The Spanish Network for the Study of Paediatric Tuberculosis has shown a lack of national consensus on the treatment of tuberculosis in children, partly due to the unavailability of paediatric presentations of antituberculosis drugs. The harmonisation of tuberculosis treatment in children is a priority in Spain. A joint action is proposed by a group of Spanish experts in childhood tuberculosis and in the area of Paediatric Pharmacology. To this end, a pTBred-led workgroup of members from five scientific bodies has been created. Drug pharmaceutical compounding in oral suspensions or oral solutions are recommended as follows: isoniazid 50mg/mL, pyrazinamide 100mg/mL, and ethambutol 50mg/mL. Raw materials, period of validity, and storage conditions are specified. Recommendations for the use of fixed-dose combination drugs are also established. If oral solutions/suspensions or fixed-dose combination drugs are not appropriate, the use of crushed tablets is recommended. Adherence to treatment and optimal dosing of antituberculosis drugs are critical in the control and eradication of TB. This multidisciplinary document provides an opportunity to promote the appropriate treatment of paediatric tuberculosis in Spain, and should become a useful tool for paediatricians and pharmacists.
La Red Española de Estudio de la Tuberculosis Pediátrica ha evidenciado una falta de consenso nacional en la administración de antituberculosos en niños, propiciada por la escasez de presentaciones pediátricas específicas. Es prioritario homogeneizar el tratamiento de la tuberculosis en niños en nuestro país. Un grupo de expertos españoles en tuberculosis infantil y en el área de medicamentos pediátricos proponen una actuación conjunta, con la finalidad de mejorar esta situación en nuestro medio. Para ello se constituye un Grupo de Trabajo, liderado por pTBred, en el que participan otras 5 sociedades e instituciones científicas. Se proponen las siguientes fórmulas magistrales en forma de suspensión o solución oral para el tratamiento de la tuberculosis en niños: isoniacida 50mg/ml, pirazinamida 100mg/ml y etambutol 50mg/ml. Se especifican materias primas, periodo de validez y condiciones de conservación y administración. Se establecen recomendaciones para el uso de fármacos combinados a dosis fijas. Si no se consigue la dosis apropiada mediante fármacos combinados a dosis fijas, y no se dispone de fórmula magistral, se recomienda la administración mediante comprimidos triturados. El adecuado cumplimiento terapéutico y la administración de dosis óptimas de los fármacos antituberculosos constituyen pilares fundamentales en el control y erradicación de la enfermedad. La oportunidad de disponer de este documento multidisciplinar en España favorecerá el correcto tratamiento de la tuberculosis pediátrica, y será una guía útil para todos los pediatras y farmacéuticos que lo precisen.
The formulation of paediatric drugs continues to be a world challenge, especially in diseases like tuberculosis that call for prolonged combined therapies with adequate adherence.1 In this document, a group of Spanish experts in childhood tuberculosis and paediatric pharmacology propose several strategies that could help facilitate the treatment of children with tuberculosis in Spain.
Between February and March 2015, the Spanish Network for the Study of Paediatric Tuberculosis (Red Española de Estudio de la Tuberculosis Pediátrica [pTBred])2 carried out the first phase of the Magistral Project,1 a survey focused on learning how antituberculosis drugs are administered to children in Spain. The survey was sent to all the member institutions of the Network. The results evinced a lack of nationwide consensus in the administration of antituberculosis drugs in children, which was promoted by the scarcity of preparations specifically formulated for children. Most paediatricians prescribe tablets that need to be crushed and dissolved, while the rest prescribe compounded formulations (CFs) whose preparation and concentration vary between pharmacy departments in different hospitals.1
The heterogeneity in the administration of these drugs could compromise the strict adherence required in the treatment of tuberculosis. In light of this situation, the pTBred proposed the elaboration of a pioneering document on the administration of antituberculosis drugs in the paediatric age group, with special emphasis on children that cannot swallow solid dosage forms.1 This document focuses on the preparation of suspensions of isoniazid, pyrazinamide and ethambutol, as liquid dosage forms are the most child-friendly. It does not include rifampicin because it is the sole first-line antituberculosis drug that is commercially available as a suspension.3 Furthermore, this document contemplates the possibility of using paediatric dosage forms with fixed concentrations of antituberculosis agents in the doses required by children adjusted by kilogram of body weight. This document was developed and agreed on by the main experts in tuberculosis and paediatric pharmacology in Spain, and is supported by renowned scientific associations.
Compounded isoniazid formulationIsoniazid is a drug with a high solubility in water,4 incompatible with sugars such as sucrose,5 and most stable at a pH of 6.6 Supplementation with pyridoxine is recommended in children that are malnourished, HIV-positive patients, exclusively-breastfed infants and pregnant women due to the interaction of isoniazid with the metabolism of this vitamin.
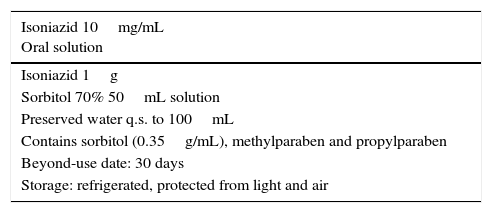
Isoniazid is not commercially available as oral suspension in Spain, but it is in other countries, and it can be procured as an imported drug at a concentration of 10mg/mL. This concentration is recommended in different formularies,7,8 and is the one most frequently prepared in CFs. However, in children that weigh more than 5kg and are unable to swallow commercially available tablets, this concentration results in the administration of high volumes of suspension.
The CFs included in the reference formularies in Spain use sorbitol as an excipient.7,8 However, its use at high doses can result in intolerance and have an osmotic laxative effect.7,9 A more concentrated formulation (50mg/mL) would allow the administration of smaller volumes with a reduced amount of sorbitol, which would facilitate dosing and adherence while reducing the risk of potential adverse effects associated with the excipients.
Due to the ease of its preparation, its excipient compatibility and its potential to improve adherence to treatment, we recommend the preparation of the CF at a concentration of 50mg/mL. Table 1 presents the formulation for both concentrations. The drug should be compounded using the raw chemical rather than commercially available preparations, as reduced activity has been observed with the use of the latter due to their lactose content.10 We recommend that its administration is routinely combined with vitamin B6 supplementation.
