Lymphomas are the third malignancy in children, and within them non-Hodgkin lymphoma (NHL) accounts for just 7% of cancers in children under 15 years. Chemotherapy is currently the treatment of choice. The objective of this study is to analyse the toxicity caused by the treatment in paediatric patients diagnosed with NHL.
Material and methodsA retrospective study was conducted on patients diagnosed with mature B-cell NHL, treated according to the LMB protocol 2001, from January 2007 to February 2014. Data concerning the diagnosis, treatment and toxicities that developed in the patients during the same period were collected.
ResultsA total of 20 mature B-cell NHL cases were diagnosed: 16 Burkitt lymphomas, 2 diffuse large cell lymphomas and 2 mature leukaemias. Almost two-thirds (65%) of patients were classified in a high grade stage (III–IV) at diagnosis. Serious infectious processes, severe myelosuppression, liver abnormalities, and mucositis were the most frequent toxicities. Overall survival was 95% (19/20). One patient died of causes unrelated to the illness.
ConclusionDespite the excellent survival rate, most patients diagnosed with NHL mature B cells experience grade III and IV toxicities during treatment.
Los linfomas son la tercera neoplasia maligna en los niños y, dentro de ellos, los linfomas no Hodgkin (LNH) representan tan solo el 7% del cáncer en menores de 15 años. La quimioterapia constituye actualmente el tratamiento de elección. El objetivo de este estudio es analizar la toxicidad secundaria al tratamiento en pacientes pediátricos diagnosticados de LNH.
Material y métodosEstudio retrospectivo de pacientes diagnosticados de LNH de células B maduras tratados según el protocolo LMB 2001, desde enero del 2007 hasta febrero del 2014. Se recogieron los datos referentes al diagnóstico, el tratamiento y las toxicidades que desarrollaron durante el mismo.
ResultadosSe diagnosticaron 20 LNH de células B maduras: 16 linfomas Burkitt, 2 linfomas difusos de células grandes y 2 leucemias maduras. Un 65% de los pacientes se clasificó al diagnóstico en un estadio de alto grado (III–IV). Los procesos infecciosos graves, la mielosupresión severa, las alteraciones hepáticas y la mucositis fueron las toxicidades más frecuentes. La supervivencia global fue del 95% (19/20). Un paciente falleció por causas no relacionadas con su enfermedad.
ConclusiónLa mayoría de los pacientes diagnosticados de LNH de células B maduras experimentan toxicidades grado III y IV durante el tratamiento, a pesar de lo cual la supervivencia es excelente.
Lymphomas are the third most common type of malignant neoplasm in children, following acute leukaemia and tumours of the central nervous system.1 Non-Hodgkin lymphomas (NHL) comprise a heterogeneous group of diseases that account for 7% of malignant disease in children less than 15 years of age in developed countries.2 At present, the most widely accepted classification of NHL is that of the World Health Organization.3 The most common subtypes in children arise from mature B-cells.4 Among them are Burkitt lymphoma, diffuse large cell lymphoma and mature cell leukaemia.5 Burkitt lymphoma is the third most common lymphoid tumour following acute lymphoblastic leukaemia and Hodgkin lymphoma in children younger than 15 years.6 They are usually characterised by a high degree of malignancy and aggressive behaviour.7 Chemotherapy, the cornerstone of treatment, is associated with considerable toxicity, especially bone marrow suppression, which calls for transfusion support and infection prophylaxis. Nausea and vomiting, as well as mucositis, are also common.8,9 In spite of these adverse effects, the prognosis is highly favourable with the currently available treatments. Survival rates have improved spectacularly in recent years thanks to new chemotherapy regimens, with 5-year survival exceeding 85%.2 The French Lymphoma Malignancy B (LMB 96) protocol has demonstrated an increase in overall and event-free survival in patients diagnosed with Burkitt lymphoma and mature leukaemia compared to other protocols.10
The aim of this study was to analyse the toxicity secondary to chemotherapy in patients with Burkitt lymphoma, diffuse large cell lymphoma and mature leukaemia treated with the LMB-2001 protocol (NHL 04).
