No studies have analysed the effectiveness of treatment for constipation in critically ill children. The aim of this study was to assess the implementation, efficacy and safety of a treatment protocol using polyethylene glycol 3350 with electrolytes (PEG 3350 + E) for constipation in critically ill children.
MethodsWe conducted a single-centre prospective study in children admitted to the paediatric intensive care unit for a minimum of 72 h and who developed constipation. Children with previous gastrointestinal disorders or diseases were excluded. The patients were treated with rectal enemas or with the oral PEG 3350 + E protocol at the discretion of the treating physician. We compared clinical and demographic variables as well as adverse events (diarrhoea, abdominal distension and electrolyte imbalances).
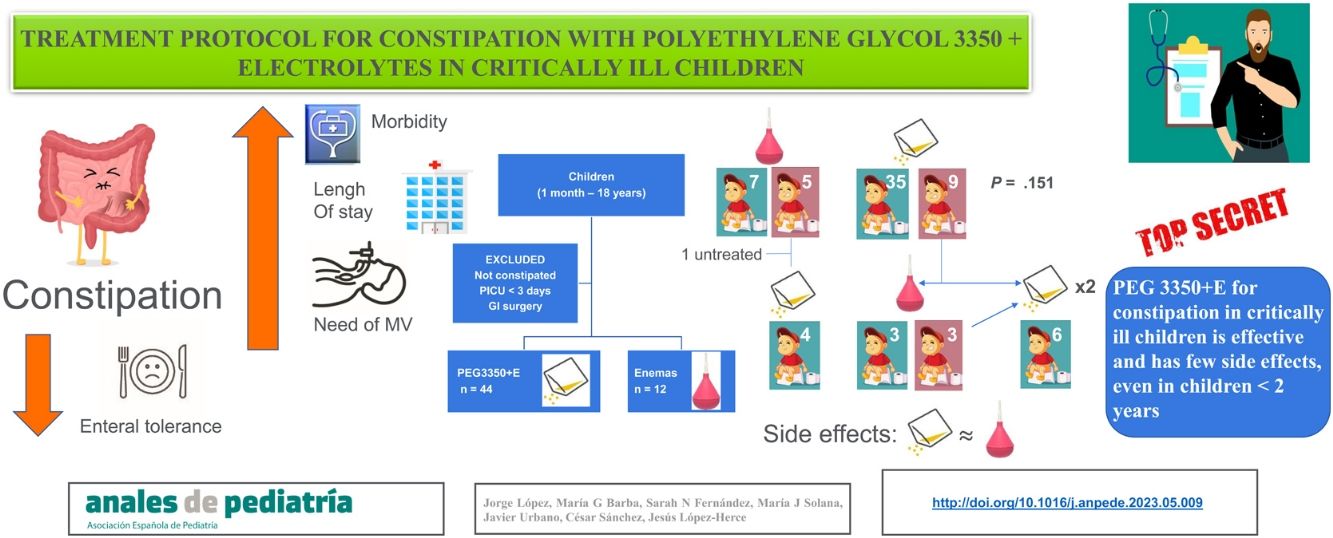
ResultsThe sample included 56 patients with a mean age of 48.2 ± 11.9 months, of who 55.4% were male. Forty-four patients (78.6%) were treated with PEG 3350 + E and 12 (21.4%) with rectal enemas. The proportion of patients that responded well to treatment was greater in the PEG 3350 + E group (79.5%) compared to the enema group (58.3%), but the difference was not statistically significant (P = .151). There were no significant differences between the groups in any of the adverse effects. Treatment with PEG 3350 + E was more effective in children aged less than 2 years (100%) compared to older children (100% vs 65.4%; P < .01), with no significant differences in the development of adverse events.
ConclusionsThe PEG 3350 + E treatment protocol for constipation in critically ill children was effective and associated with few adverse events, even in children aged less than 2 years.
Ningún estudio ha analizado la efectividad del tratamiento del estreñimiento en niños críticamente enfermos. El objetivo de este estudio fue evaluar la implementación, eficacia y seguridad de un protocolo de tratamiento con polietilenglicol 3350 con electrolitos (PEG 3350 + E) para el estreñimiento en niños en estado crítico.
