Eosinophilic oesophagitis is an emerging and chronic disorder mediated by the immune system, and is characterised by symptoms of oesophageal dysfunction and inflammation with isolated eosinophil infiltration in the oesophagus. It is more common in males and in atopic subjects, and the symptoms vary with age. In younger children, there is vomiting, abdominal pain and dietary problems, with dysphagia and food impaction in older children and adolescents. The diagnosis is based on the presence of symptoms and oesophageal inflammation with ≥15 eosinophils/high power field, and after ruling out other causes of oesophageal eosinophilia. Without treatment, the disease usually persists and can progress to fibrostenotic forms more common in adults. The treatment options included proton pump inhibitors, empirical elimination diets, and swallowed topical corticosteroids. Maintenance therapy is advisable after the induction treatment. Diet is the only treatment that is directed at the cause of the disease, on identifying the triggering food or foods. The response to the treatments requires a histological assessment due to the poor agreement between the symptoms and the oesophageal inflammation.
The practical management of Eosinophilic oesophagitis presents with challenges, due to, among other causes, the current lack of availability of specific drugs, and its approach with, occasionally complex, diet treatments. The present document, prepared by the Working Group on Eosinophilic Gastrointestinal Disorders of the Spanish Society of Paediatric Gastroenterology, Hepatology and Nutrition, has as its objective to help in the diagnostic and therapeutic approach to paediatric eosinophilic oesophagitis, based on the recent evidence-based consensus guidelines.
La esofagitis eosinofílica es una enfermedad emergente, crónica, mediada por el sistema inmune y caracterizada por síntomas de disfunción esofágica e inflamación con infiltración eosinofílica aislada en el esófago. Es más frecuente en varones y en sujetos atópicos y los síntomas varían con la edad: en niños pequeños se manifiesta con vómitos, dolor abdominal y problemas con la alimentación y en niños mayores y adolescentes con disfagia e impactación alimentaria. El diagnóstico se basa en la presencia de síntomas e inflamación esofágica con ≥ 15 eosinófilos/campo de gran aumento, tras descartar otras causas de eosinofilia esofágica. Sin tratamiento, la enfermedad suele persistir y puede evolucionar a formas fibroestenóticas más frecuentes en el adulto. Las opciones terapéuticas incluyen inhibidores de la bomba de protones, dieta de eliminación empírica y corticoides deglutidos. Tras el tratamiento de inducción es aconsejable la terapia de mantenimiento. La dieta es el único tratamiento que se dirige a la causa de la enfermedad, al identificar los alimentos desencadenantes. La respuesta a los tratamientos requiere la evaluación histológica, por la escasa concordancia entre los síntomas y la inflamación esofágica.
El manejo práctico de la esofagitis eosinofílica presenta desafíos debido, entre otras causas, a la falta de disponibilidad actual de fármacos específicos y a su abordaje con tratamientos dietéticos, en ocasiones, complejos. El presente documento, elaborado por el Grupo de Trabajo de Trastornos Gastrointestinales Eosinofílicos de la Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátricas, tiene como objetivo facilitar el abordaje diagnóstico y terapéutico de la esofagitis eosinofílica pediátrica, con base en las recientes guías de consenso basadas en la evidencia.
Eosinophilic oesophagitis (EoE) is an emerging disease. Described as a clinicopathologic syndrome in the early 1990s,1 its incidence has grown exponentially in the past few decades. In Spain, there has been an increase in the annual incidence in the paediatric age group of 19% between 2002 and 2013,2 with an incidence in 2017 of 10.6 cases per 100,000 inhabitants per year and a prevalence of 111 cases per 100,000 inhabitants.3
Eosinophilic oesophagitis is the most prevalent form of oesophagitis in the paediatric age group and is the leading cause of dysphagia and food impaction in children and adolescents. It is a chronic disease associated with significant morbidity and can have a negative impact on quality of life.
From the first consensus recommendations for the diagnosis and management of EoE published in 2007,4 new guidelines have been developed incorporating advances in diagnosis and treatment. The most recent guideline, published in 2017, was developed with evidence-based methodology and with the consensus of European societies of paediatric and adult fields of medicine.5
Eosinophilic oesophagitis is an evolving disease whose management poses challenges, among other reasons, due to the current unavailability of specific drugs for its treatment and its management with dietary approaches that are very complex in some cases.
The document presented here, developed by the Working Group on Eosinophilic Gastrointestinal Disorders of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátricas (Spanish Society of Paediatric Gastroenterology, Hepatology and Nutrition, SEGHNP), is meant to facilitate the approach to the diagnosis and management of paediatric EoE based on the most recent evidence-based consensus recommendations.