Materials and methodsWe reviewed the medical records of patients diagnosed with NHL that were treated with the NHL 04 protocol between January 2007 and February 2014. We collected data pertaining to the diagnosis and the toxicities experienced in the different cycles of treatment. Data on toxicity were gathered conforming to the datasheet included in the protocol, and events were classified according to the common toxicity criteria of the National Cancer Institute, version 2.0. We have expressed quantitative data as median and range, and qualitative data as frequency over the total number of patients (n/N). We performed the statistical analysis using the SPSS statistics® software version 19.
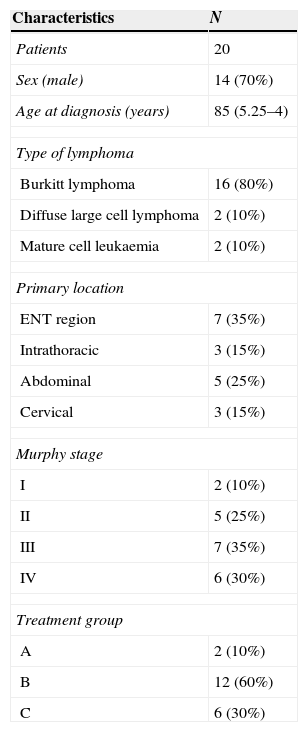
ResultsA total of 20 patients were diagnosed in our unit between January 2007 and February 2014. Table 1 summarises the characteristics of the population. Their age ranged from 3 to 16 years. Males predominated (70%). We did not identify any past medical history of interest (immunodeficiency, solid organ transplant, previous neoplasm, HIV) associated with the risk of developing a NHL. At the time of diagnosis, six patients had lymph node involvement, nine had secondary extranodal involvement, mostly at the kidney or liver, six had pleural effusion, and eight required admission to the paediatric intensive care unit (PICU) with a mean length of stay of 3.25 days. Sixty-five percent of cases were classified as high stage (III–IV).
Patient characteristics and classification by stage and treatment group.
| Characteristics | N |
|---|---|
| Patients | 20 |
| Sex (male) | 14 (70%) |
| Age at diagnosis (years) | 85 (5.25–4) |
| Type of lymphoma | |
| Burkitt lymphoma | 16 (80%) |
| Diffuse large cell lymphoma | 2 (10%) |
| Mature cell leukaemia | 2 (10%) |
| Primary location | |
| ENT region | 7 (35%) |
| Intrathoracic | 3 (15%) |
| Abdominal | 5 (25%) |
| Cervical | 3 (15%) |
| Murphy stage | |
| I | 2 (10%) |
| II | 5 (25%) |
| III | 7 (35%) |
| IV | 6 (30%) |
| Treatment group | |
| A | 2 (10%) |
| B | 12 (60%) |
| C | 6 (30%) |
Qualitative data are expressed as n (%) and quantitative data as median (range).
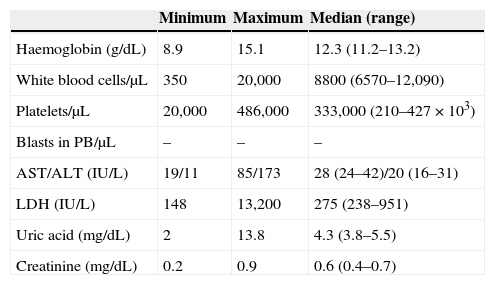
The diagnostic laboratory tests (Table 2) revealed anaemia in 4 out of 20 patients, with haemoglobin levels below 11g/dL, associated in a single case with severe thrombocytopaenia that required transfusion support. The white blood cell count revealed one patient with severe neutropaenia and six with leukocytosis. The most salient biochemistry findings corresponded to one patient with mild hypertransaminasaemia; meanwhile, up to seven patients had onset with elevated lactate dehydrogenase (LDH), of which four also had hyperuricaemia. None of the patients had abnormal creatinine levels.