Compounded isoniazid formulations. Consensus document.
| Isoniazid 10mg/mL Oral solution |
|---|
| Isoniazid 1g |
| Sorbitol 70% 50mL solution |
| Preserved water q.s. to 100mL |
| Contains sorbitol (0.35g/mL), methylparaben and propylparaben |
| Beyond-use date: 30 days |
| Storage: refrigerated, protected from light and air |
| Isoniazid 50mg/mL Oral solution |
|---|
| Isoniazid 5g |
| Sorbitol 70% 50mL solution |
| Preserved water q.s. to 100mL |
| Contains sorbitol (0.35g/mL), methylparaben and propylparaben |
| Beyond-use date: 30 days |
| Storage: refrigerated, protected from light and air |
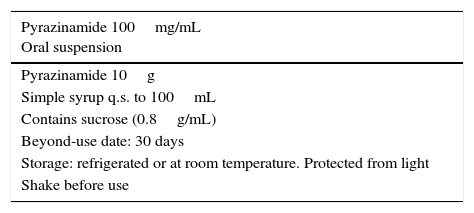
Pyrazinamide is obtained as a white crystalline powder with a low solubility in water. It forms suspensions that sediment easily and can be resuspended with shaking. Pyrazinamide is not commercially available as oral suspension in Spain,11 but it is in other countries with a concentration of 50mg/mL.
The preparation of a pyrazinamide suspension at a concentration of 10mg/mL is included in some formularies,12 although the CF provided most frequently as a reference in different formularies in Spain and other countries has a concentration of 100mg/mL.7,12–14 This is the CF proposed in this document (Table 2) due to its easy preparation and the vast documentation available for it. The raw chemical is the first choice for the starting material, although it could also be prepared using commercially available tablets, as has been done in stability studies of pyrazinamide formulations.13
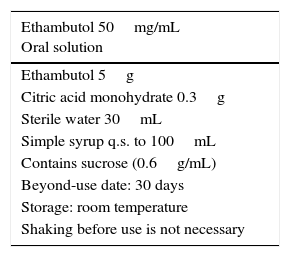
Compounded ethambutol formulationEthambutol hydrochloride is a white crystalline powder that is hygroscopic, odourless and bitter in taste.4,15–17 A 2% aqueous solution has a pH of 3.7–4. It is optically active, as it has two chiral carbons. Ethambutol is the name given to the dextrorotatory isomer. The levorotatory isomer is inactive and has been associated with optic neuropathy.15 Ethambutol has displayed good bioavailability following oral intake and it is excreted in urine mostly unaltered.16
No oral liquid formulations are commercially available in the world. In Spain, the only commercially available formulation consists of 400mg tablets, which are difficult to cut or disperse. Tablets containing 100mg are available in other countries.17
Most CFs of ethambutol have concentrations of 25, 50 or 100mg/mL, and are prepared with the raw powder or commercial tablets depending on the availability of these starting materials.18–20Table 3 presents the CF (with a concentration of 50mg/mL) recommended in this document.18–21
Combined compounded formulations. Are they possible?Due to the pathophysiology of tuberculosis, a combination of several drugs is required to cure the disease and prevent the development of drug resistance.
Promoting the development of formulations that would facilitate adherence to treatment is absolutely justified in the paediatric population. Ideally, a combined formulation of the main first-line oral antituberculosis drugs (isoniazid, rifampicin, pyrazinamide and ethambutol) would become available. However, this objective poses numerous challenges.
Compounded formulations prepared as oral solutions have shorter beyond-use dates, as active ingredients degrade faster in solution or suspension than in solid dosage forms, and there may be problems of compatibility or interactions between their components. Currently, the number of liquid formulations with two or more active ingredients that are commercially available in the world is insignificant.
Antituberculosis drugs have different physical and chemical characteristics. Isoniazid is incompatible with sugars such as sucrose and the recommended medium is a sorbitol solution.7,8 Its solubility in water is much higher than those of ethambutol and pyrazinamide, and it can form aqueous solutions. Pyrazinamide is the least soluble in water, and forms a suspension. Pyrazinamide and ethambutol can be prepared with sucrose.15,22
We did not find any evidence in the literature on the physical and chemical stability of preparations that combine three or four antituberculosis drugs in solution or suspension. These data are needed to guarantee the quality and safety of such formulations, especially in treatment regimens as complicated as those required for tuberculosis, and in populations as vulnerable as the paediatric age group.
In some countries, kits that include three drugs prepared separately in liquid form23 are commercially available. From a compounding perspective, the preparation and standardisation in mg/mL of liquid single-component antituberculosis drugs would be the most appropriate strategy for the paediatric population, as well as the safest in terms of absorption. The challenge in this approach is the optimisation of the volumes of each drug to be administered based on the age of the patient while preserving the physical and chemical stability of each agent. Such kits are not available in Spain.
Fixed-dose combinations in paediatricsPharmaceutical preparations that combine two, three or four antituberculosis drugs in a single tablet (fixed-dose combinations [FDCs]) have clear advantages compared to the separate administration of individual drugs. They promote adherence and minimise dosing errors, thus decreasing the risk of treatment failure and the selection of resistant strains. However, their manufacturing poses considerable technical challenges. In recent years, it has become possible thanks to the development of sophisticated infrastructures in pharmaceutical laboratories.24
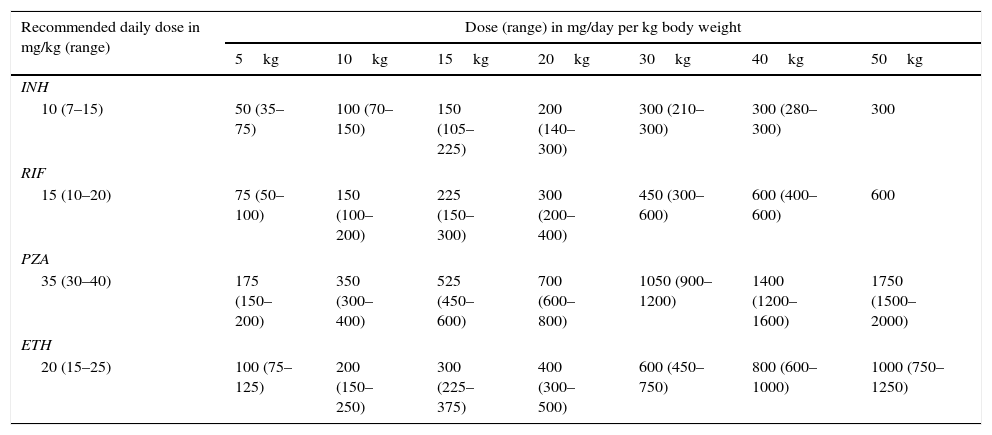
Several pharmacokinetic studies have shown that the dose of antituberculosis drugs per kilogram of body weight must be higher in children than the corresponding dose in adults.25–27 These findings, along with the low toxicity of antituberculosis drugs described in the paediatric population,28 provided the basis for the updated dosage recommendations of the WHO for the use of these agents in children in 201029 (Table 4). These are the doses currently recommended by the Committee on Medicinal Products of the Spanish Association of Paediatrics.30 In 2014 the WHO updated its recommendations once again, reducing the minimum recommended dose of isoniazid to 7mg/kg to facilitate the development of paediatric FDCs.31
Paediatric doses of first-line antituberculosis drugs based on the recommendations of the WHO.
