The recommendations included in this document will be part a series of updated reviews of the literature on respiratory support in the newborn infant. These recommendations are structured into 12 modules, and in this work module 8 is presented. Each module is the result of a consensus process amongst all members of the Surfactant and Respiratory Group of the Spanish Society of Neonatology. They represent a summary of the published papers on each specific topic, as well as the clinical experience of each one of the members of the group.
Las recomendaciones incluidas en este documento forman parte de una revisión actualizada de la asistencia respiratoria en el recién nacido. Están estructuradas en 12 módulos, y en este trabajo se presenta el módulo 8. El contenido de cada módulo es el resultado del consenso de los miembros del Grupo Respiratorio y Surfactante de la Sociedad Española de Neonatología. Representan una síntesis de los trabajos publicados y de la experiencia clínica de cada uno de los miembros del grupo.
Conventional mechanical ventilation attempts to imitate spontaneous breathing by administering tidal volumes similar to physiological volumes at normal respiration rates. When tidal volumes need to be increased in order to maintain an adequate gas exchange, this produces increases in pulmonary pressures that may foster the development of bronchopulmonary dysplasia or air leaks.
High-frequency ventilation (HFV) attempts to minimise such lung injury. It uses very small tidal volumes (smaller than the anatomical dead space) at supraphysiological frequencies (of more than 150breaths/min), thus maintaining adequate ventilation.
High-frequency ventilation was first described in 1969,1 with positive results in the animal model.
There are 3 main types of HFV based on the devices used to deliver it2:
High-frequency oscillation ventilation (HFOV). Consist in a closed circuit that maintains a continuous positive pressure with an integrated piston pump or oscillating membrane. The movements of the piston displace the air volume within the circuit towards the lung during inspiration, creating a positive pressure, and pull air away during expiration by generating a negative pressure. Thus, expiration in this type of ventilation is active. It is the type of ventilation used most frequently in our hospitals.3
High-frequency jet ventilation (HFJV). It delivers pulses of humidified gas at the level of the endotracheal tube through a jet injector. Expiration is passive.
High-frequency flow interruption ventilation (HFFIV). It is a mixed type of HFV that uses a solenoid valve that functions as a shutter, opening and closing at a high frequency.
Different types of ventilators for HFV are available in Spain. Table 1 describes some of them.
Types of high-frequency ventilators.
| Type | HFOV | HFJV | HFFIV |
|---|---|---|---|
| Ventilator | Sensormedics® Fabian® SLE 5000® Babylog® | Life Pulse® (Bunnell) | Infant Star® VN500® |
HFFIV, high-frequency flow interruption ventilation; HFJV, high-frequency jet ventilation; HFOV, high-frequency oscillation ventilation; HFV, high-frequency ventilation.
Continuous distending pressure of the lung (cmH2O) (CDP). It is the pressure maintained in the ventilator circuit and applied to the alveoli. It is used for alveolar recruitment and therefore for oxygenation.
Amplitude (ΔP). Difference in pressure above and below the CDP expressed in cmH2O (expressed as a percentage in some ventilators). It is responsible for alveolar ventilation.
Respiratory rate in hertz (Hz) (RR). Frequency of oscillations at the given amplitude; 1Hz=60cycles/min.
Ventilation and oxygenationVentilation. The elimination of CO2 is determined by the square of the tidal volume multiplied by the RR (a concept known as DCO2). Tidal volume is the greatest determinant of CO2 clearance.
Tidal volume is influenced by amplitude. Small changes in amplitude or lung compliance (and thus in tidal volume) have significant effects on ventilation. The RR is inversely correlated to tidal volume. Tidal volume increases as RR decreases.
The precise mechanism by which gas exchange takes place has yet to be elucidated. Different hypotheses have been proposed: direct alveolar ventilation, the pendelluft effect, and facilitated diffusion (convection).4
Oxygenation. The greatest determinant of oxygenation is the maintenance of functional residual capacity (FRC) through the CDP.
IndicationsPreterm newborn with respiratory distress syndromeThe various controlled trials that have compared HFV with conventional ventilation have not had the encouraging results obtained in animal experiments. They have failed to demonstrate significant improvements in the variables under study. These discrepancies in the results are most likely due to the different therapeutic strategies used, variability in clinical practices between centres, variability in the included patients, and advances in conventional mechanical ventilation.5,6
The outcomes observed in the more than 4000 infants studied in the various clinical trials comparing HFV, with a high volume strategy, and conventional mechanical ventilation, with respiratory rates of more than 60cycles per minute and minimal tidal volumes, were similar.