Laboratory values at diagnosis.
| Minimum | Maximum | Median (range) | |
|---|---|---|---|
| Haemoglobin (g/dL) | 8.9 | 15.1 | 12.3 (11.2–13.2) |
| White blood cells/μL | 350 | 20,000 | 8800 (6570–12,090) |
| Platelets/μL | 20,000 | 486,000 | 333,000 (210–427×103) |
| Blasts in PB/μL | – | – | – |
| AST/ALT (IU/L) | 19/11 | 85/173 | 28 (24–42)/20 (16–31) |
| LDH (IU/L) | 148 | 13,200 | 275 (238–951) |
| Uric acid (mg/dL) | 2 | 13.8 | 4.3 (3.8–5.5) |
| Creatinine (mg/dL) | 0.2 | 0.9 | 0.6 (0.4–0.7) |
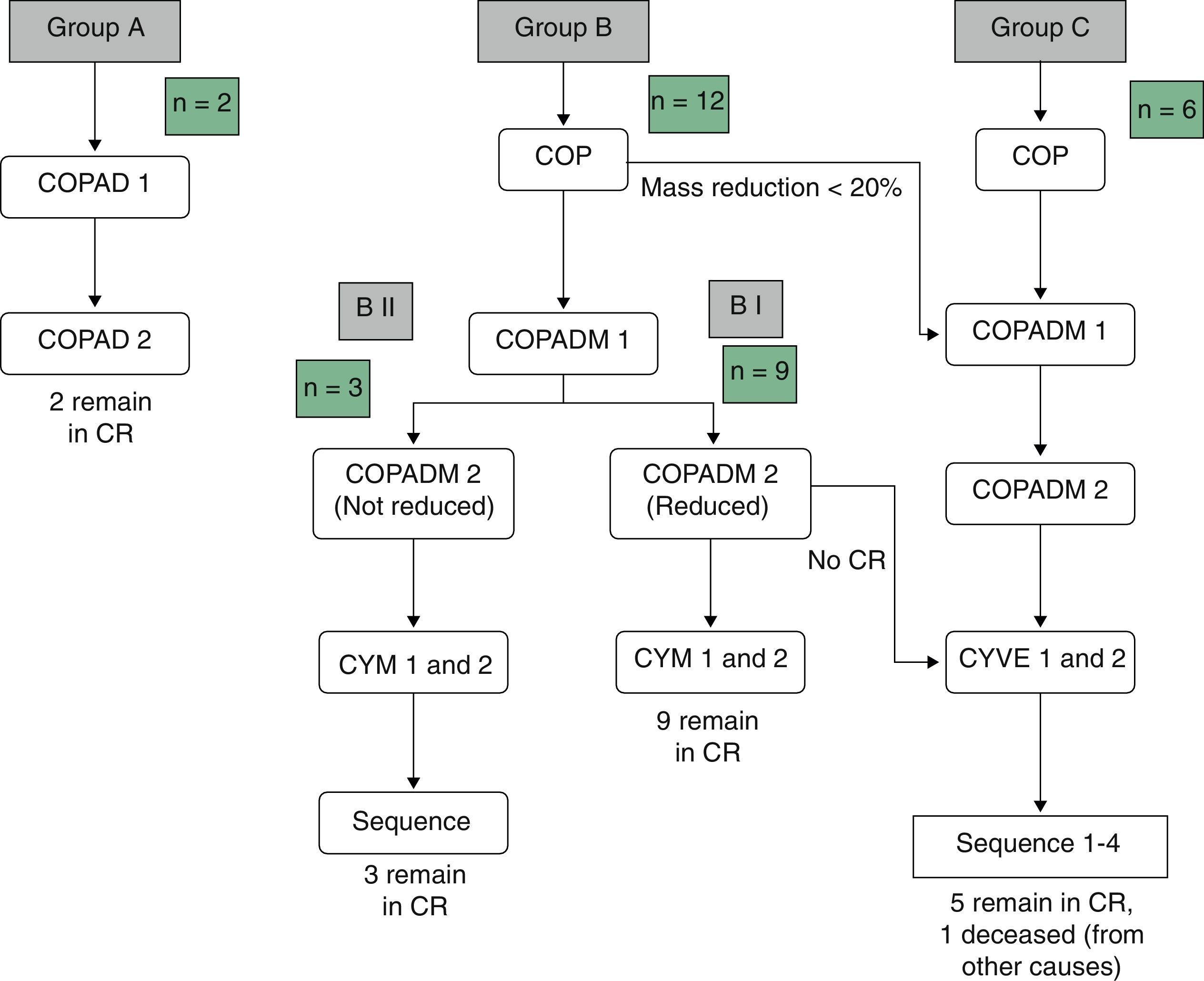
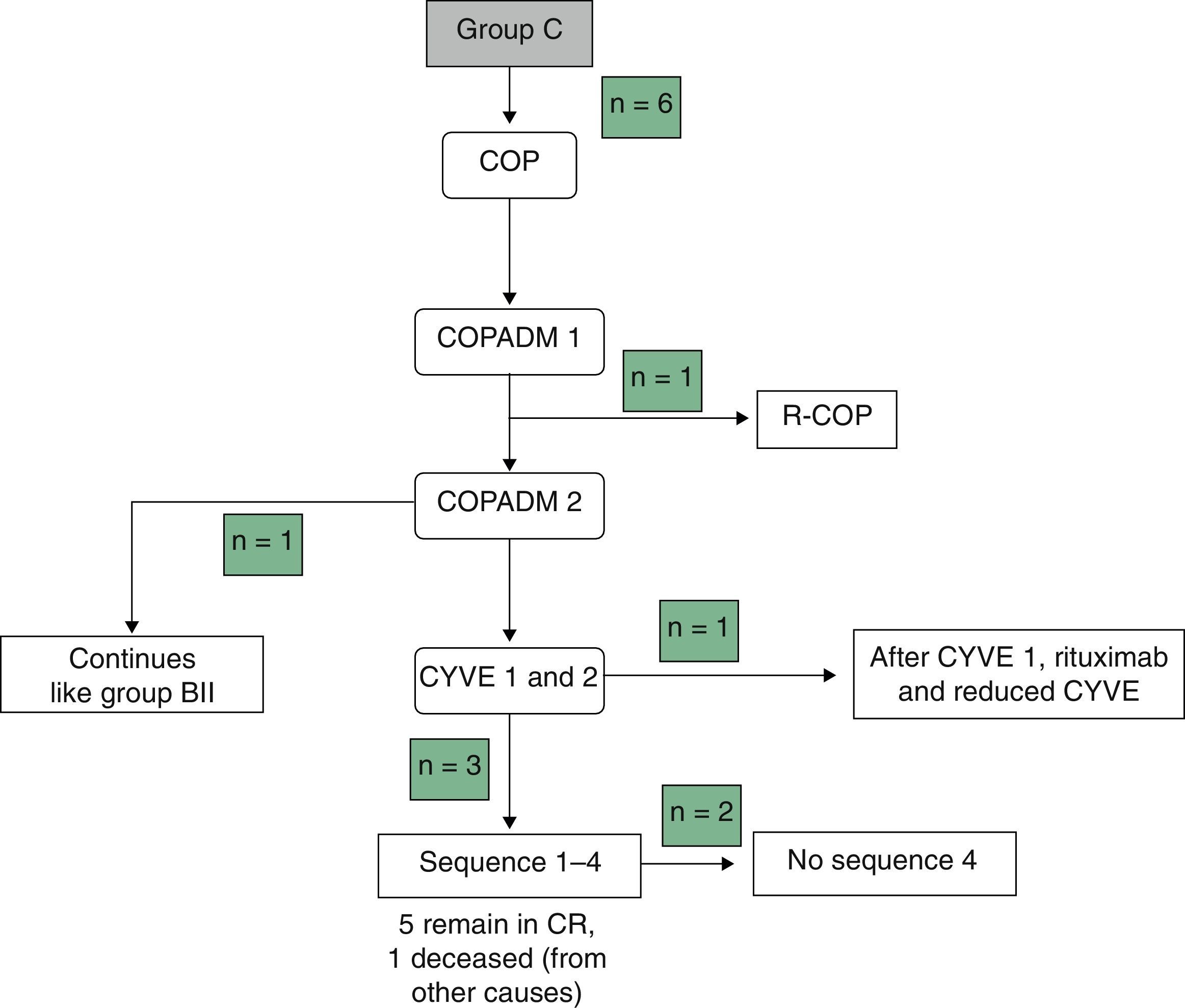
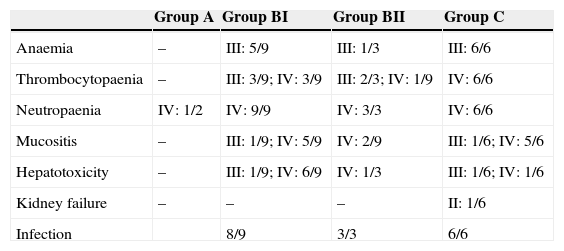
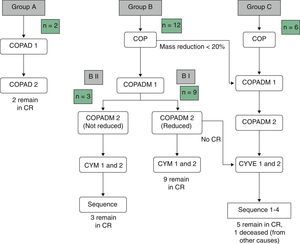
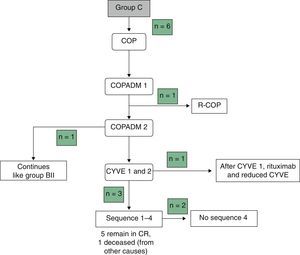
Table 3 summarises the occurrence of grade III–IV toxicities and adverse events during treatment. Two patients in the BI group developed severe complications, an acute pulmonary oedema and coagulation abnormalities, respectively. One patient in group BII developed acute liver failure. Thus, it was decided to reduce the dose in the second cycle of COPADM and then continue