| Recommended daily dose in mg/kg (range) | Dose (range) in mg/day per kg body weight | ||||||
|---|---|---|---|---|---|---|---|
| 5kg | 10kg | 15kg | 20kg | 30kg | 40kg | 50kg | |
| INH | |||||||
| 10 (7–15) | 50 (35–75) | 100 (70–150) | 150 (105–225) | 200 (140–300) | 300 (210–300) | 300 (280–300) | 300 |
| RIF | |||||||
| 15 (10–20) | 75 (50–100) | 150 (100–200) | 225 (150–300) | 300 (200–400) | 450 (300–600) | 600 (400–600) | 600 |
| PZA | |||||||
| 35 (30–40) | 175 (150–200) | 350 (300–400) | 525 (450–600) | 700 (600–800) | 1050 (900–1200) | 1400 (1200–1600) | 1750 (1500–2000) |
| ETH | |||||||
| 20 (15–25) | 100 (75–125) | 200 (150–250) | 300 (225–375) | 400 (300–500) | 600 (450–750) | 800 (600–1000) | 1000 (750–1250) |
ETH, ethambutol; INH, isoniazid; PZA, pyrazinamide; RIF, rifampicin.
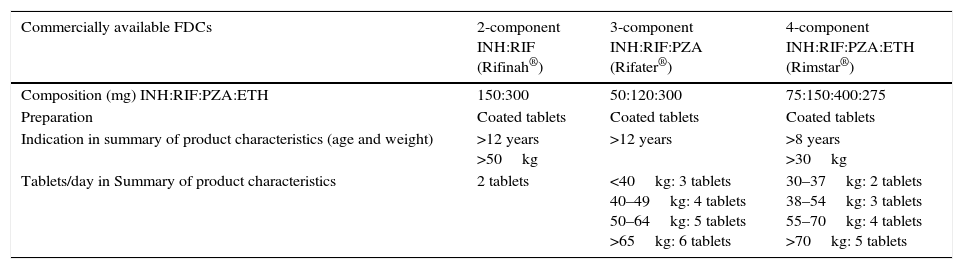
The dosing of antituberculosis FDCs available in Spain is based on the therapeutic dosage range used in adults, and these drugs are only authorised for their use starting at a certain age and/or weight (Table 5). Their off-label use in younger children often requires cutting or crushing the tablets, which carries the risk of altering drug bioavailability and especially of administering inadequate doses—suboptimal or excessive—of some of the components.
FDCs available in Spain.
| Commercially available FDCs | 2-component INH:RIF (Rifinah®) | 3-component INH:RIF:PZA (Rifater®) | 4-component INH:RIF:PZA:ETH (Rimstar®) |
|---|---|---|---|
| Composition (mg) INH:RIF:PZA:ETH | 150:300 | 50:120:300 | 75:150:400:275 |
| Preparation | Coated tablets | Coated tablets | Coated tablets |
| Indication in summary of product characteristics (age and weight) | >12 years >50kg | >12 years | >8 years >30kg |
| Tablets/day in Summary of product characteristics | 2 tablets | <40kg: 3 tablets 40–49kg: 4 tablets 50–64kg: 5 tablets >65kg: 6 tablets | 30–37kg: 2 tablets 38–54kg: 3 tablets 55–70kg: 4 tablets >70kg: 5 tablets |
ETH, ethambutol; FDC, fixed-dose combination; INH, isoniazid; PZA, pyrazinamide; RIF, rifampicin.
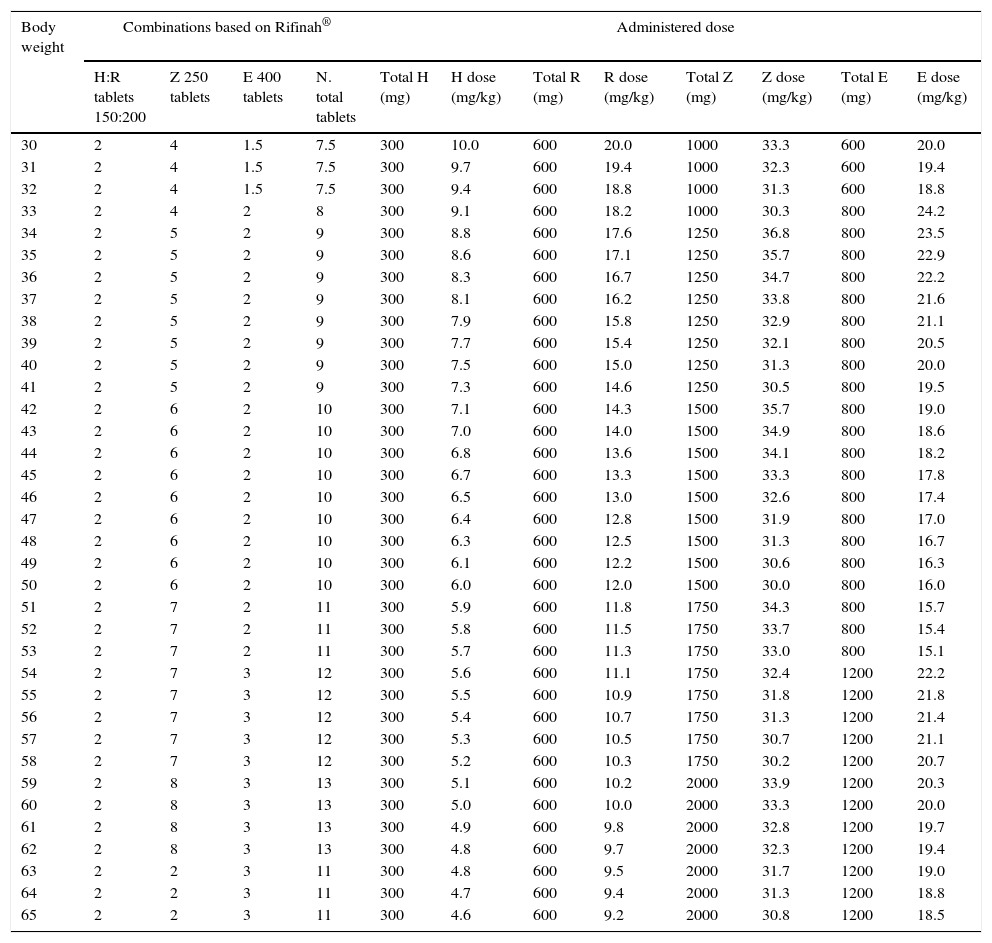
When FDCs are used within the age and weight ranges authorised in their summaries of product characteristics, only Rifinah® (isoniazid+rifampicin) is used in compliance with the paediatric doses recommended by the WHO. Conversely, the available three- and four-component FDCs do not reach the recommended dose of isoniazid, which should be corrected by the supplementary administration of this drug. Increasing the number of FDC tablets to reach the therapeutic range for isoniazid would result in the administration of excessive doses of ethambutol with Rimstar®, or rifampicin and pyrazinamide with Rifater®. In 2009, the WHO established recommendations for the use of FDCs in children based on weight, and noted that none of the combinations available at the time were appropriate for use in the paediatric age group.32
The development of FDCs for exclusive use in children require the adjustment of dosing to paediatric safety and efficacy ranges and to facilitate administration by the use of liquid or dispersible preparations. Liquid dosage forms are easier to administer, but dispersible tablets have advantages in areas with poor resources, as they simplify their conservation, transport and storage. The addition of sweeteners and flavourings, which is often needed to improve palatability, carries a risk of interaction with active ingredients.33
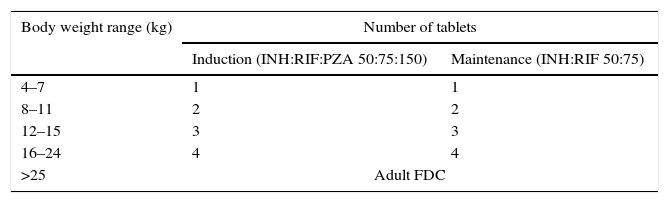
After resolving these problems, the TB Alliance and the WHO, in collaboration with UNITAID and USAID, promoted the development and distribution of the first FDCs appropriate for the treatment of tuberculosis34,35 in children weighing less than 25kg. They are dispersible tablets that contain 50mg isoniazid, 75mg rifampicin and 150mg pyrazinamide for the induction phase, and the same dosing without pyrazinamide for the maintenance phase (Table 6). The tablets are small, dissolve readily in water, and have a pleasant taste. The formulation does not include ethambutol, which must be administered separately when the antibiotic susceptibility of the strain in the patient or index case is unknown. This could be solved by developing an FDC with the same doses of isoniazid, rifampicin and pyrazinamide, but with an additional 100mg of ethambutol per tablet. Such an option would considerably improve conventional tuberculosis treatment in children.