DefinitionEosinophilic oesophagitis is defined as a chronic, local immune-mediated oesophageal disease, characterised clinically by symptoms related to oesophageal dysfunction and histologically by eosinophil-predominant inflammation. It is conceived as a clinicopathological syndrome in which clinical manifestations and pathologic data should both be considered for its characterisation.5,6
PathophysiologyEosinophilic oesophagitis is caused by a non-IgE-mediated immune response to antigens mainly, but not exclusively, from food. One of its key features is impairment of the oesophageal epithelial barrier allowing penetration of food allergens, which stimulate type 2 T helper cells promoted by thymic stromal lymphopoietin (TSLP). The release of proinflammatory cytokines, such as interleukin-13 (IL-13) and eotaxin-3 (CCL26) promotes recruitment of eosinophils to the oesophageal epithelium. Eosinophils degranulate and release toxic proteins, promoting inflammation and finally fibrosis through mediators such as transforming growth factor beta (TGF-β).
There is also evidence that EoE may run in families, as 5% to 7% of paediatric patients have first-degree relatives with EoE. Several predisposing genetic variants have been identified, some of which had been previously identified in other forms of atopy (the TSLP gene and the CAPN14 gene, encoding calpain 14) and could have a synergistic effect on other, more specific variants (IL4/KIF3A, interleukin 4/kinesin family member 3A) in the development of EoE. However, evidence from epidemiological studies supports that this familial clustering mainly results from exposure to the same environmental factors, while genetic factors play a much lesser role. Recent studies suggest that certain factors in early life, such as maternal fever, preterm birth, caesarean delivery or the use of antibiotics or acid-reducing drugs in childhood are associated with an increased risk of paediatric EoE.
DiagnosisEosinophilic oesophagitis has been described in every race and continent, but there is a slight predominance of Caucasian patients. It is also more frequent in men than women (2.45: 1).
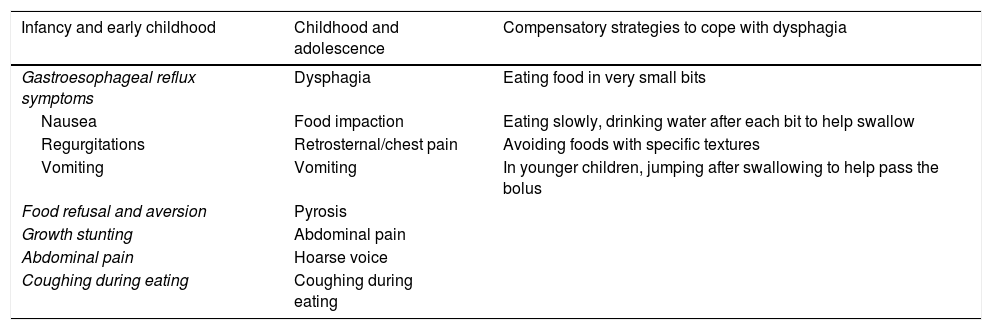
SymptomsThe clinical presentation of EoE varies depending on the age of the child and their ability to express their symptoms. A high level of suspicion for EoE is required, as patients can develop adaptive dietary behaviours (Table 1).
Symptoms suggestive of eosinophilic oesophagitis.
| Infancy and early childhood | Childhood and adolescence | Compensatory strategies to cope with dysphagia |
|---|---|---|
| Gastroesophageal reflux symptoms | Dysphagia | Eating food in very small bits |
| Nausea | Food impaction | Eating slowly, drinking water after each bit to help swallow |
| Regurgitations | Retrosternal/chest pain | Avoiding foods with specific textures |
| Vomiting | Vomiting | In younger children, jumping after swallowing to help pass the bolus |
| Food refusal and aversion | Pyrosis | |
| Growth stunting | Abdominal pain | |
| Abdominal pain | Hoarse voice | |
| Coughing during eating | Coughing during eating |
The suspicion of EoE increases with: personal history of asthma, atopic dermatitis, allergic rhinoconjunctivitis, sensitivity to air allergens, IgE-mediated food allergy, history in first-degree relative of atopy or EoE, detection of eosinophilia in peripheral blood.
In older children and adolescents, symptoms are more specific (Table 1). Food impaction may be the first symptom to develop, as solid food dysphagia may be intermittent.
Allergic comorbidities (atopic dermatitis, asthma, rhinoconjunctivitis, IgE-mediated food allergy) are more frequent in patients with EoE compared to the general population, and their presence should heighten suspicion of EoE.