MétodosEstudio prospectivo unicéntrico, incluyendo niños que ingresaron en Cuidados Intensivos Pediátricos durante más de 72 horas y que desarrollaron estreñimiento. Se excluyeron los niños con trastornos o patologías gastrointestinales previas. Los pacientes fueron tratados con enemas rectales o con PEG 3350 + E oral a criterio del médico tratante. Se compararon variables clínicas, demográficas y efectos secundarios (diarrea, distensión abdominal y desequilibrio electrolítico).
ResultadosSe estudiaron 56 pacientes de 48,2 ± 11,9 meses de edad, siendo el 55,4% varones. Cuarenta y cuatro pacientes (78,6%) fueron tratados con PEG 3350 + E y 12 pacientes (21,4%) con enemas rectales. El porcentaje de efectividad del PEG 3350 + E (79,5%) fue mayor que el de los enemas (58,3%), pero la diferencia no fue estadísticamente significativa (p = 0,151). No existieron diferencias significativas en ninguno de los efectos secundarios entre los 2 grupos. El PEG 3350 + E fue más efectivo en los niños menores de 2 años (100%) que en los mayores de esa edad (65,4%), p < 0,01, sin diferencias significativas en la aparición de efectos secundarios.
ConclusionesEl tratamiento del estreñimiento en los niños en estado crítico con PEG 3350 + E es eficaz y tiene pocos efectos secundarios, incluso en niños menores de 2 años.
Constipation is a common complication that has not been investigated in depth in critically ill patients. It is associated with an increase in morbidity, a reduction in enteral tolerance and an increase in the duration of mechanical ventilation (MV) and in the length of stay in the intensive care unit.1–3 In recent years, various studies in adult patients have highlighted the importance of establishing protocols for the prevention and treatment of constipation.4–7
Constipation is very common in children. The ROME IV criteria and the joint guidelines of the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and the North American Society For Pediatric Gastroenterology, Hepatology & Nutrition (NASPGHAN) have focused on functional constipation,8–10 but only a few studies have been devoted to constipation in critically ill children, and none of them analysed the usefulness of diagnostic and treatment protocols.3,11 Moreover, one of the main barriers to the performance of studies on constipation in critically ill patients, whether adult or paediatric, is that there is still no established definition for acute constipation.1,3
In critically ill adults, constipation is managed with the same pharmaceuticals used for functional constipation. The most widely used treatments are polyethylene glycol (PEG), lactulose, sennosides, enemas and mineral oils.6,7,12,13 In the case of opioid-induced constipation, peripherally-acting mu receptor antagonists (methylnaltrexone and naloxegol), mu and kappa receptor antagonists (axelopran) and delta receptor antagonists (bevenopran) have been used in recent years, as well as serotonin agonists such as prucalopride.14–16 These treatments have been tested thoroughly in critically ill adults.6,7 but no clinical trials have analysed the efficacy and safety of these drugs in critically ill children; only a few studies have been published, and they included few cases.17 Neostigmine, an antagonist of neuromuscular blockade agents, has also been used in some patients to reverse the effects of drugs that decrease intestinal motility.18,19
Polyethylene glycol, also known as macrogol, is a molecule that cannot be absorbed or metabolised in the intestine but can draw water towards the intestinal lumen, making the faeces more liquid and easier to expel.20–22 Alone or combined with electrolytes, PEG is the most effective drug for disimpaction and maintenance treatment for constipation,14,23–26 and there are no differences between the two formulations available for adults.13 Results in children have been similar,27 although comparator studies in the paediatric population are few and of low quality.28,29
The aim of our study was to assess the implementation, effectiveness and safety of a treatment protocol for constipation using polyethylene glycol 3350 plus electrolytes (PEG 3350 + E) in critically ill children.
Patients and methodsAfter designing a treatment protocol for constipation in critically ill children using PEG 3350 + E, we conducted an observational prospective pilot study to evaluate its implementation and efficacy over 1 year. It was conducted in a paediatric intensive care unit (PICU) with 11 beds that manages 450 admissions per year, of which approximately 65% correspond to postoperative patients. The study was approved by the Clinical Research Ethics Committee of our hospital.