With the high lung volume strategy, there was a higher incidence of air leak syndrome and there was not an increased incidence of grade III or IV intraventricular haemorrhage or periventricular leukomalacia, so HFV with high lung volume does not increase the risk of neurologic morbidity.
There is no clear evidence that HFV offers any advantages compared to conventional ventilation when used as the initial ventilation strategy in preterm infants with respiratory distress syndrome.7,8 However, 1 out of 5 very low birth weight newborns may receive HFV at some point during their stay in the intensive care unit.
The long-term followup of adolescents aged 11–14 years born before 29 weeks of gestation that had been included in a randomised trial comparing the use of HFOV versus conventional ventilation immediately after birth found that those who had received HFOV had superior lung function with no evidence of poorer functional outcomes.9
When it comes to rescue therapy, few clinical trials have studied the use of HFV as rescue therapy in preterm patients with severe respiratory distress syndrome and interstitial emphysema. The results of most favour HFV when it comes to the resolution of the respiratory problem, but have not shown differences in mortality or the incidence of bronchopulmonary dysplasia.6,9
Air leak syndrome: pulmonary interstitial emphysema, pneumothorax and bronchopleural fistulaThe studies that compared HFV and conventional ventilation when the use of surfactant was not yet widespread found that in preterm newborns with air leak syndrome, the use of HFV improved gas exchange with lower peak and mean pressures and was associated with a quicker resolution of pulmonary interstitial emphysema and decreased mortality, which suggests that this ventilation modality is an efficient tool in the management of air leak syndromes.10
Diaphragmatic herniaEvidence from retrospective and observational studies suggests that the use of HFOV could improve the incidence of bronchopulmonary dysplasia and mortality, and reduce the need for extracorporeal membrane oxygenation (ECMO) in newborns with isolated congenital diaphragmatic hernia.11,12
The first clinical trial that compared HFOV with conventional mechanical ventilation in infants with a prenatal diagnosis of congenital diaphragmatic hernia did not find a statistically significant difference in mortality or the incidence of bronchopulmonary dysplasia between the two groups. The study found a shorter duration of ventilation and a less frequent need for ECMO in the conventional ventilation group.13
Acute pulmonary disease refractory to conventional mechanical ventilation and eligible for extracorporeal membrane oxygenation (ECMO)High-frequency oscillatory ventilation is a more effective rescue therapy compared to conventional ventilation in newborns with severe and reversible pulmonary disease eligible for ECMO. It improves gas exchange in term or near-term newborns with severe respiratory failure with no apparent increase in morbidity. It has been associated with a reduced incidence of chronic lung disease and intracranial haemorrhage in infants treated successfully with HFOV compared to newborns that were refractory to it and required ECMO.
The effectiveness of HFOV in improving gas exchange depends on the underlying disease, and is greater in cases of pneumonia, meconium aspiration and surfactant deficit.14
The combined use of inhaled nitric oxide and HFOV may be more effective than therapy alone in the management of newborns with reversible severe pulmonary disease and pulmonary hypertension, reducing the use of ECMO. This improvement has been observed especially in newborns with meconium aspiration.15
Other diseasesSmall studies support the use of HFV in patients with increased intra-abdominal pressure that hinders conventional mechanical ventilation (omphalocoele, gastroschisis or necrotising enterocolitis) and pulmonary haemorrhage.16
Practical managementAs we have seen, the main indication for HFV is the need for lung recruitment. We propose the open lung strategy, that is, the use of a mean airway pressure (MAP) that maximises alveolar recruitment while avoiding atelectasis.
No objective data are available for the purpose of establishing criteria for the use of HFV. The clinical consensus is to consider the use of this modality in the following situations10:
- 1.
When peak inspiratory pressures of more than 25cmH2O are required in conventional mechanical ventilation to achieve adequate ventilation.
- 2.
When a FiO2 of more than 0.6 is needed after optimising conventional mechanical ventilation and there are signs of overdistension (pressure–volume loop, C20/C<0.8).
- 3.
Air leak.
