Hyperbilirubinaemia is one of the most frequent causes of hospital readmission during the first week of life. Its detection is still a big challenge, mainly due to the early discharge from the hospital that can be associated with a delay of the diagnosis.
The identification of those newborns at risk of developing significant hyperbilirubinaemia is one of the main priorities in the public health care system.
An approach to the management of newborn jaundice is presented in this article, following the recommendations based on the medical evidence and on the opinion of the Standards Committee of the Spanish Society of Neonatology.
La hiperbilirrubinemia representa la causa más común de reingreso hospitalario en la primera semana de vida. Su detección continúa siendo un desafío, debido especialmente al alta precoz que puede asociarse con un retraso en el diagnóstico.
La identificación de los niños con riesgo de desarrollar hiperbilirrubinemia significativa es una de las principales prioridades de la sanidad pública.
En este documento, se presenta un enfoque para el manejo de la ictericia del recién nacido, según recomendaciones basadas en la evidencia médica y en la opinión del Comité de Estándares de la Sociedad Española de Neonatología.
Hyperbilirubinaemia is a common medical problem and is usually benign in term and late preterm (PT) newborns.1 It is the most frequent cause of hospital readmission in the first week of life.2 Early discharge of healthy newborns, especially those in who breastfeeding (BF) has not been fully established, may be associated with delays in its diagnosis.3 In certain circumstances (glucose-6-phosphate dehydrogenase [G6PD] deficiency, sepsis, etc.) severe hyperbilirubinaemia may occur and can produce brain damage despite appropriate intervention.4
Much of the management of neonatal jaundice is based on low-grade evidence.5,6 This review proposes an approach to its management through evidence-based recommendations.1,7–9
MethodsWe performed a literature review searching the PubMed database (MeSH) for the following keywords: jaundice, hyperbilirubinaemia, newborn, late preterm, guidelines.
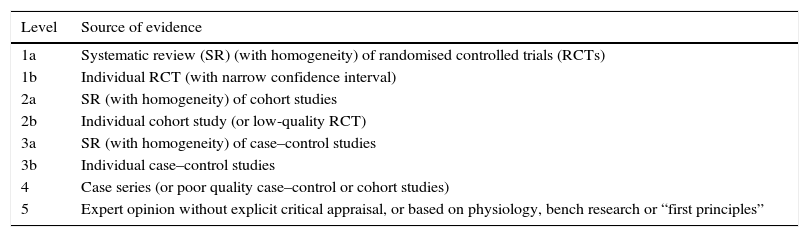
We graded the quality of the evidence with the classification established by the Center of Evidence-Based Medicine (http://www.cebm.net) (Table 1) and the strength of the recommendation based on the guidelines of the Canadian Task Force on Preventive Health Care10 (Table 2).
Levels of evidence.
| Level | Source of evidence |
|---|---|
| 1a | Systematic review (SR) (with homogeneity) of randomised controlled trials (RCTs) |
| 1b | Individual RCT (with narrow confidence interval) |
| 2a | SR (with homogeneity) of cohort studies |
| 2b | Individual cohort study (or low-quality RCT) |
| 3a | SR (with homogeneity) of case–control studies |
| 3b | Individual case–control studies |
| 4 | Case series (or poor quality case–control or cohort studies) |
| 5 | Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles” |
Grades of recommendation for specific preventive interventions.
| A. There is good evidence to recommend the clinical preventive action |
| B. There is fair evidence to recommend the clinical preventive action |
| C. The existing evidence is conflicting and does not allow to make a recommendation for or against the use of the clinical preventive action; however, other factors may influence decision-making |
| D. There is fair evidence to recommend against the clinical preventive action |
| E. There is good evidence to recommend against the clinical preventive action |
| I. There is insufficient evidence to make a recommendation, however, other factors may influence decision-making |
Breastfed newborns are at greater risk of developing hyperbilirubinaemia than newborns fed artificial formula. However, the known risks of acute bilirubin encephalopathy are very small when weighed against the benefits of BF. The primary approach to mitigating the hyperbilirubinaemia associated to BF is to ensure that BF is successful. An insufficient energy intake and/or dehydration associated with inadequate BF may contribute to the development of hyperbilirubinaemia due to an increase in the enterohepatic circulation of bilirubin.11,12
Recommendations- •
Mothers must be advised to breastfeed their newborns at least 8–12 times a day in the first days7,13 (grade I recommendation).