New paediatric FDCs for the treatment of tuberculosis in children (WHO and TB Alliance, 2015).
| Body weight range (kg) | Number of tablets | |
|---|---|---|
| Induction (INH:RIF:PZA 50:75:150) | Maintenance (INH:RIF 50:75) | |
| 4–7 | 1 | 1 |
| 8–11 | 2 | 2 |
| 12–15 | 3 | 3 |
| 16–24 | 4 | 4 |
| >25 | Adult FDC | |
ETH, ethambutol; FDC, fixed-dose combination; INH, isoniazid; PZA, pyrazinamide; RIF, rifampicin.
However, these new paediatric FDCs also have their disadvantages. Their dosages are established based on broad body weight ranges, which carries a risk of overdosing, especially in smaller children. Recommendations regarding the doses to be used in children that weigh less than four kilograms have yet to be made due to the lack of pharmacokinetic data. Starting at 25kg of body weight, the WHO recommends the administration of FDCs formulated for adults, despite the aforementioned risks of underdosing and toxicity. In our opinion, this could be resolved by administering five paediatric FDC tablets to children that weigh between 20 and 29kg, and six tablets to children that weigh between 30 and 39kg. Patients weighing 40kg or more could be treated with FDCs authorised for use in adults, supplementing them with the additional administration of single-component formulations of some drugs as needed.
The only paediatric FDCs guaranteed to meet quality, safety and efficacy standards equivalent to those applied in the European Union are those that have been prequalified by the WHO.36 Unfortunately, the combination of 50mg of isoniazid and 75mg of rifampicin (with or without 150mg of pyrazinamide) has yet to be prequalified by the WHO, and is currently undergoing evaluation. In countries with a high prevalence of tuberculosis, these formulations are distributed by the Global TB Drug Facility of the WHO. Ideally, once they are prequalified, it should be possible to obtain these new paediatric FDCs in Spain by applying for their import as a foreign drug to the Spanish Agency of Medicines and Medical Devices.
Drug administrationRegardless of their formulation, antituberculosis drugs are ideally taken in the morning, fasting and sequentially, without mixing them in a single glass, spoon or oral syringe.
The use of liquid formulations facilitates dosing adjustments for the administration of doses below those available in tablet form, so these formulations are the ideal and thus the first option recommended in this document, along with newly developed paediatric FDCs.
Ideally, tablets should be taken in a whole. If a patient has trouble swallowing them, all solid formulations of antituberculosis drugs can be manipulated to facilitate their administration. Tablets can be crushed and capsules can be opened.37–39 This option should be reserved exclusively for the following cases: when the dose approximates the contents of a solid form or half a tablet, when the child rejects liquid formulations due to their organoleptic properties, or when no other formulation is available.
Crushed tablets have a stronger flavour than larger fragments.37 This is why drugs frequently have to be mixed with foods to be given to children, although this option should be reserved only for patients with gastrointestinal intolerance. If the drug is not administered within 30min from mixing, it should be discarded.37–39 It must be taken into account that suspension of the crushed tablet may lead to aggregation, sedimentation or precipitation problems,40 with a potential loss of stability.
In the case of isoniazid, the food must be low in fat, as fats slow down its absorption, and if possible low in sugars, which would inactivate it.37–39 For infants, it can be dissolved in a spoonful of warm water and mixed with a small amount of breast milk or follow-on formula.37–39
Ethambutol can be administered mixed in a small volume of juice or apple sauce.37–40 It takes 10min to dissolve.39 Mixing it with other fluids or syrups is not recommended, as it may not be stable, or the medium may fail to mask the bitter taste of the drug.39,40 Last of all, there are no restrictions on the types of food that pyrazinamide crushed tablets can be mixed with for administration.