The study included patients aged 1 month to 18 years with constipation who were admitted to the PICU for over 72 h and whose parents or guardians signed the informed consent form. The exclusion criteria were a length of stay in the PICU of less than 72 h and the presence of gastrointestinal disorders or conditions before admission that could involve acute changes in bowel transit, such as abdominal surgery or paralytic ileus. Functional constipation was not an exclusion criterion. Constipation was defined as the absence of bowel movements for more than 3 days after admission to the PICU in patients who received total enteral nutrition (EN) for at least the preceding 24 h.3 Total EN was defined as the patient receiving more than 80% of their daily energy requirements (total kcal/kg/day), calculated by the Schofield equation, via the enteral route.
Treatment of constipationConstipation was treated with rectal enemas or with oral PEG 3350 + E as prescribed by the physician in charge of the patient. The first-line drug was selected based on the judgment of the treating physician. If the patient did not respond, the treating physician could switch to the other available treatment option (Fig. 1). The option of oral PEG without electrolytes was not contemplated in this study because it is not available at our hospital.
Rectal enemas consisted in the administration of warm saline solution (Grifols 0.9% physiological saline solution; Laboratorios Grifols SA, Barcelona, Spain), according to the customary management of constipation for children of any age. Other options, such as docusate enemas, are not recommended in Spain for children aged less than 6 years, while the use of phosphate enemas is discouraged due to the risk of hyperphosphataemia, especially in younger children. The administered volume was 5 mL/kg (to a maximum of 250 mL/enema). The daily number of enemas depended on their efficacy, with a maximum of 2 enemas within 6 h. The enema was considered effective if a bowel movement occurred within 2 h of its delivery. This therapeutic option was considered ineffective if none of the enemas achieved a bowel movement within 24 h of treatment.
The PEG 3350 + E solution was prepared by diluting a sachet of Movicol® paediatric (Norgine Pharma, Rueil Malmaison Cedex, France) containing 6.9 g of PEG 3350 + E in 20 mL of water for children weighing less than 25 kg or one sachet of Movicol® (Norgine Pharma, Rueil Malmaison Cedex, France) containing 13.8 g of PEG 3350 + E in 40 mL of water for those weighing 25 kg or more. This dilution (concentration of 0.345 g/mL), made with a smaller volume of water than recommended in the summary of product characteristics (one sachet in 62.5 mL),30 was administered at a rate of 1 mL/kg every 8 h to start (1.035 g/kg/day). Treatment with PEG 3350 + E was considered effective if a bowel movement occurred within 48 h from initiation. If it was ineffective, the dose could be doubled (2 mL/kg every 8 h) up to a maximum of 100 mL/8 h in children weighing 25 kg or more (7.5 sachets/day). If the patient had a bowel movement within the first 48 h of treatment, the dose was reduced to 1 mL/kg/day (0.345 g/kg/day). If the patient continued to have a bowel movement at least once every 48 h, the interval between doses was increased to 1 mL/kg/48 h. Treatment was discontinued once the patient had had at least 1 bowel movement every 48 h over 4 consecutive days.
Data collectionWe collected the following data: age, sex, body weight, faecal continence (defined as the voluntary passage of stool from the rectum through the anus without episodes of unintentional leakage of stool), diagnosis, clinical severity scores (Pediatric Risk of Mortality III [PRISM III], Pediatric Index of Mortality 2 [PIM 2] and Pediatric Logistic Organ Dysfunction [PELOD]),31–33 need of continuous renal replacement therapy (CRRT), extracorporeal membrane oxygenation (ECMO), treatment with opiates or vasoconstrictors (adrenalin and/or noradrenalin), MV, length of stay in the PICU and mortality.
During the PICU stay, and for a maximum of 10 days after treatment initiation, we documented the number and characteristics of bowel movements, the presence of diarrhoea and abdominal distension and electrolyte levels (sodium, potassium, chlorine, calcium, phosphorus and magnesium) to assess for potential adverse effects of the treatment. Diarrhoea was defined as more than 8 liquid bowel movements in infants aged up to 3 months, more than 4 liquid bowel movements in infants aged 3–12 months and more than 2 liquid bowel movements in children aged 12 or more months.
Statistical analysisWe analysed the efficacy of treatment and the development of adverse events in the subsets of patients aged less than 24 months and treated with PEG 3350 + E, as the drug was not approved for children aged 12–24 months of age in the summary of product characteristics30 until April 2021.