- •
A programme for breastfeeding support should be established in every health care institution that manages deliveries, with continuation in primary care (evidence level 5, grade I recommendation).
- •
Newborns that lose more than 10% of their birth weight should be assessed by a professional with specific training in breastfeeding (evidence level 5, grade I recommendation).1
- •
Routine supplementation with water or dextrose water in nondehydrated breastfed newborns is recommended against1,7 (grade D recommendation).
Visual estimation of bilirubin levels based on the degree of jaundice may lead to error, especially in newborns with dark skin pigmentation.7,14 Measurement of transcutaneous bilirubin (TcB) offers an alternative to the measurement of serum bilirubin that is non-invasive and superior to estimation based on clinical signs, and is a useful screening tool.7,12 Transcutaneous bilirubin is a measurement of the yellow colour of the blanched skin, and while it provides a good estimate of the total serum bilirubin (TSB) level, it is not a substitute for it.4 Given that phototherapy (PT) “bleaches” the skin, TcB is not a reliable measure in newborns undergoing PT or in the first hours after its discontinuation.7 Other limitations of TcB measuring devices is that they should not be used in the first 24h of life, and that they must be used with caution in newborns of less than 35 weeks’ gestation.1,8
Most studies have shown that TcB tends to underestimate TSB, especially at higher levels of TSB. Thus, measurement of TSB is recommended if4,12:
- •
The value of TcB is 70% or more of the currently recommended TSB threshold value for the use of PT.15
- •
The TcB value is >14.6mg/dL or within less than 3mg/dL of a value that indicates treatment.7,16
- •
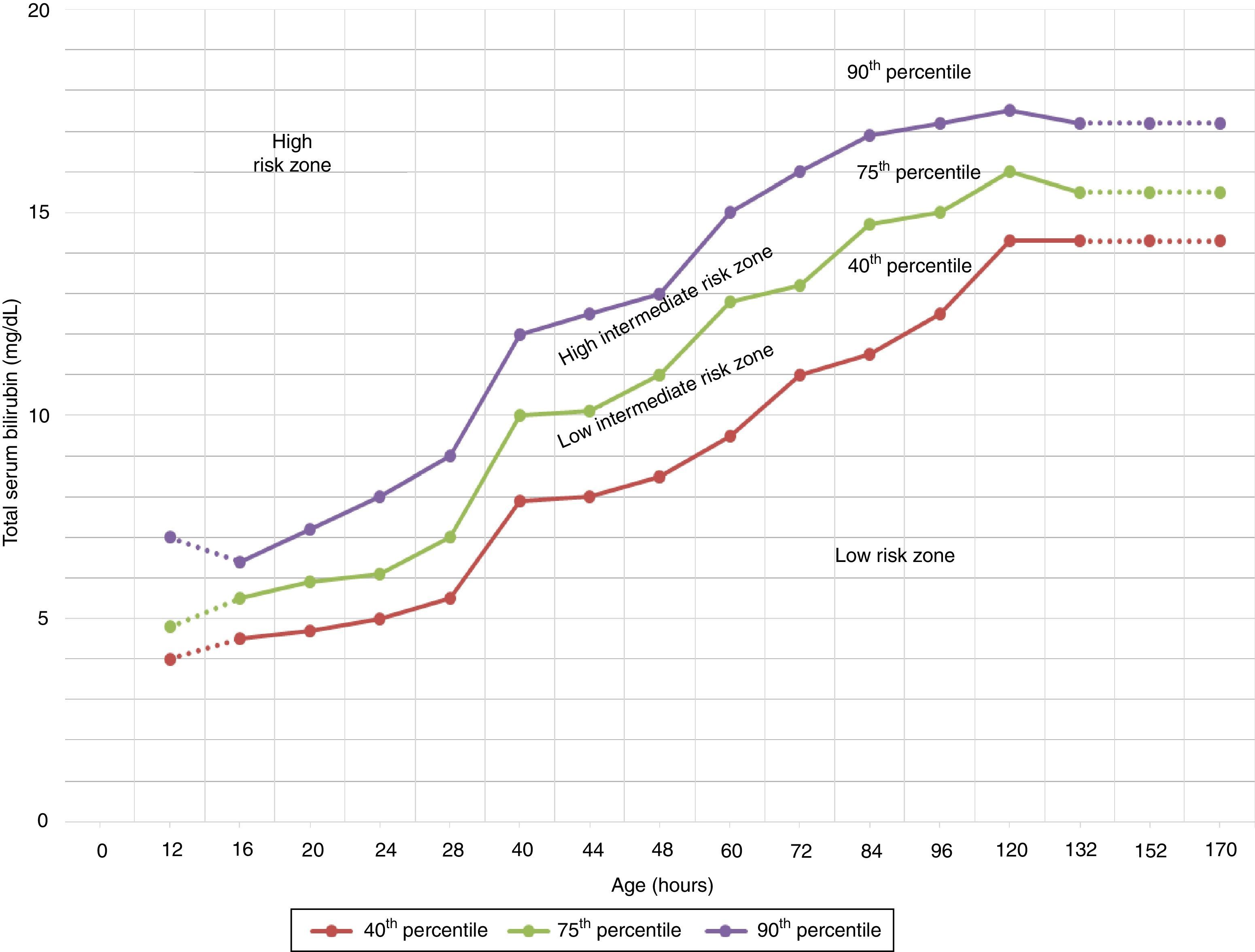
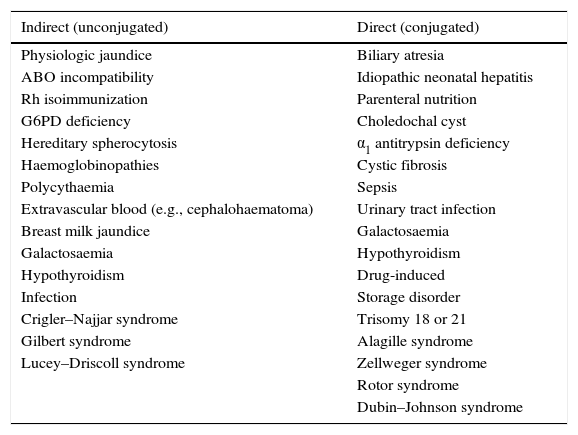
The TcB value is above the line for high intermediate risk in the Bhutani nomogram.17 (Fig. 1).
Figure 1.Nomogram by Bhutani et al. for designation of risk in 2840 well newborns at 36 or more weeks’ gestational age with birth weight of 2000g or at 35 or more weeks’ gestational age with birth weight of 2500g or more, based on the hour-specific serum bilirubin values. The serum bilirubin was obtained before discharge, and the zone in which the value fell predicted the likelihood of a subsequent bilirubin level exceeding the 95th percentile.
Source: Adapted from the American Academy of Pediatrics.7 - •
The TcB value at followup after discharge is of less than >13mg/dL.18
- •
Nurseries should have established protocols for the clinical (visual) assessment of jaundice. This assessment should be performed whenever the newborn's vital signs are measured (at least every 8–12h in the first 24h) and again, at a minimum, 24–48h later7 (grade I recommendation).
