Necrotising enterocolitis (NEC) is one of the most common and serious acquired bowel diseases a premature newborn can face. This meta-analysis was performed comparing different probiotic mixtures to ascertain their benefits as a routine tool for preventing necrotising enterocolitis and reducing late-onset sepsis and mortality in premature neonates of less than 1500g.
MethodsA systematic review of randomised controlled trials, between January 1980 and March 2014, on MEDLINE, the Cochrane Central Register of Controlled Trials, together with EMBASE, was carried out. Studies with infants <1500g or <34 weeks were selected, discarding those with Jadad scores lower than 4.
Results9 studies were selected for further investigation, pooling a total of 3521 newborns. Probiotics were found to reduce the NEC incidence (RR 0.39; 95% CI: 0.26–0.57) and mortality (RR 0.70; 95% CI: 0.52–0.93), with no difference to placebo regarding late-onset sepsis (RR 0.91; 95% CI: 0.78–1.06). Finally, when analysing the different strands, the use of a 2-probiotic combination (Lactobacillus acidophilus with Bifidobacterium bifidum) proved to be statistically significant in reducing all-cause mortality when compared to other probiotic combinations (RR 0.32; 95% CI: 0.15–0.66, NNT 20; 95% CI: 12–50).
ConclusionsProbiotics are a beneficial tool in the prevention of NEC and mortality in preterm neonates. Moreover, the combination of 2 probiotics (L. acidophilus with B. bifidum) seems to produce the greatest benefits. However, due to the differences in probiotic components and administration, it would be wise to perform a randomised controlled trial comparing different probiotic mixtures.
Los recién nacidos pretérminos y de muy bajo peso presentan mayor riesgo de enterocolitis necrosante (NEC) dado que su colonización a nivel intestinal se produce más tardíamente y es diferente respecto a los recién nacidos a término, además de presentar con mayor frecuencia otros factores favorecedores como isquemia intestinal. Se cree que los probióticos pueden modificar la microbiota y la respuesta immune de los recién nacidos, disminuyendo la incidencia de NEC.
ObjetivoRevisar los estudios realizados con diferentes probióticos y comparar diferentes combinaciones de éstos para ver si es beneficiosa su administración de forma rutinaria en recién nacidos pretérmino de menos de 1500g para evitar la enterocolitis necrosante, reducir la sepsis tardía y la mortalidad.
Material y MétodosSe llevó a cabo una revisión sistemática entre enero 1980 y marzo 2014, en MEDLINE, EMBASE y Cochrane Central Register of Controlled Trials. Se seleccionaron los estudios clínicos con recién nacidos prematuros de <1500g y/o <34 semanas descartando aquellos con puntuaciones de Jadad menores de 4.
ResultadosSe seleccionaron 9 estudios, de 24 pre-seleccionados, con un total de 3521 recién nacidos. Se observó que los probióticos reducen la incidencia de NEC (RR 0,39; 95% CI: 0,26-0,57) y la mortalidad (RR 0,70; 95% CI: 0,52-0,93). No se detectaron diferencias significativas con el placebo en la disminución de sepsis tardía (RR 0,91; 95% CI: 0,78-1,96). Finalmente, cuando se analizan las distintas cepas, la combinación de 2 probióticos (Lactobacillus acidophilus con Bifidobacterium bifidum) demostró reducir la mortalidad de forma significativa comparada con otras combinaciones de probióticos (RR 0,32; 95% CI: 0,15-0,66, NNT 20; 95% CI: 12-50).
ConclusionesLos probióticos son beneficiosos en cuanto a la prevención de NEC y reducen la mortalidad en pretérminos de menos de 1.500g. Además, la combinación de dos probióticos (Lactobacillus acidophilus con Bifidobacterium bifidum) presenta mayor beneficio. Dada la diferencia de composición de probióticos son necesarios estudios aleatorizados comparando diferentes combinaciones de probióticos.