The statistical analysis was performed with the statistical package SPSS, version 21.0 (IBM SPSS Statistics, Chicago, USA). We used the Kolmogorov–Smirnov test to assess whether the data followed a normal distribution. We have summarised quantitative variables as mean ± 2 standard deviations or median and interquartile range, as applicable, and qualitative variables absolute frequencies and percentages. We compared quantitative data with the Student t-test if they were normally distributed, and otherwise with the Mood’s median test, and compared qualitative variables with the χ2 test or the Fisher exact test as applicable. Since the study was not randomised or controlled, we fitted logistic regression models to control for baseline characteristics that differed between groups. The results are presented as odds ratios (ORs) with the corresponding 95% confidence intervals (CIs). We defined statistical significance as a P value of less than .05.
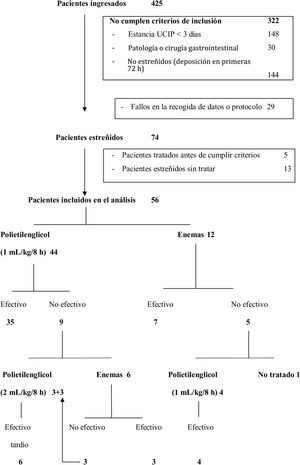
ResultsOf the 425 patients admitted over 1 year, 74 (17.4%) met the inclusion criteria, but only 56 (13.2%) were included in the final analysis. Five of the 74 potentially eligible patients (6.7%) were excluded because they had needed treatment before meeting the diagnostic criteria for constipation (3 with enemas and 2 with PEG 3350 + E), which was effective in all of them. Another 13 patients (17.5%) did not receive treatment despite meeting the diagnostic criteria for constipation. Of these 13 patients, 11 (84.6%) were discharged from the PICU and 2 (15.4%) died without treatment and before having a bowel movement, with a median length of stay in the PICU of 3 days (3–6) (Fig. 1).
The 56 patients included in the analysis had a mean age of 48.2 ± 11.9 months, and 31 (55.4%) were male. Thirty children (53.6%) had faecal incontinence. Forty-three (76.8%) were admitted for postoperative care after surgery. The reasons for admission were: postoperative care after heart surgery, 40 patients (71.4%); heart failure, 7 (12.5%); neurological disease, 4 (7.1%); respiratory disease, 2 (3.6%); septic shock, 1 (1.8%); haemolytic-uraemic syndrome, 1 (1.8%); cardiac arrest, 1 (1.8%).
The mortality risk scores at admission were: PRISM III, 11.3% (5.4–32.2); PIM 2, 28.7% (2.5–20.1); PELOD, 1.7% (1−20.8). Forty-three patients (76.8%) required MV for a median duration of 8 days (3–10), 52 (92.9%) required opiates and 29 (51.8%) required vasoactive support with adrenalin or noradrenalin. Eleven patients (19.6%) required CRRT and 9 (16.1%) required ECMO. The median length of stay in the PICU was 16 days (10.2−30.2). The mortality in the sample was 7.1% (4 patients). Enteral nutrition was initiated in the first 48 h in the PICU in 54 patients (96.4%), and the remaining 2 patients (3.6%) received parenteral nutrition for the first 48 h, followed by initiation of EN.
The initial treatment was PEG 3350 + E in 44 patients (78.6%) and enemas in the remaining 12 (21.4%) (Fig. 1). There were no differences in the baseline characteristics of the 2 groups, except in age and weight. Patients treated with PEG 3350 + E were older and had a greater body weight compared to those treated with enemas (Table 1). Treatment with PEG 3350 + E was effective in a greater proportion of patients (35/44, 79.5%) compared to enemas (7/12, 58.3%), but the difference was not statistically significant (P = .151).