- •
The ability of health care professionals to estimate bilirubin levels based on the cephalocaudal progression of jaundice is limited (evidence level 1b).14,19 Bilirubin should be measured in every jaundiced newborn, and TcB can be used as a first step.9
- •
TSB should be measured in case of a high TcB value, whenever therapeutic intervention is considered, and for every measurement of bilirubin levels following initiation of PT (evidence level 1b, grade A recommendation).4,9
- •
TcB values of more than 14.6mg/dL are not accurate and should be verified with measurement of TSB.8,16
- •
The direct serum bilirubin should not be subtracted from the TSB concentration in making decisions about exchange transfusion (ET) or PT7 (grade I recommendation).
- •
The TSB can be estimated from a venous or capillary blood sample1 (grade I recommendation). One study found that capillary TSB levels were higher than venous TSB levels, but another study found that capillary levels were lower.20,21 Collection of a venous blood sample to confirm a capillary bilirubin value is not recommended.1,7
Early neonatal jaundice is generally due to unconjugated hyperbilirubinaemia (Table 3). The presence of clinically significant hyperbilirubinaemia (that in which treatment is indicated based on current recommendations) or prolonged hyperbilirubinaemia requires investigation of its aetiology.1
Causes of neonatal hyperbilirubinaemia.
| Indirect (unconjugated) | Direct (conjugated) |
|---|---|
| Physiologic jaundice | Biliary atresia |
| ABO incompatibility | Idiopathic neonatal hepatitis |
| Rh isoimmunization | Parenteral nutrition |
| G6PD deficiency | Choledochal cyst |
| Hereditary spherocytosis | α1 antitrypsin deficiency |
| Haemoglobinopathies | Cystic fibrosis |
| Polycythaemia | Sepsis |
| Extravascular blood (e.g., cephalohaematoma) | Urinary tract infection |
| Breast milk jaundice | Galactosaemia |
| Galactosaemia | Hypothyroidism |
| Hypothyroidism | Drug-induced |
| Infection | Storage disorder |
| Crigler–Najjar syndrome | Trisomy 18 or 21 |
| Gilbert syndrome | Alagille syndrome |
| Lucey–Driscoll syndrome | Zellweger syndrome |
| Rotor syndrome | |
| Dubin–Johnson syndrome |
- •
The cause of jaundice should be investigated in newborns requiring intensive PT for significant hyperbilirubinaemia1 (grade I recommendation).
- •
All pregnant women should be tested for ABO and Rh blood types (grade D recommendation) and undergo a serum screen for unusual isoimmune antibodies (grade B recommendation).
- •
If the mother has not had prenatal blood grouping or is Rh-negative, blood grouping and a direct Coombs test of a cord blood sample of the newborn is recommended (grade B recommendation).
- •
If the maternal blood is group O, Rh-positive, it is an option to test the cord blood, but it is not required provided that there is appropriate surveillance7 (grade I recommendation).
- •
Blood group evaluation and a direct Coombs test should be performed in newborns with early jaundice or in the high intermediate risk zone (see Bhutani nomogram, Fig. 1) of mothers with blood group O (grade B recommendation).1
- •
The incidence of severe hyperbilirubinaemia is high in children with G6PD deficiency (evidence level 1b). Measurement of the G6PD level is recommended in every jaundiced newborn who is receiving PT and whose family history or ethnic origin suggest the likelihood of this deficiency, or in any newborn with a poor response to PT1,7 (evidence level 5, grade I recommendation).
- •
A measurement of direct bilirubin should be performed in infants that are jaundiced at 3 or more weeks post birth to identify cholestasis. The results of the newborn thyroid and galactosaemia screen should be checked in these infants (grade I recommendation).
- •
Perform urinalysis and urine culture in newborns with elevated direct bilirubin,22 and an additional testing to rule out sepsis if warranted by the situation7 (grade I recommendation).
- •
If the direct-reacting or conjugated bilirubin level is elevated (>1.5mg/dL or >20% of the TSB), additional evaluation for the causes of cholestasis is recommended7,8,23 (grade I recommendation).