Necrotising enterocolitis (NEC) is one of the most common and serious acquired bowel diseases a newborn can face. Although it is not fully understood, the cause is suspected to be a combination of vascular necrosis, bacterial overgrowth and metabolic insults to the immature gut wall. It is believed that after toxic or ischaemic damage to the mural wall, and using the substrate proceeding from enteral feedings, there is an overgrowth of bacteria, especially those generating gases such as methane or hydrogen, producing intramural gas (pneumatosis intestinalis) leading to a mural necrosis, gut gangrene and, finally, intestinal perforation with peritonitis. In recent years, there has also been a greater acceptance of the theory that the host's immune system also plays an important pathophysiological role in the development of NEC.1
NEC predominantly affects premature infants, with 70–85% of cases of NEC occurring in very low birth weight infants (<1500g) or infants younger than 32 weeks, whereas only 10–25% of cases are term infants or late preterm infants. The lower the gestational age, the higher the risk of NEC. Overall incidence of NEC has been reported as 7% in a study conducted by the National Institute of Child Health (NICHD) Neonatal Research Network (NRN) in a population of 11,072 very low birth weight infants (VLBW) for the period 1999–2001.2 Another study, between the years 2003 and 2007, found that the prevalence of NEC was about 11% in very premature infants who were born at between 22 and 28 weeks post-menstrual age and had birth weights of 401–1500g.3 The global mortality of NEC is approximately 20–40%, rising to 90–100% in the most severe cases, which represents 2–5% of all neonatal intensive care unit (NICU) admissions.4 In addition, between 27% and 63% of affected infants will undergo surgery5 and one third will have a perforated bowel, the short bowel syndrome being the most common complication.
Probiotics can be defined as live microorganisms which, when administered in adequate amounts, confer a health benefit for the host (WHO/FAO 2001). Members of the genera Lactobacillus and Bifidobacterium are the most commonly used, the Lactobacillus rhamnosus GG and Bifidobacterium Lactis being the strains with stronger evidence to support their use in humans. Although the origin of the human microbiota is yet unknown, it is important to note the differences after birth depend on the method of delivery, type of feeding during gestation, as well as the mother's microbiota.6 Healthy breast-fed infants have a higher amount of bifidobacteria whereas formula-fed infants tend to have a more varied range of microorganisms such as bifidobacteria, bacteroidetes, enterobacteria, streptococci and clostridia. The human microbiota will not be stable until the age of 2–3 years and, even then, might suffer modifications due to diet, disease, antibiotic use and ageing. In recent years, probiotics have been increasingly studied and used in clinical practice. Some examples of this are the improvement of intestinal function in cystic fibrosis, reducing bacterial overgrowth,7 or the shortening of the duration of acute gastroenteritis.8
VLBW infants with increased risk of NEC have different faecal bacteria colonisation, together with delayed onset of bacterial colonisation in comparison to normal term newborns.9 It is believed that probiotics may modify the microbiota and the immune response of the newborn, reducing the risk of NEC through different mechanisms; direct competition for nutrients and prebiotics, transforming certain elements present in the gut into inhibitory substances, producing growth factors and vitamins to promote a healthy microbiota, synthesising bacteriocins, competing for binding locus, increasing the production of IgA, increasing the immune response and reducing the inflammation when stimulating regulatory lymphocytes through IL-10 and TGFβ.10–12 Despite the potential benefits, such interventions have been delayed due to the theoretical risk of bacterial sepsis that could occur in VLBW or preterm infants, especially those with extremely low birth weight (<1000g). However, there is little data to support this concern. In addition, infants exposed to a NICU undergo a wide range of procedures that may modify their microbiota: antibiotic use, diet modifications, catheterisation, etc.
The use of probiotics has been analysed and studied by different scientific societies. An example would be the Spanish Society of Neonatology (SENeo), which provides the following reccommendations13:
- -
In situations with high NEC incidence, it is recommended to provide probiotic supplementation. Grade 1+.
- -
Probiotic use should be considered in neonates under 32 weeks or less than 1500g, including less than 1000g newborns. Good ethics practice.
