 | 34 months | No microorganism isolated | |||||
| Case 2 | Female | 1 | 11 years | S. pneumoniae serotype 19A | Osseous and dural defect at the cribriform plate level | Placement of ventriculoperitoneal shunt | No neurologic sequelaeNo new episodes of meningitis |
| 2 | 13 years | S. pneumoniae serotype 19A | |||||
| 3 | 14 years | S. pneumoniae serotype 6A | |||||
| Case 3 | Female | 1 | 2 months | S. aureus | Occipital dermal sinus and dermoid cyst | Complete resection of dermal sinus and dermoid cyst | No neurologic sequelaeNo new episodes of meningitis |
| 2 | 7 months | S. aureus | |||||
| Case 4 | Male | 1 | 9 days | S. bovis type i | Currarino syndrome | Surgical repair of the fistula between the rectum and the subarachnoid space | No neurologic sequelaeNo new episodes of meningitis |
| 2 | 7 months | E. faecium, K. pneumoniae y E. coli | |||||
| 3 | 7 months | C. freundii | |||||
| 4 | 7 months | E. faecium | |||||
A boy, 22 months of age, with no history of interest except for nonvaccination from 12 months of age by parental choice, was admitted to the hospital with meningitis. The CSF culture was positive for nontypeable non-beta-lactamase-producing Haemophilus influenzae. The patient responded favourably to intravenous cefotaxime. The electroencephalogram and computed tomography (CT) scan showed no abnormalities, but a moderate right-sided hypoacusis was detected in the brainstem that evoked auditory potentials, which was interpreted as a sequela of the infection.
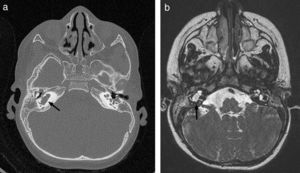
Thirty months later, he was readmitted with meningitis due to nontypeable H. influenzae. The parents reported a history of symptoms suggestive of otitis in the right ear. Since this was the second episode of bacterial meningitis, complete immunological testing was performed, the results of which were normal. The investigation included human immunodeficiency virus (HIV) serology, serum immunoglobulin levels, complement classical and alternative pathway tests, presence of spleen in ultrasound, and absence of Howell-Jolly bodies in peripheral blood. Based on the recurrence of meningitis caused by bacteria characteristic of the otic and oropharyngeal flora, we decided to rule out an anatomical defect at this level. Thus, we expanded the imaging study by a high resolution CT scan of the petrous body and a cranial MRI, which revealed a malformation of the membranous labyrinth affecting the cochlea and the vestibule in the right ear (incomplete partition type ii or Mondini dysplasia), and assumed that the source of the recurrent meningitis was a fistula to the subarachnoid space (Fig. 1).
CT scan and MRI of petrous part. (a) Axial high resolution TC scan. (b) Axial CISS (three-dimensional constructive interference in steady state) MRI. Membranous labyrinth malformation affecting the right cochlea and vestibule (incomplete partition type ii, also known as Mondini dysplasia) (black arrows).
In light of these findings, a course of vaccination against pneumococcus was initiated and a tympanostomy tube placed in the right ear to prevent the development of otitis, which could result in a new source of infection, while surgical repair of the defect was postponed.
In spite of these measures, at 34 months of age the patient was readmitted for a suspected recurrence of bacterial meningitis. Analysis of the CSF revealed markedly increased protein and white blood cell levels, with predominance of neutrophils, but on this occasion the causative agent was not isolated even with the use of molecular biology techniques. The main otoscopic findings were dislodgment of the tympanostomy tube and otitis media in the right ear.
After the symptoms of the third episode of bacterial meningitis resolved, it was decided to repair the perilymph fistula by separation of the middle and inner ear and closure of the mastoid sinus,6 and the outcome was good. The patient, currently 39 months of age, has not developed new episodes of meningitis.
Case 2A girl 11 years of age, was admitted to another hospital with fever, headache and vomiting lasting 12hours. She was diagnosed with meningitis. Streptococcus pneumoniae was isolated from the blood and CSF cultures and treatment with cefotaxime was initiated, and the patient had a good clinical response. The patient underwent a MRI scan of the brain that was normal, and was discharged with no sequelae.