In every newborn that develops jaundice in the first 24h of life, the TSB level should be measured within a maximum of 2h. If the level does not reach the threshold for treatment, it is recommended that repeat bilirubin measurements are made every 6h until the level is below the treatment threshold, either stable or falling.
If jaundice is detected in a newborn after 24h post birth, the bilirubin level (TSB or TcB) should be measured as soon as possible (within a maximum of 6h). The following monitoring scheme is proposed in case PT is not indicated8:
- a.
Well newborns with gestational age (GA) ≥38 weeks and with a bilirubin level under the threshold for PT but within 3mg/dL of the limit: repeat the bilirubin measurement 18h later in newborns with risk factors for hyperbilirubinaemia and 24h later in those with no risk factors.
- b.
Well newborns with GA ≥38 weeks and with a bilirubin level more than 3mg/dL under the threshold for PT: do not routinely repeat the measurement of bilirubin.
- •
Measure the TSB in every newborn with jaundice in the first 24h of life (grade I recommendation).
- •
Order a bilirubin measurement if jaundice seems excessive for the infant's age (grade I recommendation).
- •
Interpret all bilirubin measurements according to the newborn's age in hours1,7,24 (grade I recommendation).
Combining TSB measurement (made before 48h post birth and interpreted according to hours of postnatal age) with GA improves the prediction of future development of TSB levels of 20mg/dL or higher (evidence level 2b).25
The guidelines of the American Academy of Pediatrics (AAP) and the National Institute for Health and Care Excellence (NICE) conclude that the combination of early bilirubin measurement and clinical risk factor assessment has a high predictive value for subsequent hyperbilirubinaemia, although there is no evidence on the effectiveness of this strategy in reducing the incidence of kernicterus.5
The need for the routine measurement of bilirubin in newborns that are not visibly jaundiced remains a subject of debate, although the introduction of a pre-discharge bilirubin screen could increase health care costs, and the quality of the evidence to support its recommendation is limited (there is no evidence that it reduces the incidence of kernicterus).4
The early identification of newborns at risk of developing significant hyperbilirubinaemia is key for its prevention. The NICE guidelines have found evidence for 4 risk factors8:
- a.
Gestational age under 38 weeks.
- b.
Jaundice requiring PT in a previous sibling.
- c.
Exclusive BF.
- d.
Jaundice in the first 24h of life.
In the absence of haemolytic diseases, the GA of the newborn is the most important individual clinical risk factor.26
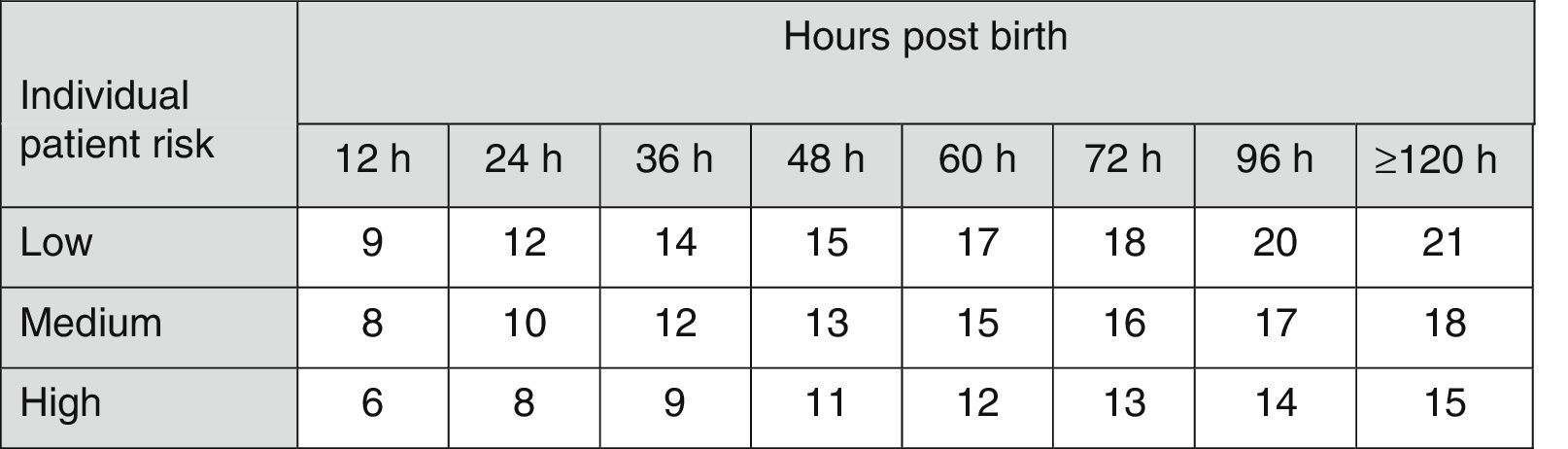
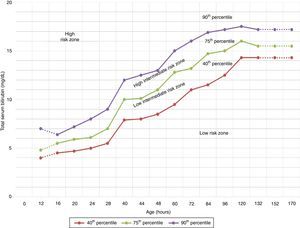
The AAP has also established risk factors for neurotoxicity secondary to hyperbilirubinaemia4 to be taken into account in therapeutic decision-making (Fig. 2).