Comparison of patient characteristics in each treatment group.
| Variable | PEG 3350 + E | Enemas | P |
|---|---|---|---|
| Demographic and clinical characteristics at admission | |||
| Age (months) | 55.2 ± 13.9 | 22.3 ± 14.9 | .003 |
| Weight (kg) | 18.1 ± 3.9 | 9.9 ± 3.9 | .039 |
| Male | 26/44 (59.1%) | 5/12 (41.7%) | .282 |
| Continence | 26/44 (59.1%) | 4/12 (33.3%) | .113 |
| Previous constipation | 13/44 (29.5%) | 1/12 (8.3%) | .258 |
| Postoperative | 34/44 (77.3%) | 9/12 (75%) | 1 |
| Severity | |||
| PRISM III (%) | 10.8 (3.3−29) | 18.5 (6.1−38.6) | .499 |
| PIM2 (%) | 9.6 (3.1−19.2) | 4 (1.8−20.4) | .544 |
| PELOD (%) | 1.7 (1−20.8) | 1.7 (1.3−20.8) | .878 |
| CRRT | 9/44 (20.5%) | 2/12 (16.7%) | 1 |
| ECMO | 6/44 (13.6%) | 3/12 (25%) | .385 |
| Vasoconstrictors (adrenalin/noradrenalin) | 21/44 (47.7%) | 8/12 (66.7%) | .244 |
| Opiates | 41/44 (93.2%) | 11/12 (91.7%) | 1 |
| Mechanical ventilation | 33/44 (75%) | 10/12 (83.3%) | .711 |
| Duration of MV (days) | 7.5 (2.2−10) | 9 (7.2−10) | .963 |
| Length of stay in PICU (days) | 16 (10.2−30.2) | 17.5 (8−29.5) | .963 |
| Mortality | 4/44 (9.1%) | 0/12 (0%) | .567 |
CRRT, continuous renal replacement techniques; ECMO, extracorporeal membrane oxygenation; MV, mechanical ventilation; PEG 3350 + E, polyethylene glycol 3350 with electrolytes; PELOD, Paediatric Logistic Organ Dysfunction; PICU, paediatric intensive care unit; PIM2, Paediatric Index of Mortality 2; PRISM III, Paediatric Risk of Mortality III.
Quantitative variables are expressed as mean ± 2 standard deviations (SD) or median (interquartile range), depending on whether they are normally distributed or not, and qualitative variables are expressed as fraction (percentage).
Significant differences are presented in boldface.
Four out of the 5 patients in whom enemas were not effective were subsequently treated with PEG 3350 + E, which was effective in all. The other patient had a spontaneous bowel movement 24 h later (Fig. 1). Of the 9 patients initially treated with PEG 3350 + E who did not respond to this treatment, 6 received enemas, which were effective in 3 (50%). The other 3 patients for whom enemas were also ineffective, and the 3 patients who were not treated with enemas after the initial failure of PEG 3350 + E, received increased doses of PEG 3350 + E, per the orders of the physician in charge, and all had bowel movements within 7–13 days of PICU admission (Fig. 1). Subsequently, all patients had adequate bowel movements (at least 1 normal bowel movement every 48 h), allowing tapering of the treatment dose.
There were no significant differences in the development of any adverse event between the 2 groups (Table 2).
Comparison of gastrointestinal adverse events and electrolyte disturbances in patients treated with polyethylene glycol and with enemas, adjusted for age.
| Variable | PEG 3350 + E | Enemas | P | aOR (95% CI) |
|---|---|---|---|---|
| Diarrhoea | 18/43 (41.9%) | 2/12 (16.7%) | .176 | 4.14 (0.76−22.59) |
| Abdominal distension | 7/44 (15.9%) | 4/12 (33.3%) | .224 | 0.36 (0.07−1.72) |
| Need to withdraw EN temporarily | 7/44 (15.9%) | 2/12 (16.7%) | 1 | 0.86 (0.14−5.3) |
| Hyponatraemia (Na < 130 mEq/L) | 3/44 (6.8%) | 1/12 (8.3%) | 1 | 1.84 (0.15−21.99) |
| Hypokalaemia (K < 3 mEq/L) | 14/44 (31.8%) | 3/12 (25%) | .738 | 1.18 (0.26−5.45) |
| Hypochloraemia (Cl < 96 mEq/L) | 5/44 (11.4%) | 2/12 (16.7%) | .635 | 0.87 (0.14−5.56) |
| Hypophosphataemiaa | 4/44 (9.1%) | 1/12 (8.3%) | 1 | 1.24 (0.11−13.44) |
| Hypomagnesaemia (Mg < 1.6 mEq/L) | 8/42 (19%) | 0/12 (0%) | .176 | b |
| Hypernatraemia (Na ≥ 150 mEq/L) | 5/44 (11.4%) | 2/12 (16.7%) | .635 | 1.03 (0.16−6.56) |
| Hyperkalaemia (K ≥ 5.5 mEq/L) | 3/44 (6.8%) | 1/12 (8.3%) | 1 | 1.66 (0.14−19.31) |
| Hypermagnesaemia (Mg ≥ 2.5 mEq/L) | 7/42 (16.7%) | 2/12 (16.7%) | 1 | 1.48 (0.25−8.86) |
aOR, odds ratio adjusted for age; CI, confidence interval; EN, enteral nutrition; PEG 3350 + E, polyethylene glycol 3350 with electrolytes.