At 13 years of age, she had a new episode of meningitis due to S. pneumoniae. Since this was the second meningitis, we screened for immunodeficiencies (negative HIV serology; normal results for serum immunoglobulins and classical and alternative complement pathways; presence of spleen in ultrasound; absence of Howell-Jolly bodies) and administered a first dose of pneumococcal vaccine. Since the only relevant history reported was recurrent otitis media, CT of the cochlea and petrous body was performed, and both structures appeared normal.
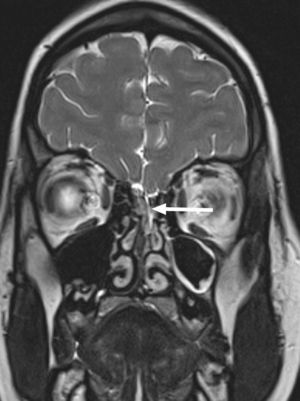
At 14 years of age, she was admitted to our hospital for a new episode of pneumococcal meningitis with bacteraemia. At that point, expanded immunological testing was performed that ruled out innate immune defects, as well as an MRI due to suspicion of an anatomical defect, which revealed a bony and dural defect at the level of the cribriform plate (Fig. 2). The patient underwent surgery to repair the anatomical defect, and has remained asymptomatic to date (4 years).
Case 3A girl 2 months of age, with no history of interest, presented with a fever of 39°C lasting a few hours and no other symptoms. The investigation of the fever without source included a lumbar puncture, and the CSF analysis revealed increased white blood cells with predominance of neutrophils, decreased glucose, and increased total protein. The microbiological study of the CSF yielded a single colony of Staphylococcus aureus (S. aureus) sensitive to cloxacillin, confirmed by a second culture. A 3-week course of intravenous cloxacillin was administered, and colonisation of the patient and her family members by S. aureus was ruled out. Expanded testing was performed during admission, including echocardiography, fundus examination, abdominal ultrasound and cranial MRI, all of which were normal.
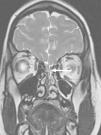
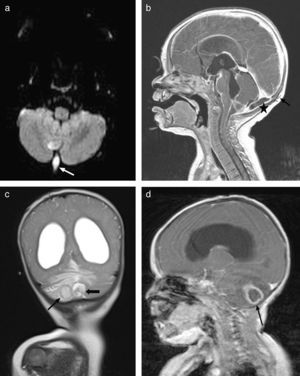
At 7 months the patient presented with febricula, a bulging fontanelle, vomiting and irritability, and underwent a lumbar puncture that showed cytochemical findings similar to those of the previous episode with isolation of a few colonies of cloxacillin-sensitive S. aureus. This time there was evidence of colonisation of the mother by S. aureus. Since this was the second episode of meningitis by this pathogen and an anatomical defect was suspected, another MRI scan was performed. The scan revealed a pattern in the posterior fossa suggestive of a dermal sinus complicated by a small subdural empyema, as well as a nonresorptive hydrocephalus secondary to inflammation (Fig. 3). At this point, an external ventricular drain was placed to drain the subdural and cerebellar abscesses, and the dermal sinus was resected. Still, the condition of the patient deteriorated, so a new scan was done, showing abscesses at the occipital and cerebellar levels (Fig. 3b) that required drainage. During the surgery, a dermoid cyst was found and completely resected. S. aureus was isolated again, and the patient was treated with a 7-week course of cloxacillin. The hydrocephalus was managed by placement of a ventriculoperitoneal shunt. The patient, currently 11 months of age, has not developed any new infections since.
(a) Axial diffusion MRI showing an infected dermal sinus in the posterior fossa (white arrow) with restricted diffusion. (b) Post-contrast sagittal T1 MRI showing the subcutaneous dermal sinus tract (black arrow), complicated by a small subdural empyema in the posterior fossa (asterisk). (c) Coronal T2 MRI showing nonresorptive hydrocephalus secondary to leptomeningitis and 2 patterns compatible with cerebellar abscesses associated with oedema. (d) Post-contrast sagittal T1 MRI showing hydrocephalus and ring enhancement in intraparenchymal cerebellar abscesses (black arrow).
A boy 7 months of age, with a history of meningitis and sepsis due to Streptococcus bovis at 9 days of life presented with fever lasting 12h and general malaise. Suspicion of meningitis led to performing a lumbar puncture, and the CSF findings were compatible with bacterial meningitis. Empirical treatment with intravenous cefotaxime, vancomycin and dexamethasone was initiated. The CSF culture was positive for Enterococcus faecium, Klebsiella pneumoniae and Escherichia coli sensitive to the administered treatment.