In all cases, the amount of food added should be small, guaranteeing that the child consumes the full dose.37–39
In case of vomiting, the dose can be retaken if less than 30min elapsed since its administration. When a dose is skipped accidentally, the drug should be administered as soon as possible if at least 12h remain to the next dose.37–40
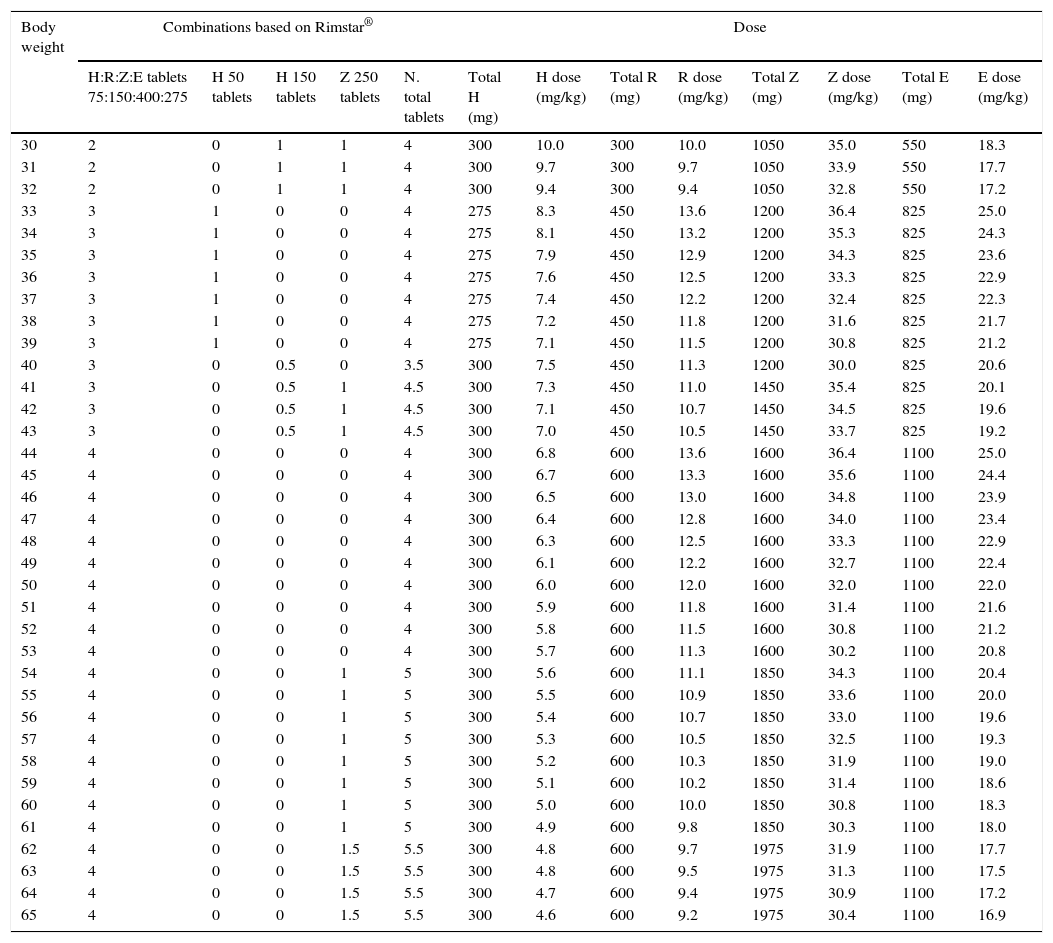
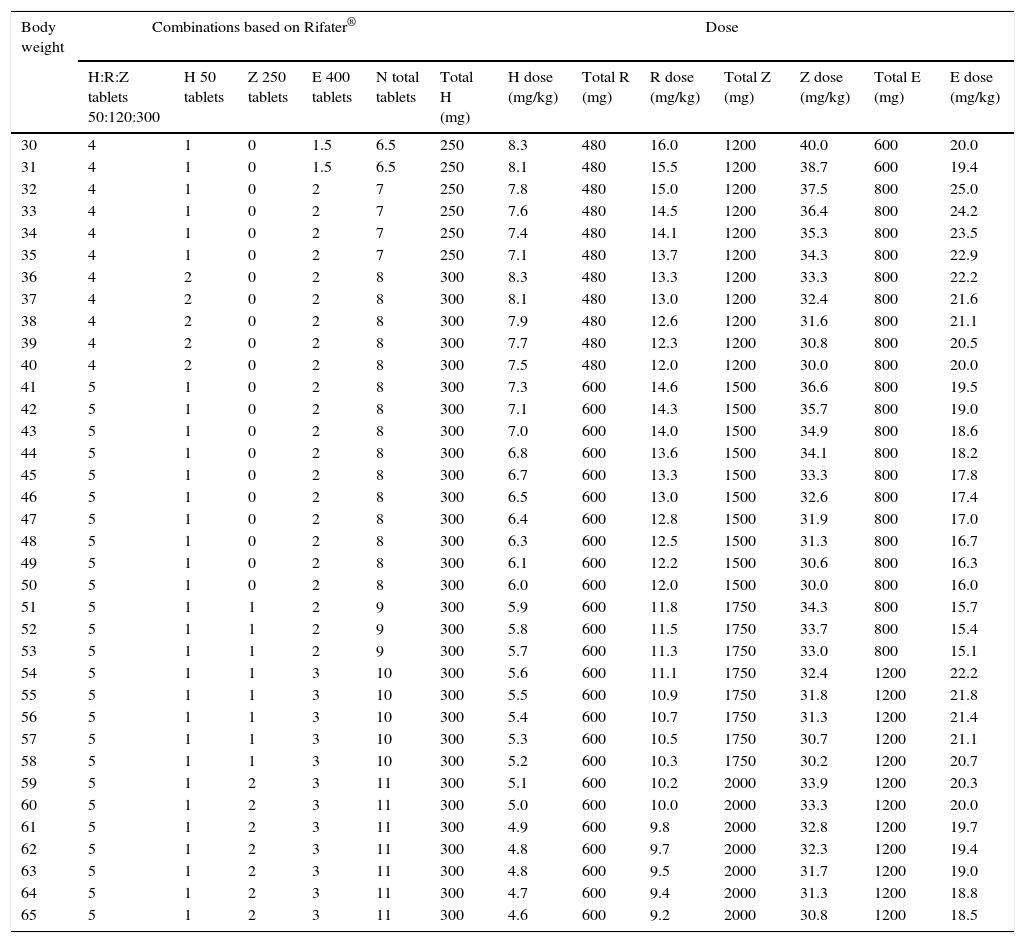
Fixed-dose combinations for adults are prepared as coated tablets. The excipients used in the coating are widely used in tablet manufacturing processes, so it is not expected that they would interfere with drug absorption after crushing. Tables 7–9 summarise the recommended doses for the use of adult FDCs in children that weigh more than 30kg.
Recommended dosing of Rimstar® for children that weigh more than 30kg.