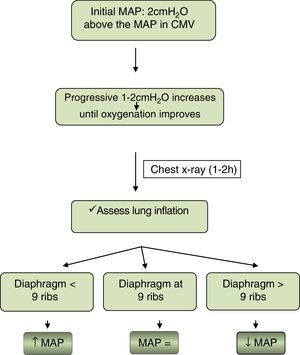
Oxygenation. Fig. 1 proposes an algorithm to guide the initiation and maintenance of HFV with the aim of maximising lung volume while avoiding hyperinflation:
In cases of air leak, HFV will be initiated with the same CDP applied in conventional mechanical ventilation with a conservative approach, tolerating higher FiO2 and pCO2 values.
Different high-frequency ventilators may not produce the same degree of hyperinflation at the same MAP. Furthermore, other signs of hyperinflation should be taken into account, such as a flattened diaphragm or compressed heart contours.
Ventilation. The initial amplitude should be of 40–50% (depends on the type of ventilator used) and adjusted in 10% increments to maintain a tidal volume between 1.5 and 2cc/kg and/or a pCO2 adequate for the individual patient. The RR will be determined based on the weight of the patient: rates of 12–15Hz can be used in patients with weights of less than 1500g, while approximately 10Hz can be applied to late preterm or term newborns. Lower rates may be needed in cases of severe lung disease.
There are ventilators that offer a volume guarantee option for HFOV. The hypothetical benefit of this option compared to maintaining the pCO2 levels within an optimal range remains to be demonstrated.17
Table 2 proposes an oxygenation and ventilation protocol for HFV.
Oxygenation and ventilation protocol for HFV.
| Poor oxygenation | Adequate oxygenation | Hypoventilation | Hyperventilation | |
|---|---|---|---|---|
| 1. | ↑ FiO2 | ↓ FiO2 (up to 0.4–0.5) | ↑ ΔP | ↓ ΔP |
| 2. | ↑ MAP (1–2cmH2O) | ↓ MAP (1–2cmH2O) | ↓ RR | ↑ RR |
HFV, high-frequency ventilation; FiO2, administered fraction of inspired oxygen; MAP, mean airway pressure; RR, respiratory rate; ΔP, amplitude.
Weaning. Once lung recruitment has been achieved and oxygen requirements have dropped to approximately 0.3–0.35, progressive decreases in MAP will be attempted every 12h, maintaining the FiO2 within the desired threshold. The speed of weaning will depend on the disease and developmental stage of the patient.
Once the MAP reaches values of 10–12cmH2O, extubation will be contemplated. Extubation can be performed following a switch to conventional mechanical ventilation or directly from HFV (with MAP values of 8–10cmH2O).
Special care. The following recommendations constitute a general guideline for the optimal management of patients receiving HFV:
- •
The tidal volume delivered to the patient varies with changes in pulmonary conditions, so close monitoring of pO2 and pCO2 by means of transcutaneous sensors is recommended.
- •
Lung expansion should be assessed by chest radiography whenever substantial changes in MAP are made.
- •
Arterial blood pressure and cardiac output should be optimised, including their exhaustive monitoring and contemplating the administration of fluids and/or inotropic support for their management.
- •
Sedoanalgesia with or without muscle relaxants may be needed in some occasions when the respiratory efforts of the patient interfere with ventilation.
- •
Endotracheal suctioning is indicated in case of decreased tactile fremitus, CO2 levels increase or oxygenation decreases with no other apparent cause. The use of closed suction systems is recommended to prevent lung derecruitment during disconnection from the ventilator.
Haemodynamic complications. When a high MAP is needed to achieve lung recruitment, certain complications may result from the increased intrathoracic pressure, such as increased central venous pressure or decreased venous return or cardiac output.
Air trapping.
Recommendations- 1.
HFV is used as rescue therapy in patients with severe lung disease in whom treatment with conventional mechanical ventilation has failed (B).
- 2.
HFV is more effective in combination with inhaled nitric oxide (B).
- 3.
HFV offers no advantages compared to conventional ventilation in the initial respiratory management of respiratory distress syndrome in preterm newborns (A).
- 4.
The MAP optimisation strategy may be most appropriate (B).