On the eighth day of admission, the fever and malaise recurred. Another lumbar puncture was performed, and CSF findings suggested a new episode of bacterial meningitis. Citrobacter freundii was isolated from the CSF culture, and treatment was switched to intravenous meropenem.
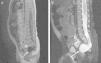
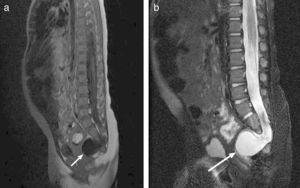
After 6 days of treatment with meropenem, the symptoms recurred, and E. faecium was isolated once again from the culture. The progression of the patient prompted immunological testing (HIV serology tests, white blood cell counts, immunoglobulins, complement pathways, presence of spleen in ultrasound), which yielded normal results. Once immune disorders were ruled out, and in light of the persistence of meningitis caused by enteric bacteria, an initial abdominal and soft tissue ultrasound was performed (along the spinal cord) that showed no abnormalities, and was followed by an MRI that showed partial agenesis of the right side of the sacrum starting at S3, a presacral meningocele below S2, and after administration of gadolinium, a CSF fistula (Fig. 4), leading to a diagnosis of Currarino syndrome and surgical repair of the anatomical defect. The patient, currently 41 months of age, has not developed further infections to date, and his psychomotor development is normal.
DiscussionRecurrent bacterial meningitis is a rare disease in children, and few data are available on its incidence. A review of 463 children with bacterial meningitis found 6 patients with recurrent episodes, corresponding to an incidence of 1.3%. The pathogen identified most frequently was S. pneumoniae. An otorhinolaryngological abnormality was confirmed in 2 cases, a primary immunodeficiency in another 2, and no predisposing factor was found in the rest.2 A study of meningitis in 696 adults identified 34 recurrent episodes (4.8%) and estimated their annual incidence at 0.12 cases per 100,000 adults.7
Anatomical defects and primary and secondary immunodeficiencies should be ruled out as underlying causes in patients with recurrent meningitis.8 The identified bacterium can give clues to locate the anatomical defect or to rule out immune defects. In this regard, a review by Trebuegge and Curtis of 132 cases of recurrent meningitis in children and adults from case reports and series published between 1988 and 2008 recommends a diagnostic approach that includes all the investigations performed in the cases presented in this article. The abnormalities described most frequently in the cases reviewed by Trebuegge and Curtis were complement deficiencies, inner ear abnormalities (Mondini dysplasia), dermoid cysts, meningoceles and meningomyeloceles.3
In the cases presented in this article, we describe 4 patients with different congenital anatomical malformations that posed challenges to early detection.
Mondini dysplasia is a defect in cochlear development that occurs in the first trimester of gestation and results in a cochlea with fewer turns than normal, with the apical and middle turns undifferentiated and forming a cystic apex that may cause deafness, vestibular abnormalities and a predisposition for infections of the central nervous system.9 Its radiological diagnosis requires a CT scan of the temporal bone,10 as it may be missed in a cranial CT scan. In case 1, the history of otitis media preceding the episodes, the concomitant unilateral hypoacusis and the repeated presence of a bacterium commonly found in the nasopharyngeal flora called for ruling out this malformation. Its surgical management can be more or less aggressive depending on the previous auditory functioning of the patient, with the purpose of closing the CSF fistula.11 The need for a cochlear implant should only be considered in cases of bilateral deafness, since there is a risk of CSF gusher (perilymph leakage) during the intervention.12,13
When it comes to defects of the cribriform plate, a clinical and anatomical study of spontaneous cerebrospinal fluid rhinorrhoea by Tóth et al. describes 29 patients, of which 4 had malformations at this locus. The study found a correlation between increases in intracranial pressure (intermittent or persistent) and the formation of CSF fistulae in the frontonasal areas, predisposed by the abnormal fusion of the different bony components.14 In the case we present here, anatomical malformations of the inner ear and primary and acquired immunodeficiencies were ruled out. The presence of a microorganism characteristic of the nasopharyngeal area in the CSF prompted a specific radiological examination that led to confirmation of an anatomical defect at the frontonasal level. The patient had abnormal CSF dynamics, so the treatment involved placing a ventriculoperitoneal shunt to reduce intracranial pressure, achieving closure of the fistula, with no further recurrence of meningitis.