treatment with the BII group protocol. Five of the six patients in group C experienced significant toxicities, the most severe of which included: a massive enterocolitis requiring haemodiafiltration, so that the intensity of chemotherapy was reduced in the following cycle along with the addition of rituximab; one invasive candidiasis that, along with other complications that included grade III neurologic toxicity, justified the administration of rituximab after the first cycle of cytarabine by continuous infusion over 12h, high-dose cytarabine and etoposide (CYVE), and discontinuation of treatment after the second cycle; one infection by zygomycetes concurrent with COPADM 1 that required continuing treatment with the regimen proposed for group BII; convulsive seizures that required treatment with levetiracetam; one episode of acute respiratory failure secondary to massive atelectasis in the context of pneumonia; paralytic ileus, syndrome of inappropriate antidiuretic hormone secretion and cardiotoxicity during sequence 3, due to which sequence 4 was not administered. In short, all patients in groups A and BI could complete the treatment according to protocol and one patient in BII received a reduced-dose COPADM but then continued treatment according to plan (Fig. 1). However, of the six patients allocated to group C, only one completed the full protocol (Fig. 2).
Frequency of adverse effects during treatment.
| Group A | Group BI | Group BII | Group C | |
|---|---|---|---|---|
| Anaemia | – | III: 5/9 | III: 1/3 | III: 6/6 |
| Thrombocytopaenia | – | III: 3/9; IV: 3/9 | III: 2/3; IV: 1/9 | IV: 6/6 |
| Neutropaenia | IV: 1/2 | IV: 9/9 | IV: 3/3 | IV: 6/6 |
| Mucositis | – | III: 1/9; IV: 5/9 | IV: 2/9 | III: 1/6; IV: 5/6 |
| Hepatotoxicity | – | III: 1/9; IV: 6/9 | IV: 1/3 | III: 1/6; IV: 1/6 |
| Kidney failure | – | – | – | II: 1/6 |
| Infection | 8/9 | 3/3 | 6/6 |
The table shows toxicity grades 3–4 and adverse events during treatment. The toxicity and adverse events associated with chemotherapy were evaluated and classified according to the Common Toxicity Criteria of the National Cancer Institute, version 2.0. The data are expressed as n/N.