EndoscopyThe upper endoscopy is the initial diagnostic test to perform when EoE is suspected. It may reveal characteristic endoscopic findings, although these are not pathognomonic.
Abnormal findings may include signs of inflammation, such as oedema (with attenuation or loss of the vascular pattern), exudates or whitish plaques or linear furrows, or signs of fibrostenosis, such as concentric rings (trachealization) and narrowing. Fibrostenotic findings are more frequent in adolescents and adults, reflecting disease progression towards oesophageal remodelling. Another common finding is a friable mucosa with a crepe paper appearance or a very narrow oesophagus.7 The grading system known as the endoscopic reference score (EREFS) has standardised the description of endoscopic findings, although studies analysing the concordance of this score with histological findings have yielded contradictory results.8
The mucosa may appear normal in 10% to 30% of cases, so it is essential to obtain oesophageal biopsy samples in all patients with suspected EoE.
HistologyThe gold-standard for diagnosis is evidence of elevated eosinophil counts in biopsy samples of oesophageal mucosa. Consensus guidelines have established a count of at least 15 eosinophils per high power field (eos/HPF) for diagnosis of EoE. Other characteristic histological findings are eosinophil sheets, eosinophil microabscesses, basal zone hyperplasia, intercellular oedema or spongiosis, eosinophil degranulation and lengthening of papillae in the squamous epithelium.
The eosinophilic infiltration of the oesophagus may be patchy, which calls for the collection of biopsy samples from both the proximal and distal oesophagus (at least 6 in total) to improve the diagnostic yield.5,6 At the time of diagnosis, the presence of eosinophilic infiltration in the stomach and duodenum must be ruled out by examination of biopsy samples.
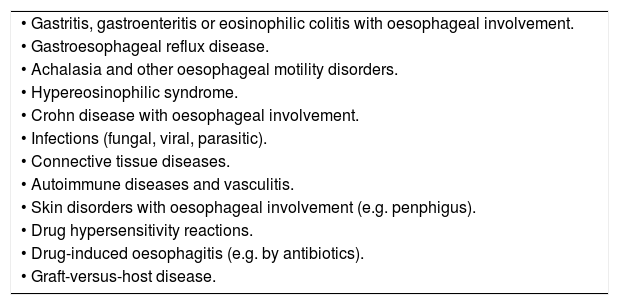
Diagnostic criteriaDiagnosis of EoE requires fulfilment of all of the following criteria:
- (a)
Symptoms of oesophageal dysfunction,
- (b)
Eosinophilic oesophageal inflammation with ≥15 eos/HPF, with extension limited to the oesophagus, and
- (c)
Ruling out other possible causes of oesophageal eosinophilia (Table 2).
Table 2.Diseases associated with eosinophilic oesophagitis.
• Gastritis, gastroenteritis or eosinophilic colitis with oesophageal involvement. • Gastroesophageal reflux disease. • Achalasia and other oesophageal motility disorders. • Hypereosinophilic syndrome. • Crohn disease with oesophageal involvement. • Infections (fungal, viral, parasitic). • Connective tissue diseases. • Autoimmune diseases and vasculitis. • Skin disorders with oesophageal involvement (e.g. penphigus). • Drug hypersensitivity reactions. • Drug-induced oesophagitis (e.g. by antibiotics). • Graft-versus-host disease.
Recent consensus guidelines have identified proton pump inhibitors (PPIs) as one possible treatment of EoE.5,6 Therefore, these agents are no longer considered to play a role in the diagnostic process, and the term PPI-responsive oesophageal eosinophilia is no longer used, as the patients it refers have the same clinical, endoscopic, histological and genetic characteristics of patients that do not respond to this therapy.
Natural historyWhen untreated, EoE typically manifests with persistent inflammation and may progress to oesophageal remodelling with stenosis, more frequently found in adult patients.9 Although there may be different phenotypes with different courses of disease, no clinical, endoscopic or histological criteria are available to identify them.