- -
Probiotic use requires a strict monitoring. Good ethics practice.
- -
Evidence suggests the use of species from the Lactobacillus and Bifidobacterium genres, due to their effect in reducing NEC, although the species, dose and duration must be adapted according to each case. Good ethics practice.
- -
Start the administration as soon as possible and maintain it 6 weeks or until discharge. Good ethics practice.
- -
Stop probiotic supplementation in case of NEC or gastrointestinal disease. Good ethics practice.
Up to the present, there have been few studies comparing the effects of specific strands of probiotics, alone or in combination, on NEC, late-onset sepsis and mortality. The objective of this study is to compare different probiotic mixtures to prevent necrotising enterocolitis and reduce late-onset sepsis and mortality in premature infants under 1500g.
MethodsStudy searchIn order to find the maximum number of studies connecting the use of probiotics and NEC prevention, a thorough search of MEDLINE, the Cochrane Central Register of Controlled Trials, together with EMBASE, was carried out. The MEDLINE search was performed through PubMed (www.ncbi.nlm.nih.gov/pubmed) using the MeSH terms “neonate”, “Infant, Newborn”, “probiotic” and “enterocolitis, necrotising”. EMBASE was searched using the same method as the MEDLINE database. Studies were considered from January 1980 to March 2014.
In addition, a manual search of different relevant studies and published reviews also took place.
Selection of studiesOut of all the available studies, only the randomised controlled studies that had NEC outcome, very low birth weight infants (<1500g) or <34 weeks of gestational age newborns were selected. Moreover, those that did not have a Jadad score, a study quality evaluation scale equal or higher than 4, or did not have blinded randomisation, were discarded (Table 1). Finally, of the remaining studies, those that had a control group of infants treated without probiotics and a group of neonates treated with bacterial probiotics, all mixed with donor, human or formula milk, were ultimately selected.
Jadad scale.
| Item | Characteristics | Scoring |
|---|---|---|
| Randomisation | Described | 1 point |
| Correct randomisation | 1 additional point | |
| Incorrect randomisation | 1 deducted | |
| Double blinding | Described | 1 point |
| Correct randomisation | 1 additional point | |
| Incorrect randomisation | 1 point deducted | |
| Withdrawals and dropouts | Number and reasons for withdrawal are stated | 1 point |
To reduce the risk of bias, all studies were observed using the Jadad Score, together with the standard methods of the Cochrane Collaboration and Neonatal Review Group. Information such as method of randomisation, blinding and reporting of all outcomes, as well as withdrawal and dropouts, was taken into account. If information was missing, it was extracted from pre-existing meta-analyses or further contact with the authors was established.
Statistical analysis and measurement of treatment effectAll data were analysed with standardised meta-analysis protocols. For dichotomous outcomes, the relative risk (RR) and numbers needed to treat (NNT), in conjunction with their 95% confidence intervals, were calculated. For continuous outcomes, the median difference and standard deviation were calculated. The severity of NEC was classified using the modified Bell's criteria.14 Only severe NEC, stages II and III, were considered in the analysis. Sepsis was considered when positive blood or cerebrospinal fluid cultures taken beyond 5 days of age.
When comparing the effects of the different probiotic strands, inference to 1000 neonates per group was calculated and then compared with chi-square analysis, alongside their 95% confidence intervals.
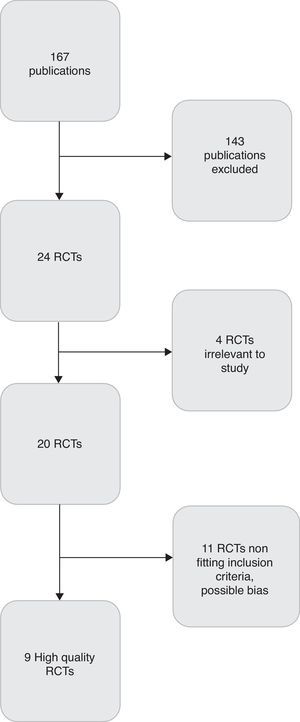
ResultsA total of 24 publications were selected to undergo further study, of which 9 were selected for their high quality and reduced risk of bias (Fig. 1). It is important to note that trials were highly variable according to their inclusion criteria, risk of NEC, sample size, as well as the dose, the type and the duration of probiotics given. The characteristics of these trials are summarised in Table 2.