In groups B and C, 89% (16/18) of the patients required red blood cell transfusion support and 60% (11/18) platelet transfusion support. Eighty percent (16/20) of all patients were prescribed transfusion support with granulocyte colony stimulating factors. During treatment, four patients had to be admitted to the PICU (3 from group C and 1 from group BII). One hundred percent of patients in groups B and C developed clinically significant infectious adverse events.
The survival rate of the whole group was 95%. Of the 20 diagnosed patients, only one died, and it was from causes unrelated to the disease. The duration of follow-up from the time of diagnosis was of 2.9 years.
DiscussionLymphomas are the third most common type of malignant neoplasm in children and account for approximately 15% of childhood cancers.4 Between 7% and 10% of lymphomas are NHLs, which are most common in children under age 10. In the past two decades, the incidence of NHL has increased in adolescents and young adults, while it has remained stable in individuals younger than 15 years.11 Mature B-cell NHLs account for more than 50% of NHL cases in children and adolescents.12
The incidence of NHL is higher in males. Congenital and acquired immunodeficiencies (HIV, post-transplant immunosuppression) are the main known risk factors. Also, Epstein–Barr virus has been associated with most cases of NHL in immunosuppressed patients.13–15
From a clinical standpoint, NHLs are aggressive tumours that may present as oncological emergencies (tumour lysis syndrome, superior or inferior vena cava syndrome, airway obstruction, bowel obstruction, cardiac tamponade secondary to pleural effusion) or have milder presentations, depending on their location in the body.1
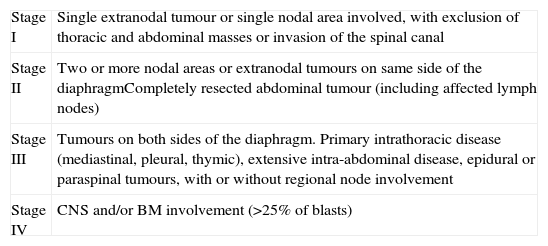
The main prognostic factor in NHL is its stage. The Murphy staging system of the St. Jude Children's Research Hospital is the one used most widely (Table 4).16
Murphy staging.
| Stage I | Single extranodal tumour or single nodal area involved, with exclusion of thoracic and abdominal masses or invasion of the spinal canal |
| Stage II | Two or more nodal areas or extranodal tumours on same side of the diaphragmCompletely resected abdominal tumour (including affected lymph nodes) |
| Stage III | Tumours on both sides of the diaphragm. Primary intrathoracic disease (mediastinal, pleural, thymic), extensive intra-abdominal disease, epidural or paraspinal tumours, with or without regional node involvement |
| Stage IV | CNS and/or BM involvement (>25% of blasts) |
Chemotherapy is the cornerstone of treatment. Paediatric studies have shown that radiotherapy does not improve outcome in early stages or when used for central nervous system prophylaxis.17,18 Furthermore, outcomes similar to those obtained by radiotherapy have been observed in children with CNS involvement treated with systemic and intrathecal chemotherapy.19
Different chemotherapy regimens have shown similar results, with overall survival rates in excess of 80%. Nevertheless, the toxicity associated with treatment carries significant morbidity, and therefore less toxic therapeutic modalities need to be developed. Rituximab, an anti-CD20 monoclonal antibody used in adults, is showing promising results in paediatric patients.12 Spain is participating in a European intergroup study that aims to assess the efficacy and safety of rituximab in high-risk children and adolescents with NHL or B-cell lymphoblastic leukaemia (Inter-B-NHL ritux 2010).