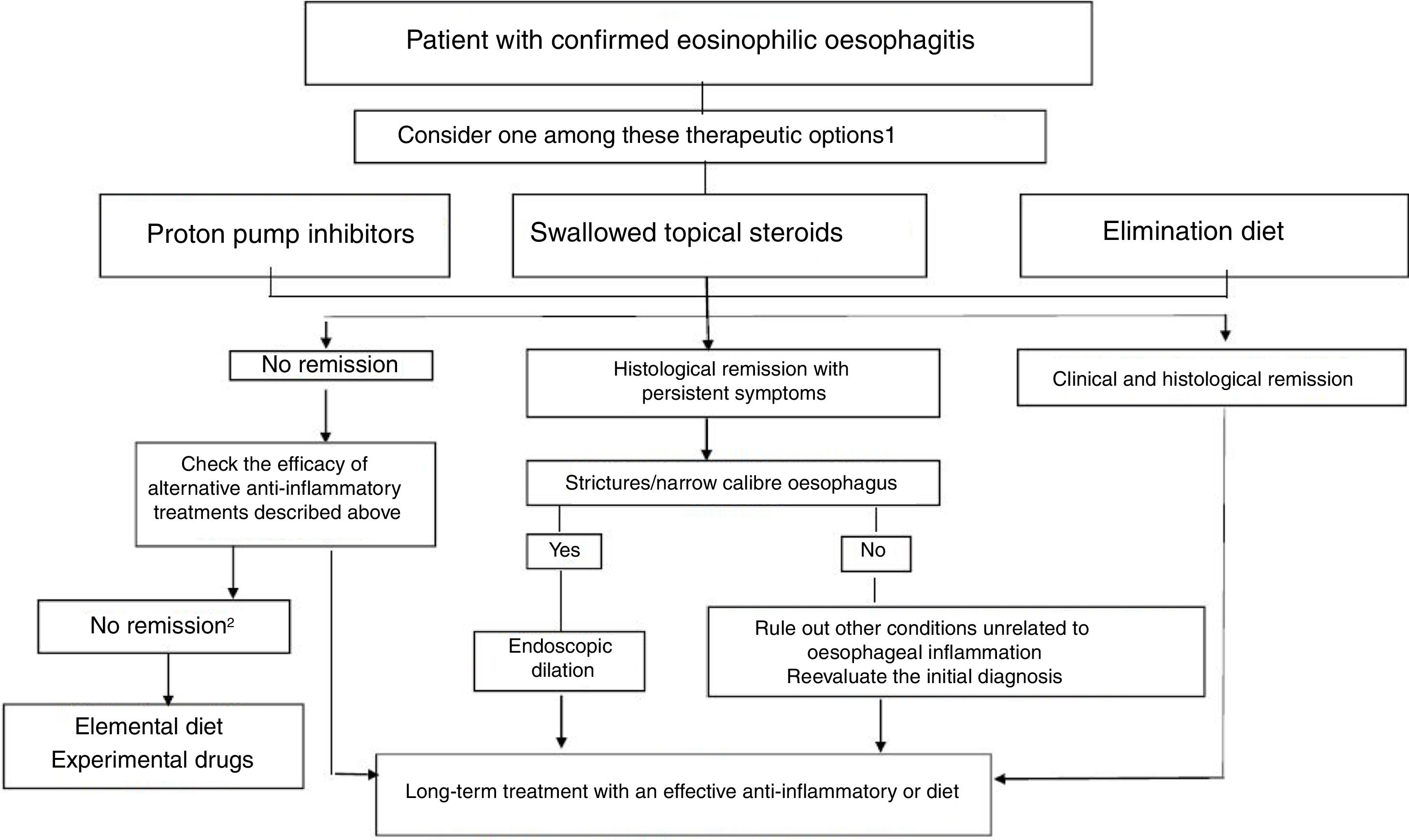
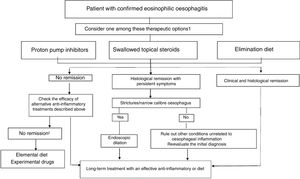
TreatmentThe goals of treatment are symptom alleviation or resolution and histological remission for prevention of fibrostenotic complications. Based on the most recent consensus guidelines, both PPIs and elimination diets can be considered first-line treatments (Fig. 1).5 Treatment must be individualised. Following the induction phase, which lasts 8 to 12 weeks, the patient must undergo an endoscopic and histological evaluation, as the correlation between symptoms and histological activity is not strong.
Therapeutic algorithm proposed for eosinophilic esophagitis. (Source: Lucendo A et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5:335–58). 1 In patienteswith persistent symtoms under anti-inflammatory therapy, endoscopic dilation should be considered. 2 Refer the patient to an EoE center.
After this initial phase, maintenance therapy is usually necessary, and it is particularly advisable in patients that experience recurring symptoms quickly or with a history of food impaction or oesophageal stenosis.