| Body weight | Combinations based on Rimstar® | Dose | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H:R:Z:E tablets 75:150:400:275 | H 50 tablets | H 150 tablets | Z 250 tablets | N. total tablets | Total H (mg) | H dose (mg/kg) | Total R (mg) | R dose (mg/kg) | Total Z (mg) | Z dose (mg/kg) | Total E (mg) | E dose (mg/kg) | |
| 30 | 2 | 0 | 1 | 1 | 4 | 300 | 10.0 | 300 | 10.0 | 1050 | 35.0 | 550 | 18.3 |
| 31 | 2 | 0 | 1 | 1 | 4 | 300 | 9.7 | 300 | 9.7 | 1050 | 33.9 | 550 | 17.7 |
| 32 | 2 | 0 | 1 | 1 | 4 | 300 | 9.4 | 300 | 9.4 | 1050 | 32.8 | 550 | 17.2 |
| 33 | 3 | 1 | 0 | 0 | 4 | 275 | 8.3 | 450 | 13.6 | 1200 | 36.4 | 825 | 25.0 |
| 34 | 3 | 1 | 0 | 0 | 4 | 275 | 8.1 | 450 | 13.2 | 1200 | 35.3 | 825 | 24.3 |
| 35 | 3 | 1 | 0 | 0 | 4 | 275 | 7.9 | 450 | 12.9 | 1200 | 34.3 | 825 | 23.6 |
| 36 | 3 | 1 | 0 | 0 | 4 | 275 | 7.6 | 450 | 12.5 | 1200 | 33.3 | 825 | 22.9 |
| 37 | 3 | 1 | 0 | 0 | 4 | 275 | 7.4 | 450 | 12.2 | 1200 | 32.4 | 825 | 22.3 |
| 38 | 3 | 1 | 0 | 0 | 4 | 275 | 7.2 | 450 | 11.8 | 1200 | 31.6 | 825 | 21.7 |
| 39 | 3 | 1 | 0 | 0 | 4 | 275 | 7.1 | 450 | 11.5 | 1200 | 30.8 | 825 | 21.2 |
| 40 | 3 | 0 | 0.5 | 0 | 3.5 | 300 | 7.5 | 450 | 11.3 | 1200 | 30.0 | 825 | 20.6 |
| 41 | 3 | 0 | 0.5 | 1 | 4.5 | 300 | 7.3 | 450 | 11.0 | 1450 | 35.4 | 825 | 20.1 |
| 42 | 3 | 0 | 0.5 | 1 | 4.5 | 300 | 7.1 | 450 | 10.7 | 1450 | 34.5 | 825 | 19.6 |
| 43 | 3 | 0 | 0.5 | 1 | 4.5 | 300 | 7.0 | 450 | 10.5 | 1450 | 33.7 | 825 | 19.2 |
| 44 | 4 | 0 | 0 | 0 | 4 | 300 | 6.8 | 600 | 13.6 | 1600 | 36.4 | 1100 | 25.0 |
| 45 | 4 | 0 | 0 | 0 | 4 | 300 | 6.7 | 600 | 13.3 | 1600 | 35.6 | 1100 | 24.4 |
| 46 | 4 | 0 | 0 | 0 | 4 | 300 | 6.5 | 600 | 13.0 | 1600 | 34.8 | 1100 | 23.9 |
| 47 | 4 | 0 | 0 | 0 | 4 | 300 | 6.4 | 600 | 12.8 | 1600 | 34.0 | 1100 | 23.4 |
| 48 | 4 | 0 | 0 | 0 | 4 | 300 | 6.3 | 600 | 12.5 | 1600 | 33.3 | 1100 | 22.9 |
| 49 | 4 | 0 | 0 | 0 | 4 | 300 | 6.1 | 600 | 12.2 | 1600 | 32.7 | 1100 | 22.4 |
| 50 | 4 | 0 | 0 | 0 | 4 | 300 | 6.0 | 600 | 12.0 | 1600 | 32.0 | 1100 | 22.0 |
| 51 | 4 | 0 | 0 | 0 | 4 | 300 | 5.9 | 600 | 11.8 | 1600 | 31.4 | 1100 | 21.6 |
| 52 | 4 | 0 | 0 | 0 | 4 | 300 | 5.8 | 600 | 11.5 | 1600 | 30.8 | 1100 | 21.2 |
| 53 | 4 | 0 | 0 | 0 | 4 | 300 | 5.7 | 600 | 11.3 | 1600 | 30.2 | 1100 | 20.8 |
| 54 | 4 | 0 | 0 | 1 | 5 | 300 | 5.6 | 600 | 11.1 | 1850 | 34.3 | 1100 | 20.4 |
| 55 | 4 | 0 | 0 | 1 | 5 | 300 | 5.5 | 600 | 10.9 | 1850 | 33.6 | 1100 | 20.0 |
| 56 | 4 | 0 | 0 | 1 | 5 | 300 | 5.4 | 600 | 10.7 | 1850 | 33.0 | 1100 | 19.6 |
| 57 | 4 | 0 | 0 | 1 | 5 | 300 | 5.3 | 600 | 10.5 | 1850 | 32.5 | 1100 | 19.3 |
| 58 | 4 | 0 | 0 | 1 | 5 | 300 | 5.2 | 600 | 10.3 | 1850 | 31.9 | 1100 | 19.0 |
| 59 | 4 | 0 | 0 | 1 | 5 | 300 | 5.1 | 600 | 10.2 | 1850 | 31.4 | 1100 | 18.6 |
| 60 | 4 | 0 | 0 | 1 | 5 | 300 | 5.0 | 600 | 10.0 | 1850 | 30.8 | 1100 | 18.3 |
| 61 | 4 | 0 | 0 | 1 | 5 | 300 | 4.9 | 600 | 9.8 | 1850 | 30.3 | 1100 | 18.0 |
| 62 | 4 | 0 | 0 | 1.5 | 5.5 | 300 | 4.8 | 600 | 9.7 | 1975 | 31.9 | 1100 | 17.7 |
| 63 | 4 | 0 | 0 | 1.5 | 5.5 | 300 | 4.8 | 600 | 9.5 | 1975 | 31.3 | 1100 | 17.5 |
| 64 | 4 | 0 | 0 | 1.5 | 5.5 | 300 | 4.7 | 600 | 9.4 | 1975 | 30.9 | 1100 | 17.2 |
| 65 | 4 | 0 | 0 | 1.5 | 5.5 | 300 | 4.6 | 600 | 9.2 | 1975 | 30.4 | 1100 | 16.9 |
Recommended dosing of Rifater® for children that weigh more than 30kg.