Indications for PT in newborns of 35 or more weeks’ gestation. These guidelines are based on limited evidence and the levels shown are approximations. Intensive PT should be used when the TBS concentration exceeds the level shown in the corresponding cell (values expressed in mg/dL). Patients at low risk: GA ≥38 weeks and well. Patients at medium risk: GA ≥38 weeks+risk factors for neurotoxicity, or GA of 35–37+6 weeks and well. Patients at high risk: GA of 35–37+6 weeks with risk factors for neurotoxicity. Risk factors for neurotoxicity: isoimmune haemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis or albumin <3g/dL. GA, gestational age; G6PD, glucose-6-phosphate dehydrogenase; PT, intensive phototherapy; TSB, total serum bilirubin.
The nomogram developed by Bhutani et al. (Fig. 1) has been widely used to predict which children are or are not at risk of hyperbilirubinaemia.12 However, some studies have shown that some newborns with a pre-discharge TcB in the low risk or low intermediate risk zone can reach TSB levels requiring PT.27
Recommendations- •
Every newborn should be assessed for the risk of developing hyperbilirubinaemia before discharge. This assessment is particularly important in infants who are discharged before the age of 72h7 (grade I recommendation).
- •
All hospitals should provide parents with written and verbal information at the time of discharge, which should include an explanation of jaundice and how to monitor infants for it7 (grade I recommendation).
- •
All newborns should be evaluated by a health care professional in the first few days after discharge to assess their wellbeing and the presence of jaundice. The timing and location of this assessment should be determined based on the length of stay in the nursery and the presence of risk factors for hyperbilirubinaemia7 (grade I recommendation).
- •
All newborns discharged before 24h post birth should be checked up within the next 24h (grade I recommendation).
- •
If the pre-discharge TSB level does not require immediate intervention, its value should be documented at the time it is obtained along with the risk zone, providing parents with a copy. The followup should be individualised based on the risk profile (grade I recommendation).
- •
Patients with TSB concentrations associated with high risk should be monitored more closely, with followup within the next 24–48h and a low threshold for performance of TSB or TcB measurements1 (grade I recommendation).
- •
Some newborns discharged before 48h post birth may require two follow-up visits, the first between 24 and 72h and the second between 72 and 120h (grade I recommendation).
- •
If appropriate followup cannot be ensured in the presence of elevated risk, it may be necessary to delay discharge from hospital until appropriate followup can be ensured or the period of greatest risk has passed (72–96h) (grade I recommendation).