In Spain, mature B-cell NHLs (Burkitt, diffuse large cell lymphoma and mature leukaemia) are currently treated with the B-NHL-04 protocol (LMB 2001). This protocol stratifies patients into three treatment groups (A, B or C) depending on stage and tumour resection status.
The current protocol is a modification of the French LMB 89 protocol (FAB/LMB 96) that stratifies patients in group B depending on their response to chemotherapy, reducing the intensity in patients that respond well, and maintaining or increasing intensity of treatment in patients that respond poorly or that cannot maintain appropriate time intervals between cycles.
The French-American-British Lymphome Malins de Burkitt study (FAB/LMB 96) was the first randomised prospective study of international scope19 to analyse the reduction of intensity and duration of treatment in children and adolescents with mature B cell lymphomas in order to minimise toxicity, both acute and delayed, and optimise survival.20
Grade III and IV toxicities were observed in the three treatment groups, especially stomatitis and infections. Still, 4-year overall survival was excellent in group A (98.3%),20 somewhat lower in group B (95.6%)21 and reached 82% in group C.19
Another study made a retrospective analysis of survival in paediatric patients diagnosed with Burkitt lymphoma and mature B-cell leukaemia and the toxicity associated with the LMB 96 protocol compared to other treatment protocols. The overall survival in the LMB group was higher, 86% versus 68.2%. The toxicity associated to treatment in the LMB group included febrile neutropaenia in 87% of patients, severe infection in 27%, and stomatitis caused by toxicity greater than grade II in 39%10.
The incidence, biological behaviour, and response to treatment are different in children than in adults. Adolescents constitute a separate group with yet unknown characteristics that are treated according to paediatric or adult protocols depending on the country of origin and the different groups under study. Burkhardt et al. observed that the 5-year event-free survival was lower in adolescents compared to patients younger than 15 years when both groups were treated according to the Non-Hodgkin Lymphoma-Berlin-Frankfurt-Münster (NHL-BFM) protocol for childhood NHL (79%±2% vs 85%±1%) (P=.014).22
Age between 15 and 21 years has been considered a factor predictive of poorer prognosis in mature B-cell NHL. However, the FAB LMB 96 study found no association between age and increased treatment failure, while the stage of NHL at diagnosis, mediastinal location, and bone marrow or central nervous system involvement were identified as independent risk factors.23
Consistent with other published studies, we observed a clear increase in toxicity in advanced stages in our series, with predominance of severe episodes of infection, severe bone marrow suppression, liver disorders and mucositis. Among the limitations of our study is its small sample size, in spite of which we could already observe difficulties in having the patients complete the chemotherapy regimen proposed by the protocol, as the intensity had to be reduced in up to 30% of patients, switching to the regimen corresponding to a lower stage or even discontinuing treatment. Nevertheless, the prognosis is excellent, and our series showed a very high survival rate (95%).
As was the case of the previous protocol, future studies should assess the importance of toxicity in patients treated with this new protocol and the need to intensify or reduce the chemotherapy regimen depending on the stage of disease. Furthermore, studies with long-term follow-up are required to observe future complications. Ideally, these studies would be multicentre and of international scope in order to include a greater number of paediatric patients.
In conclusion, the prognosis of paediatric patients with mature B-cell NHL is excellent despite the high toxicity observed, especially in patients with advanced stages at diagnosis due to the high intensity of chemotherapy. In the near future, we need to study ways to reduce the intensity of treatment and thus its toxicity without decreasing the survival of these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Baena-Gómez M, Mora Matilla M, Lassaletta Atienza A, Andión Catalán M, Hernández Marqués C, Madero López L. Linfoma no Hodgkin: excelentes resultados a expensas de elevada toxicidad del tratamiento. An Pediatr (Barc). 2015;82:381–387.
Previous presentation: this study was presented at the VII Congreso de la SEHOP; May 22–24, 2014; Las Palmas de Gran Canaria, Spain.