Variables are expressed as fraction (percentage).
None of the patients had hypocalcaemia, hypercalcaemia, hyperchloraemia or hyperphosphataemia.
The percentage of patients with diarrhoea was higher in the PEG 3350 + E group (41.9% vs 16.7%; P = .176). The diarrhoea was mild in every case and none of the patients required specific treatment for it (other than reducing the PEG 3350 + E dose or the number of enemas) or changes to or withdrawal of EN. The percentage of patients with abdominal pain or distension was higher, although not significantly, in the enema group (33.3% vs 15.9%; P = .224). Abdominal distension was not significant and only required temporary withdrawal of EN in one patient treated with PEG 3350 + E. In the other patients who required temporary discontinuation of EN, constipation was not the reason for it. The incidence of electrolyte imbalance was high in both groups, but the imbalance was not severe and could be corrected with minimal adjustments to diuretic treatment and optimizing nutritional supplementation.
Children aged less than 2 years responded better to treatment with PEG 3350 + E (100%) compared to older children (65.4%) (P < .01). There were no significant differences in the incidence of adverse events, although hypernatremia and hyperkalaemia developed more frequently in children aged less than 2 years (Table 3).
Comparison by age group of treatment effectiveness and adverse events in patients treated with PEG 3350 + E.
| Variable | <2 years | ≥2 years | P |
|---|---|---|---|
| Effectiveness | 18/18 (100%) | 17/26 (65.4%) | .006 |
| Diarrhoea | 8/17 (47.1%) | 10/26 (38.5%) | .576 |
| Abdominal distension | 1/18 (5.6%) | 6/26 (23.1%) | .211 |
| Need to withdraw EN temporarily | 2/18 (11.1%) | 5/26 (19.2%) | .682 |
| Hyponatraemia (Na < 130 mEq/L) | 2/18 (11.1%) | 1/26 (3.8%) | .558 |
| Hypokalaemia (K < 3 mEq/L) | 5/18 (27.8%) | 9/26 (34.6%) | .632 |
| Hypochloraemia (Cl < 96 mEq/L) | 3/18 (16.7%) | 2/26 (7.7%) | .386 |
| Hypophosphataemiaa | 2/18 (11.1%) | 2/26 (7.7%) | 1 |
| Hypomagnesaemia (Mg < 1.6 mEq/L) | 3/17 (17.6%) | 5/25 (20%) | 1 |
| Hypernatraemia (Na ≥ 150 mEq/L) | 4/18 (22.2%) | 1/26 (3.8%) | .142 |
| Hyperkalaemia (K ≥ 5.5 mEq/L) | 3/18 (16.7%) | 0/26 (0%) | .062 |
| Hypermagnesaemia (Mg ≥ 2.5 mEq/L) | 4/17 (23.5%) | 3/25 (12%) | .413 |
EN, enteral nutrition; PEG 3350 + E, polyethylene glycol 3350 with electrolytes.
Variables are expressed as fraction (percentage).
None of the patients had hypocalcaemia, hypercalcaemia, hyperchloraemia or hyperphosphataemia.
Our study is the first to evaluate the implementation of a treatment protocol for constipation in critically ill children and analyse the effectiveness and safety of PEG 3350 + E.
Early initiation of EN, together with active monitoring of bowel transit to allow early treatment, if necessary, are essential factors for preventing the side effects of constipation.3–7,11 However, of the 74 patients identified as potentially constipated, 18 (24.3%) were mismanaged with over- or undertreatment (Fig. 1), emphasising the complexity of trying to change clinical practice and the need to monitor the implementation of protocols on an ongoing basis.4,5 From our point of view, a treatment protocol for constipation makes the staff more vigilant for this problem. This would enable early initiation of treatment if necessary, which may be able to reduce the complications of constipation.