- •
Patients with isoimmunization are at risk of severe anaemia with a delayed onset. Measurement of haemoglobin is recommended at 2 weeks if it was low at discharge and at 4 weeks if it was normal (evidence level 5, grade I recommendation).1
Phototherapy decreases the progression to severe hyperbilirubinaemia in newborns with moderate hyperbilirubinaemia (evidence level 1a).1
Conventional PT: A single bank of fluorescent lights. It is less effective (the intensity is reduced).1 The minimal recommended spectral irradiance is 8–12μW/cm2/nm.28
Intensive PT: Involves the application of a high intensity of light in the 430–490nm range (usually 30μW/cm2/nm or higher) to the greatest possible surface area. The most effective light sources for intensive PT are those that use high-intensity special blue fluorescent tubes or a specially designed light-emitting diode (LED phototherapy).29,30 Intensive PT can also be delivered using 2 conventional PT units or reducing the distance to the newborn to up to 10cm, except when using halogen lamps, which cannot be positioned any closer than directed by the manufacturer. Intensive PT is recommended for every newborn with significant hyperbilirubinaemia or at very high risk of developing it.1
There is evidence that a high-intensity light-emitting diode (super LED) can deliver more efficient intensive PT compared to special blue fluorescent tubes and is a safe rescue therapy for severe hyperbilirubinaemia.31
The current guidelines of the AAP for the use of intensive PT in newborns of 35 or more weeks’ gestation (Fig. 2)4,7 have been widely adopted in many countries.
The serum concentration of bilirubin should be assessed 2–6h after initiation of PT.1 Once the bilirubin level is stable or decreasing, measurements should be repeated every 6–12h.8
Nutrition during PT: Enteral feeds should continue. The interruption of BF and/or oral fluid supplementation as part of the treatment of hyperbilirubinaemia is associated with decreased duration of BF (evidence level 2b)32,33; furthermore, continued BF in newborns receiving PT is not associated with adverse clinical outcomes.34 In patients requiring intensive PT, administration of enteral feeds should continue, with maternal expressed milk being the food of choice, and lactation should be supported so that BF can resume once treatment is completed.8
Recommendations- •
Conventional PT is an option if the TSB concentration is 2–3mg/dL lower than the threshold for intensive PT1 (grade I recommendation).
- •
Another option is using a fibre optic blanket, although this is less effective that conventional PT and requires longer treatment (evidence level 1a).35 The advantage is that it allows breastfeeding of the newborn without interrupting PT and it does not require the use of eye patches.1
- •
Phototherapy should be discontinued once bilirubin levels are below the threshold for treatment in 2 measurements taken 6–12h apart (evidence level 1b, grade A recommendation).9,36
- •
Total serum bilirubin should be measured 12–24h after discontinuation of PT to assess for rebound of hyperbilirubinaemia, especially in patients born preterm or with haemolysis; discharge from hospital need not be delayed to observe the newborn for rebound (evidence level 4, grade I recommendation).37
- •