| Body weight | Combinations based on Rifater® | Dose | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H:R:Z tablets 50:120:300 | H 50 tablets | Z 250 tablets | E 400 tablets | N total tablets | Total H (mg) | H dose (mg/kg) | Total R (mg) | R dose (mg/kg) | Total Z (mg) | Z dose (mg/kg) | Total E (mg) | E dose (mg/kg) | |
| 30 | 4 | 1 | 0 | 1.5 | 6.5 | 250 | 8.3 | 480 | 16.0 | 1200 | 40.0 | 600 | 20.0 |
| 31 | 4 | 1 | 0 | 1.5 | 6.5 | 250 | 8.1 | 480 | 15.5 | 1200 | 38.7 | 600 | 19.4 |
| 32 | 4 | 1 | 0 | 2 | 7 | 250 | 7.8 | 480 | 15.0 | 1200 | 37.5 | 800 | 25.0 |
| 33 | 4 | 1 | 0 | 2 | 7 | 250 | 7.6 | 480 | 14.5 | 1200 | 36.4 | 800 | 24.2 |
| 34 | 4 | 1 | 0 | 2 | 7 | 250 | 7.4 | 480 | 14.1 | 1200 | 35.3 | 800 | 23.5 |
| 35 | 4 | 1 | 0 | 2 | 7 | 250 | 7.1 | 480 | 13.7 | 1200 | 34.3 | 800 | 22.9 |
| 36 | 4 | 2 | 0 | 2 | 8 | 300 | 8.3 | 480 | 13.3 | 1200 | 33.3 | 800 | 22.2 |
| 37 | 4 | 2 | 0 | 2 | 8 | 300 | 8.1 | 480 | 13.0 | 1200 | 32.4 | 800 | 21.6 |
| 38 | 4 | 2 | 0 | 2 | 8 | 300 | 7.9 | 480 | 12.6 | 1200 | 31.6 | 800 | 21.1 |
| 39 | 4 | 2 | 0 | 2 | 8 | 300 | 7.7 | 480 | 12.3 | 1200 | 30.8 | 800 | 20.5 |
| 40 | 4 | 2 | 0 | 2 | 8 | 300 | 7.5 | 480 | 12.0 | 1200 | 30.0 | 800 | 20.0 |
| 41 | 5 | 1 | 0 | 2 | 8 | 300 | 7.3 | 600 | 14.6 | 1500 | 36.6 | 800 | 19.5 |
| 42 | 5 | 1 | 0 | 2 | 8 | 300 | 7.1 | 600 | 14.3 | 1500 | 35.7 | 800 | 19.0 |
| 43 | 5 | 1 | 0 | 2 | 8 | 300 | 7.0 | 600 | 14.0 | 1500 | 34.9 | 800 | 18.6 |
| 44 | 5 | 1 | 0 | 2 | 8 | 300 | 6.8 | 600 | 13.6 | 1500 | 34.1 | 800 | 18.2 |
| 45 | 5 | 1 | 0 | 2 | 8 | 300 | 6.7 | 600 | 13.3 | 1500 | 33.3 | 800 | 17.8 |
| 46 | 5 | 1 | 0 | 2 | 8 | 300 | 6.5 | 600 | 13.0 | 1500 | 32.6 | 800 | 17.4 |
| 47 | 5 | 1 | 0 | 2 | 8 | 300 | 6.4 | 600 | 12.8 | 1500 | 31.9 | 800 | 17.0 |
| 48 | 5 | 1 | 0 | 2 | 8 | 300 | 6.3 | 600 | 12.5 | 1500 | 31.3 | 800 | 16.7 |
| 49 | 5 | 1 | 0 | 2 | 8 | 300 | 6.1 | 600 | 12.2 | 1500 | 30.6 | 800 | 16.3 |
| 50 | 5 | 1 | 0 | 2 | 8 | 300 | 6.0 | 600 | 12.0 | 1500 | 30.0 | 800 | 16.0 |
| 51 | 5 | 1 | 1 | 2 | 9 | 300 | 5.9 | 600 | 11.8 | 1750 | 34.3 | 800 | 15.7 |
| 52 | 5 | 1 | 1 | 2 | 9 | 300 | 5.8 | 600 | 11.5 | 1750 | 33.7 | 800 | 15.4 |
| 53 | 5 | 1 | 1 | 2 | 9 | 300 | 5.7 | 600 | 11.3 | 1750 | 33.0 | 800 | 15.1 |
| 54 | 5 | 1 | 1 | 3 | 10 | 300 | 5.6 | 600 | 11.1 | 1750 | 32.4 | 1200 | 22.2 |
| 55 | 5 | 1 | 1 | 3 | 10 | 300 | 5.5 | 600 | 10.9 | 1750 | 31.8 | 1200 | 21.8 |
| 56 | 5 | 1 | 1 | 3 | 10 | 300 | 5.4 | 600 | 10.7 | 1750 | 31.3 | 1200 | 21.4 |
| 57 | 5 | 1 | 1 | 3 | 10 | 300 | 5.3 | 600 | 10.5 | 1750 | 30.7 | 1200 | 21.1 |
| 58 | 5 | 1 | 1 | 3 | 10 | 300 | 5.2 | 600 | 10.3 | 1750 | 30.2 | 1200 | 20.7 |
| 59 | 5 | 1 | 2 | 3 | 11 | 300 | 5.1 | 600 | 10.2 | 2000 | 33.9 | 1200 | 20.3 |
| 60 | 5 | 1 | 2 | 3 | 11 | 300 | 5.0 | 600 | 10.0 | 2000 | 33.3 | 1200 | 20.0 |
| 61 | 5 | 1 | 2 | 3 | 11 | 300 | 4.9 | 600 | 9.8 | 2000 | 32.8 | 1200 | 19.7 |
| 62 | 5 | 1 | 2 | 3 | 11 | 300 | 4.8 | 600 | 9.7 | 2000 | 32.3 | 1200 | 19.4 |
| 63 | 5 | 1 | 2 | 3 | 11 | 300 | 4.8 | 600 | 9.5 | 2000 | 31.7 | 1200 | 19.0 |
| 64 | 5 | 1 | 2 | 3 | 11 | 300 | 4.7 | 600 | 9.4 | 2000 | 31.3 | 1200 | 18.8 |
| 65 | 5 | 1 | 2 | 3 | 11 | 300 | 4.6 | 600 | 9.2 | 2000 | 30.8 | 1200 | 18.5 |
Recommended dosing of Rifinah® for children that weigh more than 30kg.