Characteristics of selected studies for meta-analysis.
| Trial | Birth weight and gestational age | Probiotic agents | Dosage and duration | Type of feeding | Primary outcome |
|---|---|---|---|---|---|
| Dani et al. (2002)15 | <1500g | L. rhamnosus GG | 6×109CFU/24h until discharge | Breastfeed, formula or donor milk | UTI, sepsis, NEC |
| Lin et al. (2005)16 | <1500g | L. acidophilus and B. infantis | 125mg/kg twice daily | Breastfeed or donor milk | NEC, death |
| Lin et al. (2008)17 | <34 weeks and <1500g | L. acidophilus and B. bifidum | 2×109CFU/24h for 6 weeks | Breastfeed or formula | NEC, death |
| Manzoni et al. (2009)18 | <1500g | L. rhamnosus GG | 6×109CFU/24h for 4 weeks | Breastfeed, formula or donor milk | Late-onset sepsis |
| Rougé et al. (2009)19 | <32 weeks and <1500g | L. rhamnosus GG and B. longum | 1×109 CFU/24hours until discharge | Breastfeed, formula or donor milk | Enteral feed intake at day 14 |
| Samanta et al. (2009)20 | <34 weeks and <1500g | B. infantis, B. bifidum, L. acidophilus, B. longum | 2.5×109CFU/12h until discharge | Breastfeed | NEC, death, sepsis |
| Sari et al. (2010)21 | <33 weeks and <1500g | L. sporogenes | 3.5×108CFU/24h until discharge | Breastfeed or formula milk | NEC, death |
| Bragard et al. (2011)22 | <1500g | L. casei and B. breve | 3.5×107–3.5×109CFU/24h until infant 30 days of life | Breastfeed, formula or donor milk | NEC |
| Jacobs et al. (2013)23 | <32 weeks and <1500g | B. infantis, S. termophilus and B. lactis | 1×109CFU/24h until discharge | Breastfeed or formula milk | Late-onset sepsis |
Data on severe NEC were present in all 9 studies. There were a total of 3521 newborn infants divided into two groups: the probiotic group and the control group. In the probiotic group, there were a total of 1756 neonates whereas the control group had a total of 1765 infants. There was a statistically significant reduction in the relative risk of developing NEC in the probiotic group in comparison to the control group, RR 0.39; 95% CI: 0.26–0.57. The newborns needed to treat with probiotics to prevent 1 case of NEC were 33; 95% CI: 23–54 (Table 3).
Probiotics vs control, severe NEC (stage II-III) in <1500g infants.
| Studies | NEC probiotic | NEC control | Relative Risk |
|---|---|---|---|
| Dani et al. (2002) | 4/295 | 8/290 | 0.49 [0.15–1.61] |
| Lin et al. (2005) | 2/180 | 10/187 | 0.21 [0.05–0.94] |
| Lin et al. (2008) | 4/217 | 14/217 | 0.29 [0.10–0.85] |
| Manzoni et al. (2009) | 0/151 | 3/153 | 0.05 [0.00–0.90] |
| Rougé et al. (2009) | 2/45 | 1/49 | 2.18 [0.2–23.21] |
| Samanta et al. (2009) | 5/91 | 15/95 | 0.34 [0.13–0.92] |
| Sari et al. (2010) | 6/110 | 9/111 | 0.12 [0.01–2.19] |
| Braga et al. (2011) | 0/119 | 4/112 | 0.10 [0.01–1.92] |
| Jacobs et al. (2013) | 11/548 | 24/551 | 0.56 [0.31–0.99] |
| Global | 34/1756 | 88/1765 | 0.39 [0.26–0.57] |
| NNT | 33 [23–54] | ||
In order to study if the probiotic strand had any influence on NEC reduction, data from the Dani 2002 and Manzoni 2009 vs the Lin 2005 and Lin 2008 vs Samanta 2009 and Jacobs 2013 were analysed. In the first two, L. rhamnosus GG (Bivos®) was used, whilst in the following two, they used Lactobacillus acidophilus with Bifidobacterium bifidum (Infloran®). Finally, in the last two studies, three or more probiotics were used (Bifidobacterium infantis, Streptococcus termophilus, B. lactis, B. bifidum, Bifidobacterium longum and L. acidophilus).