Proton pump inhibitorsIn addition to inhibiting the secretion of acid, PPIs have an anti-inflammatory effect on the oesophagus, reducing cytokine levels after Th2 activation. It has been demonstrated that PPIs block binding of the signal transducer and activator of transcription STAT6 to the eotaxin-3 promoter, decreasing the translation of the protein,10 restoring the integrity of the damaged oesophageal mucosa in these patients11 and reversing the inflammatory transcriptome.12
A systematic review found a 50% rate of histological remission following PPI therapy in children and adults, with no appreciable differences based on the PPI used.13 The first prospective study analysing the response to PPI therapy in the paediatric population (following treatment with esomeprazole at a dose of 1mg/kg twice a day for 8 weeks) found a 68.6% rate of remission and a clinical response rate of 80%.14 As for long-term response maintenance, the same group found a rate of histological and clinical remission of 70% in paediatric patients at 1 year of follow-up with administration of esomeprazole at a dose of 1mg/kg/day.15
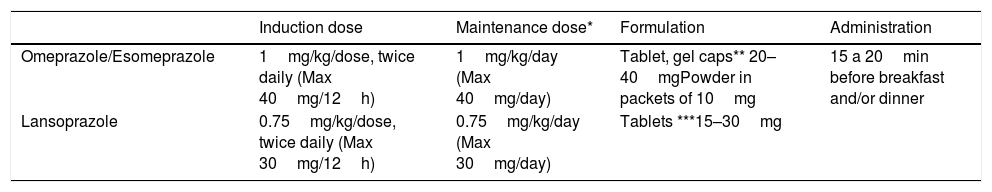
Although the optimal dose changes depending on the drug (Table 3), in general, administration of 1 to 2mg/kg/day divided in 2 doses is recommended for induction therapy. High doses may be associated with a greater response rate. When it comes to maintenance, a dose of 1mg/kg/day has proven efficacious, although attempts should be made to taper it down to the minimum effective dose. At present, predicting the response to PPI therapy is not possible, although recent evidence suggests that it may be associated with specific cytochrome CYP2C19 and STAT6 polymorphisms.16
Proton pump inhibitor therapy for eosinophilic oesophagitis.
| Induction dose | Maintenance dose* | Formulation | Administration | |
|---|---|---|---|---|
| Omeprazole/Esomeprazole | 1mg/kg/dose, twice daily (Max 40mg/12h) | 1mg/kg/day (Max 40mg/day) | Tablet, gel caps** 20–40mgPowder in packets of 10mg | 15 a 20min before breakfast and/or dinner |
| Lansoprazole | 0.75mg/kg/dose, twice daily (Max 30mg/12h) | 0.75mg/kg/day (Max 30mg/day) | Tablets ***15–30mg |
As for the long-term safety of PPI therapy, studies in adults have demonstrated that it is safe for use between 5 and 12 years,17 but there are few data on the paediatric population. Nevertheless, side effects tend to be mild, infrequent and transient and mainly consist of headache, diarrhoea and in some cases urticaria. There is evidence suggesting that prolonged use of PPIs may increase the risk of gastrointestinal and respiratory infections and iron deficiency.
Proton pump inhibitors tend to be less effective than swallowed steroids, but due to their easy administration and favourable safety profile they are a good option for treatment of paediatric EoE.
Dietary treatmentDietary treatment is the sole approach that targets the cause of the disease. Its ultimate goal is to identify which food or foods trigger oesophageal inflammation and to eliminate them from the diet in the long term. The strategy includes the induction of clinical and histological remission with an initial diet that is more restrictive, followed by sequential reintroduction of eliminated foods with clinical and endoscopic evaluations to identify the culprit food or foods. The advantage of dietary treatment is that it can achieve long-term remission while avoiding the potential adverse effects of pharmacotherapy. However, it is important to consider that it may have a negative impact on quality of life,18 although to date no studies have been published with the primary objective of assessing quality of life throughout dietary treatment.
The viability of dietary treatment must be assessed in patients who already are on restrictive diets for management of IgE-mediated food allergy. Dietary treatment requires patients and families to be motivated, and adherence to it is poorer in the adolescent population.
Elemental dietConsists in avoiding all foods and substituting a nutritionally complete formula with free amino acids. It achieves histological remission in up to 90% of patients with EoE.19 Its poor palatability, which often requires the use of a nasogastric tube, low adherence to treatment, the considerable difficulty in reintroducing foods (which requires multiple endoscopies), the psychological and social impact and its high cost are factors that substantially restrict its prescription.
Th elemental diet is recommended for only short periods of time, in young children with EoE and an important nutritional impact and/or very severe symptoms that preclude a natural diet.