| Body weight | Combinations based on Rifinah® | Administered dose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H:R tablets 150:200 | Z 250 tablets | E 400 tablets | N. total tablets | Total H (mg) | H dose (mg/kg) | Total R (mg) | R dose (mg/kg) | Total Z (mg) | Z dose (mg/kg) | Total E (mg) | E dose (mg/kg) | |
| 30 | 2 | 4 | 1.5 | 7.5 | 300 | 10.0 | 600 | 20.0 | 1000 | 33.3 | 600 | 20.0 |
| 31 | 2 | 4 | 1.5 | 7.5 | 300 | 9.7 | 600 | 19.4 | 1000 | 32.3 | 600 | 19.4 |
| 32 | 2 | 4 | 1.5 | 7.5 | 300 | 9.4 | 600 | 18.8 | 1000 | 31.3 | 600 | 18.8 |
| 33 | 2 | 4 | 2 | 8 | 300 | 9.1 | 600 | 18.2 | 1000 | 30.3 | 800 | 24.2 |
| 34 | 2 | 5 | 2 | 9 | 300 | 8.8 | 600 | 17.6 | 1250 | 36.8 | 800 | 23.5 |
| 35 | 2 | 5 | 2 | 9 | 300 | 8.6 | 600 | 17.1 | 1250 | 35.7 | 800 | 22.9 |
| 36 | 2 | 5 | 2 | 9 | 300 | 8.3 | 600 | 16.7 | 1250 | 34.7 | 800 | 22.2 |
| 37 | 2 | 5 | 2 | 9 | 300 | 8.1 | 600 | 16.2 | 1250 | 33.8 | 800 | 21.6 |
| 38 | 2 | 5 | 2 | 9 | 300 | 7.9 | 600 | 15.8 | 1250 | 32.9 | 800 | 21.1 |
| 39 | 2 | 5 | 2 | 9 | 300 | 7.7 | 600 | 15.4 | 1250 | 32.1 | 800 | 20.5 |
| 40 | 2 | 5 | 2 | 9 | 300 | 7.5 | 600 | 15.0 | 1250 | 31.3 | 800 | 20.0 |
| 41 | 2 | 5 | 2 | 9 | 300 | 7.3 | 600 | 14.6 | 1250 | 30.5 | 800 | 19.5 |
| 42 | 2 | 6 | 2 | 10 | 300 | 7.1 | 600 | 14.3 | 1500 | 35.7 | 800 | 19.0 |
| 43 | 2 | 6 | 2 | 10 | 300 | 7.0 | 600 | 14.0 | 1500 | 34.9 | 800 | 18.6 |
| 44 | 2 | 6 | 2 | 10 | 300 | 6.8 | 600 | 13.6 | 1500 | 34.1 | 800 | 18.2 |
| 45 | 2 | 6 | 2 | 10 | 300 | 6.7 | 600 | 13.3 | 1500 | 33.3 | 800 | 17.8 |
| 46 | 2 | 6 | 2 | 10 | 300 | 6.5 | 600 | 13.0 | 1500 | 32.6 | 800 | 17.4 |
| 47 | 2 | 6 | 2 | 10 | 300 | 6.4 | 600 | 12.8 | 1500 | 31.9 | 800 | 17.0 |
| 48 | 2 | 6 | 2 | 10 | 300 | 6.3 | 600 | 12.5 | 1500 | 31.3 | 800 | 16.7 |
| 49 | 2 | 6 | 2 | 10 | 300 | 6.1 | 600 | 12.2 | 1500 | 30.6 | 800 | 16.3 |
| 50 | 2 | 6 | 2 | 10 | 300 | 6.0 | 600 | 12.0 | 1500 | 30.0 | 800 | 16.0 |
| 51 | 2 | 7 | 2 | 11 | 300 | 5.9 | 600 | 11.8 | 1750 | 34.3 | 800 | 15.7 |
| 52 | 2 | 7 | 2 | 11 | 300 | 5.8 | 600 | 11.5 | 1750 | 33.7 | 800 | 15.4 |
| 53 | 2 | 7 | 2 | 11 | 300 | 5.7 | 600 | 11.3 | 1750 | 33.0 | 800 | 15.1 |
| 54 | 2 | 7 | 3 | 12 | 300 | 5.6 | 600 | 11.1 | 1750 | 32.4 | 1200 | 22.2 |
| 55 | 2 | 7 | 3 | 12 | 300 | 5.5 | 600 | 10.9 | 1750 | 31.8 | 1200 | 21.8 |
| 56 | 2 | 7 | 3 | 12 | 300 | 5.4 | 600 | 10.7 | 1750 | 31.3 | 1200 | 21.4 |
| 57 | 2 | 7 | 3 | 12 | 300 | 5.3 | 600 | 10.5 | 1750 | 30.7 | 1200 | 21.1 |
| 58 | 2 | 7 | 3 | 12 | 300 | 5.2 | 600 | 10.3 | 1750 | 30.2 | 1200 | 20.7 |
| 59 | 2 | 8 | 3 | 13 | 300 | 5.1 | 600 | 10.2 | 2000 | 33.9 | 1200 | 20.3 |
| 60 | 2 | 8 | 3 | 13 | 300 | 5.0 | 600 | 10.0 | 2000 | 33.3 | 1200 | 20.0 |
| 61 | 2 | 8 | 3 | 13 | 300 | 4.9 | 600 | 9.8 | 2000 | 32.8 | 1200 | 19.7 |
| 62 | 2 | 8 | 3 | 13 | 300 | 4.8 | 600 | 9.7 | 2000 | 32.3 | 1200 | 19.4 |
| 63 | 2 | 2 | 3 | 11 | 300 | 4.8 | 600 | 9.5 | 2000 | 31.7 | 1200 | 19.0 |
| 64 | 2 | 2 | 3 | 11 | 300 | 4.7 | 600 | 9.4 | 2000 | 31.3 | 1200 | 18.8 |
| 65 | 2 | 2 | 3 | 11 | 300 | 4.6 | 600 | 9.2 | 2000 | 30.8 | 1200 | 18.5 |
We present a document developed by a multidisciplinary group of experts that provides recommendations for the treatment of tuberculosis in children, with especial emphasis on those that have not yet developed the ability to swallow solid dosage forms. The document proposes the preparation of CFs of isoniazid, pyrazinamide and ethambutol at the most suitable concentrations for their use in children, provides recommendations on the use of the FDCs authorised for use in Spain, and urges the competent authorities to expedite the introduction of new paediatric FDCs in Spain once they complete the prequalification process. In cases in which the use of liquid formulations is not possible, the document proposes the use of crushed tablets, to be mixed with food in patients with gastrointestinal intolerance.
We believe that this document offers an unprecedented opportunity in Spain, and that it could be very useful to paediatricians and pharmacists. Strict adherence to treatment and guaranteeing the administration of optimal doses of drugs are essential to the control and eradication of tuberculosis. Achieving these goals in the paediatric population is the ultimate purpose of this document.
FundingThis project has been approved by all the undersigning societies and institutions. The project was funded by a 2013 Research Grant from the AEP.
Conflict of interestThe authors have no conflict of interest to declare.
Validation of the daily single-unit dose of isoniazid at 10mg/kg body weight in infants aged less than 3 months. Ref. PI13/01740.
Phase IIA open clinical trial assessing the absorption of an isoniazid 10mg/mL suspension for the treatment of tuberculosis infection in patients aged less than 6 years. Ref. ICI14/00228.
Roi Piñeiro Péreza–c, Begoña Santiago Garcíaa,b, Belén Rodríguez Marrodánd, Fernando Baquero Artigaoa,b, Cecilia M. Fernández-Llamazaresc,d, María Goretti López-Ramosd, Joan Vinent Genestard, David Gómez-Pastrana Durána,e, María del Carmen Dávila Pousad, Antoni Noguera Juliana,b, Cristina Calvo Reya–c, Neus Altet Gómeza,e and María José Mellado Peñaa–c
a Red Española de Estudio de la Tuberculosis Pediátrica (pTBred)
b Sociedad Española de Infectología Pediátrica (SEIP)
c Comité de Medicamentos de la Asociación Española de Pediatría (CM-AEP)
d Sociedad Española de Farmacia Hospitalaria (SEFH)
e Sociedad Española de Neumología Pediátrica (SENP)
The members of the Working Group of the Magistral Project of the pTBred are listed in Appendix A.
Please cite this article as: Piñeiro Pérez R, Santiago García B, Rodríguez Marrodán B, Baquero-Artigao F, Fernández-Llamazares CM, Goretti López-Ramos M, et al. Recomendaciones para la elaboración y administración de fármacos antituberculosos en niños. Segunda fase del Proyecto Magistral de la Red Española de Estudio de la Tuberculosis Pediátrica (pTBred). An Pediatr (Barc). 2016;85:323.