Our data shows that both PEG 3350 + E and rectal enemas helped a high percentage of critically ill children to have bowel movements. The percentage of patients who responded to PEG 3350 + E was higher compared to enemas, although the difference was not statistically significant. The number of patients treated with enemas was very low, and studies of larger scope specifically designed to compare their efficacy are required.
In children with functional constipation, PEG with or without electrolytes has been found to be more efficacious than other oral laxatives.28,29 However, in other studies enemas have shown some superiority compared to PEG, with no significant differences.20,22,34 One additional advantage of PEG is that the oral use of laxatives can be easier and more comfortable for patients and for healthcare personnel than the use of rectal enemas.
The most suitable dose of PEG for children is not known. The PEG 3350 + E dose used in our study (1 g/kg/day) was at the lower limit of the range recommended by some authors35 and lower than the dose of 1.5 g/kg/day used by Bekkali et al.20
Enemas were used in younger children, probably because the clinicians in charge felt more comfortable prescribing enemas for younger children and/or because PEG350 + E was not approved for use in infants. However, in our study we used PEG 3350 + E in children as young as 2 months (off-label),30 with superior effectiveness and no increase in the incidence of adverse events. We also used a lower volume of water than recommended in the summary of product characteristics30 because a vast majority of our patients required fluid restriction, and this does not seem to have resulted in a decreased effectiveness of PEG 3350 + E compared to previous studies.20,22,25–29
Adverse eventsIn our study, there were no serious adverse events associated with the treatment of constipation. Diarrhoea is the most common side effect of laxative drugs.20–22 The incidence of diarrhoea in patients treated with PEG 3350 + E was high (41.9%), but the diarrhoea was mild, self-limiting and did not affect enteral tolerance, as previously described in critically ill adults.6 Abdominal pain and distension have also been described with both drugs, but are more strongly associated with the use of enemas.20 In our study, abdominal distension was also found in a high proportion of patients treated with enemas (33.3%), but it was not clinically significant.
In children aged less than 2 years treated with PEG 3350 + E, the percentage of hyperkalaemia and hypernatraemia was higher, but these differences were not significant. Although we were not able to establish a clear causal relationship with the treatment, we recommend close monitoring of electrolyte levels in young critically ill children treated with PEG 3350 + E.
LimitationsThe main limitation of this study is that it was not a randomised controlled clinical trial and there was no untreated control group. Based on the results of previous studies on the impact of constipation on critically ill children,3 we did not consider it appropriate to conduct a clinical trial with an untreated control group. We therefore decided to conduct an observational pilot study to analyse the efficacy of the oral laxative protocol.
Secondly, the number of patients initially treated with enemas was too low to identify statistically significant differences between treatments, and age and weight differences may have been a source of bias in the comparison of efficacy. However, the comparison of these two treatments gives us a general idea of their efficacy and potential adverse effects.
In addition, there is no widely accepted definition of constipation in critically ill patients.3,36,37
Last of all, our study was conducted in a single centre and most of the sample consisted of postoperative, medically complex patients, and therefore other studies are required to assess the external validity of our protocol for treatment of other types of critically ill children.
ConclusionOur results show that the treatment protocol for constipation in critically ill children using PEG 3350 + E is effective and has few adverse effects, even in children aged less than 2 years. The outcomes achieved with PEG 3350 + E were not inferior to those achieved rectal enemas.
This pilot study could serve as a basis for future randomised controlled clinical trials analysing efficacy and safety in larger samples, which would allow the development of diagnostic and therapeutic protocols for management of constipation in critically ill children. Studies on the efficacy and safety of early treatment in critically ill children at high risk of constipation are also needed.
FundingThis research did not receive specific financial support from any funding agencies in the public, private or not-for-profit sectors.
The study was conducted in the framework of the Primary Care Interventions to Prevent Maternal and Child Chronic Diseases of Perinatal and Development Origin Network (RICORS) project (RD21/0012/0011), Instituto de Salud Carlos III, Madrid, Spain (without financial support from grants).
We thank José María Bellón for his invaluable help in the statistical analysis. Also, Rocío García, Andreina Ferreira and Tatiana Vilchez for their help with English-language medical editing.