Ex-utero intrapartum treatment (EXIT) is a procedure that allows the establishment of a patent foetal airway before the delivery is complete, while the newborn is still supported by the uteroplacental circulation. It offers a safe time interval to access the foetal airway in cases of severe extrinsic compression.18,19
ProcedurePerformance of EXIT requires a multidisciplinary team comprising obstetricians, neonatologists, anaesthesiologists, paediatric surgeons and nurses. The procedure is conducted in the operating theatre. The mother is placed supine on the operating table, with slight left lateral decubitus positioning. Tocolytics are administered to the mother before the intervention. An epidural catheter is placed on the mother for intraoperative and postoperative pain relief. Induction of anaesthesia is followed by rapid-sequence intubation and assisted ventilation. Maternal arterial blood pressure must be maintained at adequate levels to ensure placental perfusion. Maternal oxygenation is optimised to avoid foetal hypoxia. A muscle relaxant and fentanyl are delivered directly to the foetus with a transuterine intramuscular injection. Hysterotomy is subsequently performed. Once the uterus is open, the head and arms of the foetus are exposed, and intubation of the airway can be attempted.
Foetal monitoring includes continuous pulse oximetry with placement of a sensor in the exposed arm, and ultrasound examination of umbilical cord blood flow and heart rate.
Once the procedure has finished (it can take up to 150min), the umbilical cord is clamped and the newborn is placed in the resuscitation crib for neonatal care and treatment.19,20
IndicationsLarge cervical or pharyngeal masses such as teratomas, cystic hygromas, haemangiomas or lymphangiomas. Although these lesions are rare, they can cause compression of the foetal upper airway and substantially complicate resuscitation in the delivery room. If airway patency cannot be achieved, the newborn may develop acidosis or hypoxia and, as a consequence, suffer irreversible brain damage or death.
This complication is all the more tragic if we consider that many of these patients develop normally if the isolated malformation can be overcome.21 This requires a prior accurate and detailed prenatal diagnosis, evaluating the degree of airway compression and the complexity of neonatal resuscitation.
Ultrasound can be used to detect indirect signs of secondary airway and oesophageal obstruction, such as polyhydramnios, increased lung volume, inversion of the diaphragm, ascites and foetal hydrops (CHAOS). Magnetic resonance imaging allows a more detailed evaluation, facilitating the planning of EXIT and other necessary interventions (intubation, tracheotomy, puncture of the mass in cases of lymphangioma or resection).22
Other indications for EXIT are22,23:
- 1.
Ultrasound-guided percutaneous puncture during the EXIT procedure in cases of giant cervical lymphangioma.
- 2.
Reversion of foetal tracheal obstruction in congenital diaphragmatic hernia.
- 3.
Thoracic abnormalities: massive pleural effusion and large congenital cystic adenoid malformation.
- 4.
Closure of thoracoamniotic shunts. Thoracotomy and lobectomy of the lung.
- 5.
Central access line placement prior to ECMO.
Planning performance of EXIT is indicated in cases of severe foetal airway obstruction (A).
Extracorporeal membrane oxygenation (ECMO)Extracorporeal membrane oxygenation is a technique that can sustain vital functions by the artificial replacement of the heart, lungs or both for a period of time until the recovery of native cardiac and/or respiratory function.
This technique must be used in patients with reversible conditions.24
Components of the ECMO circuitThe main components of the circuit are a venous drainage cannula, venous access line, pump, oxygenator, arterial access line and arterial or venous return cannula, depending on the type of support.
PhysiologyExtracorporeal membrane oxygenation is achieved by drainage of venous blood followed by the exchange of CO2 and O2 through an artificial membrane (oxygenator), with a pump pushing the blood through and returning it to the systemic circulation, either venous (venovenous ECMO) or arterial (venoarterial ECMO).
Venovenous ECMO provides respiratory support, and venoarterial ECMO respiratory as well as cardiac support. The support can be partial or total, depending on the needs of the patient.
Indications and contraindicationsMost newborns who need ECMO are patients with pulmonary hypertension that experience respiratory failure, leading to sustained hypoxaemia.25
The primary cause varies and has an impact on the final efficacy of ECMO. Congenital diaphragmatic hernia, meconium aspiration syndrome, persistent pulmonary hypertension and respiratory distress syndrome are the respiratory diseases for which ECMO is used most frequently.26
Indications25,27Oxygenation index (OI)=mean airway pressure×FiO2×100
Oxygenation index ≥40: indicated.