Allergy-test-guided dietIt consists in eliminating from the diet foods for which the patient has a positive allergy test result (skin prick, CAP-RAST and/or patch test). It is the dietary strategy that generally has the lowest documented efficacy, with only approximately 45% of patients responding to it, and a high variability in the results reported by different centres.19 The results of available allergy tests have exhibited very poor agreement with the foods identified with the method of elimination and reintroduction combined with histological evaluations,20 as reported in a document developed by members of the European Academy of Allergy and Clinical Immunology (EAACI).21 For this reason, food allergy test-guided elimination diets are not recommended for treatment of paediatric EoE.
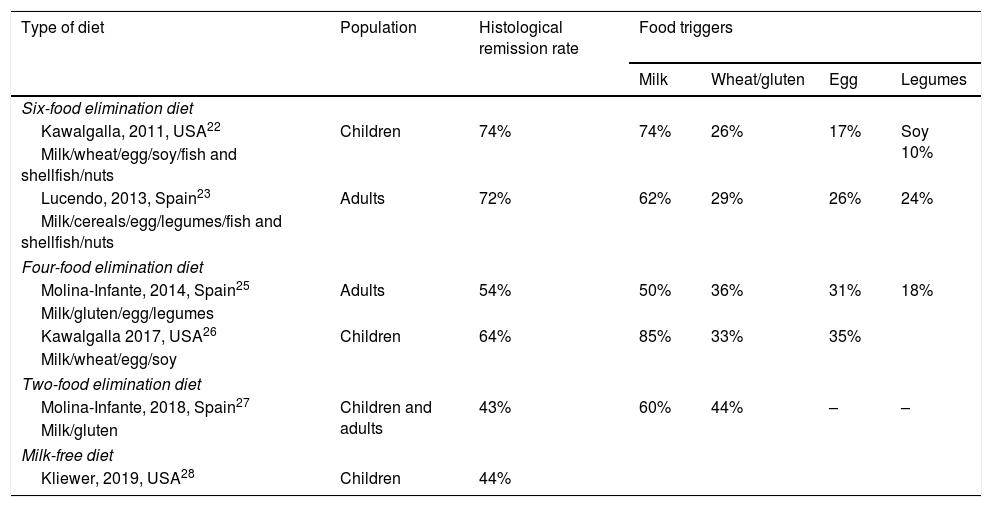
Empiric dietsIn this approach, the foods most commonly associated with EoE are eliminated regardless of food allergy tests. A research group in the United States was the first to try an empiric diet with elimination of 6 food groups (cow's milk, egg, wheat, soy, fish-shellfish and nuts), achieving a histological remission rate of 74% (Table 4).22 These outcomes were replicated in studies conducted in Spain.19,23
Efficacy of empiric diets used for treatment of eosinophilic oesophagitis in prospective studies.
| Type of diet | Population | Histological remission rate | Food triggers | |||
|---|---|---|---|---|---|---|
| Milk | Wheat/gluten | Egg | Legumes | |||
| Six-food elimination diet | ||||||
| Kawalgalla, 2011, USA22 | Children | 74% | 74% | 26% | 17% | Soy 10% |
| Milk/wheat/egg/soy/fish and shellfish/nuts | ||||||
| Lucendo, 2013, Spain23 | Adults | 72% | 62% | 29% | 26% | 24% |
| Milk/cereals/egg/legumes/fish and shellfish/nuts | ||||||
| Four-food elimination diet | ||||||
| Molina-Infante, 2014, Spain25 | Adults | 54% | 50% | 36% | 31% | 18% |
| Milk/gluten/egg/legumes | ||||||
| Kawalgalla 2017, USA26 | Children | 64% | 85% | 33% | 35% | |
| Milk/wheat/egg/soy | ||||||
| Two-food elimination diet | ||||||
| Molina-Infante, 2018, Spain27 | Children and adults | 43% | 60% | 44% | – | – |
| Milk/gluten | ||||||
| Milk-free diet | ||||||
| Kliewer, 2019, USA28 | Children | 44% | ||||
The sequential reintroduction of each of the 6 eliminated food groups accompanied by an endoscopic and histological evaluation every 8 to 12 weeks allows the accurate identification of the foods that trigger EoE in each patient,24 but it requires imposing very restrictive diets and performance of many endoscopies. Furthermore, these studies have found that up to 75% of responders had only 1 or 2 trigger foods, most frequently cow's milk, followed by eggs or gluten and less frequently soy and legumes (Table 4). For this reason, the empiric six-food elimination diet is not currently recommended in the dietary management of EoE.