Breastfeeding should be maintained during PT (grade A recommendation).1 Routine supplementation of breastfed infants with formula, water or dextrose water for treatment of jaundice is not recommended (evidence level 1b, grade E recommendation).7,9,33
- •
Administration of supplemental fluids (via the oral or intravenous routes) to breastfed newborns should be restricted to those at high risk of progressing to ET (evidence level 1b, grade A recommendation).1,38
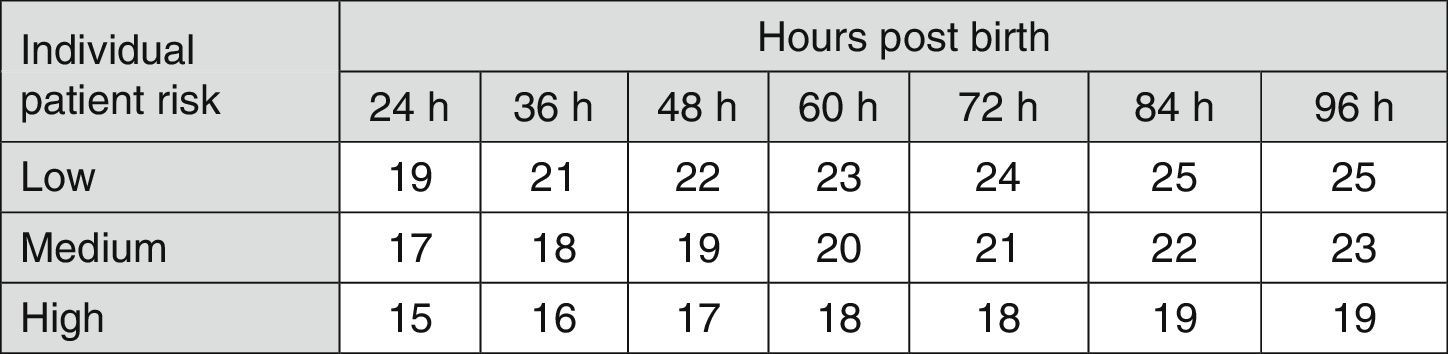
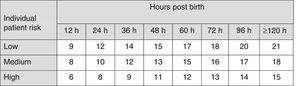
Fig. 3 shows the recommendations for exchange transfusion therapy.7
Indications for exchange transfusion in newborns of 35 or more weeks’ gestation. These guidelines are based on limited evidence and the levels shown are approximations. Exchange transfusion should be performed when the TSB concentration exceeds the level shown in the corresponding cell (values expressed in mg/dL). Patients at low risk: GA ≥38 weeks and well. Patients at medium risk: GA ≥38 weeks+risk factors, or 35–37+6 weeks and well. Patients at high risk: GA 35–37+6 weeks with risk factors. Risk factors: isoimmune haemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis or albumin <3g/dL. The indication in the first 24h is uncertain and varies depending on clinical circumstances and the response to phototherapy. GA, gestational age; G6PD, glucose-6-phosphate dehydrogenase; TSB, total serum bilirubin.
- •
Newborns with a TSB level above the threshold for ET shown in Fig. 3 should have immediate intensive PT and referred to a tertiary care hospital (grade B recommendation).1
- •
Every newborn with jaundice and clinical manifestations of acute bilirubin encephalopathy should have an immediate ET, even if the TSB concentration is falling (evidence level 4, grade I recommendation).1,7,8
- •
Discontinuation of enteral feeding during ET and for 6h following the end of the procedure is recommended (evidence level 5, grade I recommendation).9
In newborns with isoimmune haemolytic disease, administration of specific intravenous immunoglobulin (0.5–1g/kg over 2–4h) reduces the level of bilirubin and the need for ET (evidence level 1a).1,9,39
RecommendationsExposure to sunlightAlthough sunlight provides sufficient irradiance in the 425–475nm band to provide phototherapy, the practical difficulties involved in safely exposing a newborn to sunlight avoiding sunburn preclude the use of sunlight as a safe therapeutic tool, and it is not recommended.7 The randomised controlled trial by Kumar published in 2016 showed that the use of sunlight with especial filter may be as effective as conventional PT in term and late preterm newborns with mild jaundice.40
ConclusionsThe identification of newborns at risk of developing significant hyperbilirubinaemia and the prevention of bilirubin encephalopathy continue to be public health priorities. The combination of GA and pre-discharge hour-specific bilirubin levels can be used with considerable confidence to measure the risk of severe hyperbilirubinaemia in most newborns. An appropriate followup can prevent most cases of kernicterus.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sánchez-Redondo Sánchez-Gabriel MD, Leante Castellanos JL, Benavente Fernández I, Pérez Muñuzuri A, Rite Gracia S, Ruiz Campillo CW, et al. Recomendaciones para la prevención, la detección y el manejo de la hiperbilirrubinemia en los recién nacidos con 35 o más semanas de edad gestacional. An Pediatr (Barc). 2017;87:294.e1–294.e8.