Oxygenation index ≥20: consider use.
ContraindicationsAbsolute- 1.
Lethal chromosomal disorders
- 2.
Severe irreversible brain damage
- 3.
Grade III or greater intraventricular haemorrhage
- 1.
Irreversible organ damage (possibility of transplantation)
- 2.
Body weight<2kg
- 3.
Postmenstrual age<34 weeks
- 4.
Disease with a high probability of poor prognosis
The efficacy of ECMO is based on the ability of the patient to recover from lung disease in a short period of time (14–21 days).
From the second week of ECMO, the risks and complications associated with the technique (clot formation, nosocomial infection, mechanical problems in the circuit, etc.) increase. Most facilities accept a maximum duration of ECMO of 20–30 days, although improvements in the use of this technique have resulted in increases in this time interval.
Discontinuation of ECMOThe main indication for discontinuation is the recovery of adequate pulmonary and cardiac function. As oxygenation improves, the patient is progressively weaned off support until it is fully withdrawn.
ComplicationsTable 3 enumerates potential complications of ECMO.27
Mechanical and patient-related complications in extracorporeal membrane oxygenation (ECMO).
| Mechanical complications | Patient complications |
|---|---|
| Clots in circuit | Haemolysis |
| Cannula-related | Dialysis/Haemofiltration |
| Air in circuit | Intracranial haemorrhage |
| Oxygenator failure | Seizures |
| Pump failure | Hypertension |
| Heater failure | Infection |
| Circuit break | Arrhythmia |
Extracorporeal membrane oxygenation is indicated in severe neonatal pulmonary disease that can be reversed with a short period of use (B).
Conflict of interestsThe authors have no conflict of interests to declare.
Dr Félix Castillo Salinas. Department of Neonatology, Hospital Universitario Vall d’Hebrón, Barcelona. Dr Dolores Elorza Fernández. Department of Neonatology, Hospital Universitario La Paz, Madrid. Dr Antonio Gutiérrez Laso. Department of Neonatology, Hospital Universitario La Fe, Valencia. Dr Julio Moreno Hernando. Department of Neonatology, Hospital Universitario Sant Joan de Déu, Barcelona. Dr Gerardo Bustos Lozano. Department of Neonatology, Hospital Universitario 12 de Octubre, Madrid. Dr Manuel Gresa Muñoz. Department of Neonatology, Hospital Materno-Insular Las Palmas, Las Palmas. Dr Xavier Miracle Echegoyen. Department of Neonatology, Hospital Clínic-Maternidad, Barcelona. Dr Jon López de Heredia Goya. Department of Neonatology, Hospital de Cruces, Barakaldo. Dr Marta Aguar Carrascosa. Department of Neonatology, Hospital Universitario La Fe, Valencia. Dr José Ramón Fernández Lorenzo, Department of Neonatology, Complejo Universitario de Vigo. Dr María del Mar Serrano. Hospital Materno-Infantil Carlos Haya, Málaga. Dr Ana Concheiro Guisan. Department of Neonatology, Complejo Hospitalario Universitario de Vigo. Dr Cristina Carrasco Carrasco. Department of Neonatology, Hospital Universitario Sant Joan de Déu, Barcelona. Dr Juan José Comuñas Gómez. Department of Neonatology, Hospital Universitario Vall d’Hebrón, Barcelona. Dr María Teresa Moral Pumarega. Hospital Universitario 12 de Octubre, Madrid. Dr Ana María Sánchez Torres. Department of Neonatology, Hospital Universitario La Paz, Madrid. Dr María Luisa Franco. Department of Neonatology, Hospital Universitario Gregorio Marañón, Madrid.
Los miembros del Grupo Respiratorio y Surfactante (RESPISURF) de la Sociedad Española de Neonatología se presentan en anexo.
Please cite this article as: Castillo Salinas F, Elorza Fernández D, Gutiérrez Laso A, Moreno Hernando J, Bustos Lozano G, Gresa Muñoz M, et al. Recomendaciones para la asistencia respiratoria en el recién nacido (IV). Ventilación de alta frecuencia, ex-utero intrapartum treatment (EXIT), oxigenador de membrana extracorpórea (ECMO). An Pediatr (Barc). 2017;87:295.e1–295.e7.