Other authors have used less restrictive diets, such as the four-food elimination diet (milk, wheat, egg and soy) with an efficacy of up to 64%.25,26 A recent study assessed the efficacy of a stepwise approach that started with the elimination of 2 foods (milk and gluten), then increased to 4 foods (milk, gluten, egg and legumes) if the patient did not respond, and finally 6 foods (further eliminating fish/shellfish and nuts), achieving remission rates in children of 40%, 57% and 76% in each respective step. Compared to the diet that eliminates all 6 food groups from the outset, this stepwise approach allowed reducing the number of endoscopies performed and the time to diagnosis by 20%. An additional advantage is that 90% of responders to an empiric diet with elimination of 2 or 4 foods only reacted to 1 or 2 food triggers. These patients are good candidates for long-term dietary therapy, unlike patients that needed to step up to the elimination of 6 foods, who reacted to multiple food triggers.27
Cow's milk is the food most frequently involved in paediatric EoE. Preliminary prospective studies evaluating the efficacy of the exclusive elimination of cow's milk have found a remission rate of 44% associated with a positive impact on quality of life.28
In the long term, a few studies have confirmed persistence of histological remission for at least 3 or 4 years without drugs, but also an attrition rate of approximately 50%.26,29
In sort, less restrictive empiric diets are the first line treatment for management of EoE, starting with the elimination of 2 foods and stepping up to elimination of 4 foods in patients that do not respond and remain motivated to identify the involved allergens.30
Safety of elimination dietsElimination diets can affect the intake of macronutrients and micronutrients. Nutritional follow-up is necessary during the process of diagnosis, especially in case of the most restrictive diets and in patients with previous elimination of several foods due to IgE-mediated allergy. Supplementation with micronutrients and/or elemental formulas is recommended in these cases to achieve the recommended dietary allowance.31,32
There have been reports of cases of moderate-to-severe IgE-mediated allergic reactions following the reintroduction of previously eliminated foods. Although these reactions are infrequent, assessment by an allergist should be considered, in addition to the controlled and safe reintroduction of foods, especially in children at high risk of atopy and after prolonged elimination.33
Appendix B (online supplemental material) details the elimination diets for the most frequent food triggers, as well as strategies to replace the eliminated foods to maintain balanced nutrition.
Swallowed topical corticosteroidsTopical steroid administration is preferred over administration of systemic steroids. Swallowed fluticasone propionate has exhibited a similar efficacy to that of oral prednisone in a randomised controlled trial in children. However, systemic steroids were significantly associated with more severe adverse events.34 The use of systemic steroids should only be considered in emergency patients with severe dysphagia and significant weight loss or severe stenosis.
Several meta-analyses have confirmed the efficacy of topical steroid therapy, with variable remission rates averaging 68% for fluticasone and 76% for budesonide.35 The agents used most frequently in clinical trials are nebulised fluticasone (suspended for inhalation in a pressurised canister) and budesonide in a viscous solution. Viscous formulations achieve higher rates of histological remission compared to aerosol formulations because they remain in contact with the oesophageal mucosa for longer periods of time, especially the distal oesophagus.36
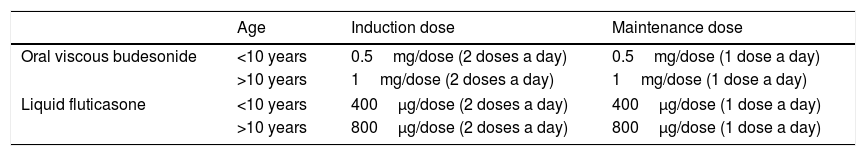
The steroids used in current clinical practice are the formulations developed for other diseases, such as asthma or rhinitis. The most widely used steroids are oral viscous budesonide and liquid fluticasone (Table 5). The first topical steroid specifically designed for EoE in adult patients has been recently authorised by the European Medicines Agency. It is budesonide prepared as orodispersible tablets containing a 1mg dose. The efficacy and safety of this formulation was proven in a randomised controlled trial in adults, which found histological remission rates of more than 90% in both the proximal and distal oesophagus.37 At present, this drug is marketed in several countries in Europe, but is not yet available in Spain.
Swallowed topical steroids for treatment of eosinophilic oesophagitis.
| Age | Induction dose | Maintenance dose | |
|---|---|---|---|
| Oral viscous budesonide | <10 years | 0.5mg/dose (2 doses a day) | 0.5mg/dose (1 dose a day) |
| >10 years | 1mg/dose (2 doses a day) | 1mg/dose (1 dose a day) | |
| Liquid fluticasone | <10 years | 400μg/dose (2 doses a day) | 400μg/dose (1 dose a day) |
| >10 years | 800μg/dose (2 doses a day) | 800μg/dose (1 dose a day) | |
2 doses a day: after breakfast and before bed.
1 dose a day: before bed.