Dermal sinuses, involved in the third case, are congenital defects involving incomplete neural tube closure that results in a variety of communications between the skin and the central nervous system. They can be located anywhere between the nasal root and the conus medullaris, and are found most frequently in the lumbosacral region, followed by the occipital region.15 In the latter case the communication is usually intradural, allowing the direct entry of bacteria from the skin flora and the subsequent development of meningitis, most frequently due to S. aureus.16 The presence of hair may pose difficulties to the early detection of occipital dermal lesions. Where such a lesion is found, performance of an MRI is recommended for diagnosis and to determine the extension of the dermal sinus and its potential association with cystic tumours and venous anomalies,17 allowing for early surgical management and for the prevention of future complications. It is frequently associated with dermoid or epidermoid cysts, and more rarely to teratomas. Patients with an occipital dermoid cyst associated with a dermal sinus may develop meningitis and/or abscesses as the earliest clinical manifestation due to the abscessation of the dermoid cyst itself or the formation of secondary abscesses.18,19
There are 25 descriptions of meningitis cases associated to dermoid and epidermoid cysts in the literature. In all these cases, there was a dermal sinus connecting with the central nervous system.20 There are also descriptions of recurrent aseptic meningitis associated to these cysts. Their high keratin content produces an inflammatory reaction that can trigger conditions ranging from mild leptomeningitis to granulomatous meningitis, ependymitis and posterior radiculitis.21 In a review of the medical records of 18 patients with meningitis associated to occipital dermal sinuses, Naderi et al. recommend the prophylactic surgical resection of all cranial dermal sinuses and early resection when the patients are symptomatic.16
In the case presented here, the recurrence of meningitis caused by S. aureus was indicative of craniospinal dysraphism. When the dermal sinus was found, the family history was re-examined, and the mother reported an occipital mass present since birth that had not been examined. The symptoms did not resolve until the dermoid cyst and the dermal sinus were fully resected, and the abscesses were drained while the patient received antibiotic therapy. Drainage and antibiotic treatment alone are not appropriate, as they do not eliminate the source of infection.22
The last case presented, of polymicrobial meningitis caused by gut bacteria, some of them anaerobic, evinces the importance of the prompt determination of the cause of meningitis to avoid severe neurologic sequelae.23
The Currarino syndrome is defined by the following clinical triad: anorectal malformation, in 30% of cases a rectal stenosis (diagnosed a posteriori); sacrococcygeal bony defect, which is pathognomonic in case of a scimitar sacrum; and presacral mass, which is an anterior meningocele in 60% of cases.24 While constipation is the most frequent symptom, it may be associated with recurrent meningitis, which carries a high mortality rate (56%) due to rectomeningeal fistula.25 Fifty percent of cases are due to an autosomal dominant mutation in the HLXB9 gene (7q36). This gene is involved in caudal development and its defects cause the anomalous separation of the neuroectoderm and neuroendoderm before the development of the notochord. Genetic testing for the mutation in family members is recommended.26 In 80% of the cases, the diagnosis is made in the first decade of life by imaging tests. The management of asymptomatic children is conservative, with laboratory monitoring of alpha-foetoprotein due to the possibility of malignisation of the presacral mass. In symptomatic children, the treatment is based on surgical repair of the anatomical defect.24
To conclude, we want to underscore that the close collaboration of clinician and radiologist is essential in cases like the four presented here. The bacterium helps find the source of infection, but knowledge of the various anatomical defects that facilitate the development of meningitis is needed to perform the most appropriate imaging test in each situation, and knowledge of the normal flora of different regions is needed for the prescription of empirical antibiotic treatment. In a patient with recurrent meningitis and a normal evaluation of the immune system, a previous imaging test with normal results should not preclude a more thorough investigation if there is a strong clinical suspicion of an underlying anatomical defect.
Based on our experience with these cases and the cases described in the literature, we conclude that an anatomical defect causing recurrent episodes of meningitis should be treated surgically. At present, there are no studies that demonstrate the efficacy of antibiotic prophylaxis in patients with congenital anatomical defects that cannot be resolved with surgery.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Morgenstern Isaak A, Bach Faig A, Martínez S, Martín-Nalda A, Vázquez Méndez E, Pumarola Segura F, et al. Meningitis recurrente por defectos anatómicos: la bacteria indica su origen. An Pediatr (Barc). 2015;82:388–396.