Do not drink or eat anything, brush teeth or rinse mouth for 30 to 60min following administration.
Budesonide: avoid intake of grapefruit or grapefruit juice, as it inhibits the activity of cytochrome CYP3A required for first-pass metabolism.
Oral viscous budesonide preparation:
- -
Formulation prepared by the patient before each dose: budesonide suspension for inhalation with a nebuliser
- ∘
0.5mg dose: 1 ampoule (2mL) budesonide 0.25mg/mL mixed with 4–5g maltodextrin
- ∘
1mg dose: 1 ampoule (2mL) budesonide 0.5mg/mL mixed with 4–5g of maltodextrin
- ∘
- -
Magistral formula prepared in the pharmacy with a concentration of 0.2mg/mL or 0.5mg/mL.
Oral liquid fluticasone:
Nasal ampoules of 400μg fluticasone administered orally (it is best to release the contents of the ampoule at the base of the tongue to improve delivery of the drug to the oesophagus).
The recommended induction dose depends on the age of the child (<10 years or ≥10 years) and the steroid used (Table 5).
In patients that initially respond to topical steroids, long-term treatment is effective in maintaining remission in a proportion of them. A prospective study in children that responded to nebulised fluticasone found that long-term administration of doses similar to those used in the induction phase achieved sustained remission in 63% of the patients at 2 years of follow-up.38 In patients that respond to induction therapy, maintenance therapy with half the initial dose is recommended (Table 5).
Treatment with topical steroids appears to be safe and not cause adverse events. Most clinical trials have not found differences compared to placebo in the incidence of adverse events with the exception of oesophageal candidiasis, observed in 5% to 26% of the patients,36,39 most frequently asymptomatic and a chance finding in the follow-up endoscopic examinations.
There is little evidence on the risk of adrenal suppression induced by long-term topical steroid therapy in children. Most of the data is from observational studies with small sample sizes.40 Until more information is available, it may be advisable to monitor cortisol levels to prevent adrenal insufficiency in children with EoE if they are given high doses of topical steroids for long periods of time or receive concomitant steroid therapy through other routes (oral, inhaled or nasal).5
Oesophageal dilationIn paediatric patients, stenosis is usually the product of inflammation and responds to anti-inflammatory therapy, so dilation is reserved for very select cases with severe oesophageal stenosis that persists after this treatment.
Other treatmentsThere is a dearth of data on the management of patients refractory to the three treatments described above. Combination therapy with PPIs and steroids may be useful in some cases, although there is little evidence on the subject. Different studies have analysed the efficacy of the monoclonal antibodies anti-IL 5 (mepolizumab and reslizumab), anti-IL13 and anti-IL 4 receptor α (dupilumab) in children and adults with EoE, and dupilumab has proven most efficacious. Biological therapy may be considered in cases of refractory EoE.
Members of the Working Group on Eosinophilic Gastrointestinal Disorders of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátricas (SEGHNP):
Carmen Alonso Vicente. Hospital Clínico Universitario de Valladolid
Marina Álvarez Beltrán. Hospital Universitario Maternoinfantil Vall d’ Hebron. Barcelona
Josefa Barrio Torres. Hospital Universitario de Fuenlabrada. Madrid
Patricia Barros García. Complejo Hospitalario Universitario de Cáceres.
Gemma Colomé Rivero. Hospital de Nens de Barcelona
Francisco Javier Eizaguirre Arocena. Hospital Universitario Donostia. San Sebastian
Beatriz Fernández Caamaño. Hospital Alvaro Cunqueiro. Vigo
Enrique La Orden Izquierdo. Hospital Universitario Infanta Elena. Valdemoro, Madrid
Rosaura Leis Trabazo. Hospital Clínico Universitario de Santiago-USC
Helena Lorenzo Garrido. Hospital Universitario Basurto. Bilbao
Enrique Medina Benítez. Hospital Universitario Doce de Octubre. Madrid
Montserrat Montraveta Querol. Hospital Germans Trias i Pujol. Badalona.
Raquel Vecino López. Hospital Clínico Universitario. Madrid
Appendix A details the members of the Working Group on Eosinophilic Gastrointestinal Disorders of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátricas (SEGHNP).
Please cite this article as: Gutiérrez Junquera C, Fernández Fernández S, Domínguez-Ortega G, Vila Miravet V, García Puig R, García Romero R, et al. Recomendaciones para el diagnóstico y manejo práctico de la esofagitis eosinofílica pediátrica: Diagnóstico y tratamiento de la esofagitis eosinofílica pediátrica. An Pediatr (Barc). 2020;92:379.