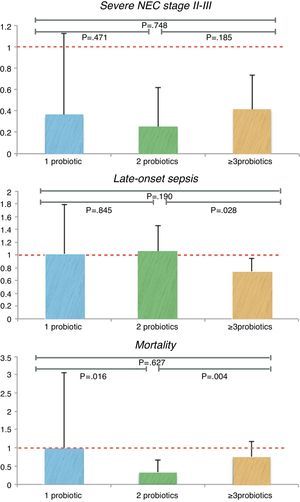
When studying the three different groups, 446 newborns were treated with Bivos®, 397 with Infloran® and 639 neonates with 3 or more probiotics. The 1st group of only 1 probiotic showed no statistically significant reduction in NEC incidence, whereas the 2nd of 2 probiotics and the 3rd of 3 or more probiotics did have a significant reduction in relative risk (Table 4). As for the NNT, in the 2nd group the NNT was the lowest of the three, NNT 22; 95% CI: 14–54, followed by the 3rd group, NNT 28; 95% CI: 17–75. Once summarised to the same number of cases, and after applying chi-square analysis, there was no statistically significant difference between them (Fig. 2).
Probiotic strand associated differences.
| Probiotic | Control | Relative risk | NNT | |
|---|---|---|---|---|
| NEC (stage II-III) | ||||
| 1 probiotic (Bivos®) | 4/446 | 11/443 | 0.36 [0.12–1.13] | – |
| 2 Probiotics (Infloran®) | 6/397 | 24/404 | 0.26 [0.11–0.62] | 22 [14–54] |
| ≥3 probiotics | 16/639 | 39/646 | 0.41 [0.23–0.73] | 28 [17–75] |
| Mortality | ||||
| 1 probiotic (Bivos®) | 6/446 | 6/443 | 0.99 [0.32–3.06] | – |
| 2 Probiotics (Infloran®) | 9/397 | 29/404 | 0.32 [0.15–0.66] | 20 [12–50] |
| ≥3 probiotics | 31/639 | 42/646 | 0.74 [0.47–1.17] | – |
| Sepsis | ||||
| 1 probiotic (Bivos®) | 21/446 | 21/443 | 0.99 [0.55–1.79] | – |
| 2 Probiotics (Infloran®) | 62/397 | 60/404 | 1.05 [0.76–1.46] | – |
| ≥3 probiotics | 85/639 | 117/646 | 0.73 [0.57–0.95] | 21 [11–119] |
In all the publications, there was data referring to sepsis studies through different cultures such as blood, LCR and peritoneal fluid, as well as bronchial secretions, if present. There were 1756 infants in the probiotic group and 1765 newborns in the control group. Amongst these patients, there was no statistically significant difference in relative risk, RR 0.91; 95% CI: 0.78–1.06(Table 5). There was a discrete advantage in using 3 or more probiotics, when compared to the other combinations, RR 0.73; 95% CI: 0.57–0.95. These differences are still present between the 2 probiotics group and the 3 or more probiotic group (p=0.028), with the chi-square analysis (Fig. 2).
Probiotic vs control culture positive sepsis.
| Studies | Sepsis probiotic | Sepsis control | Relative risk |
|---|---|---|---|
| Dani et al. (2002) | 14/295 | 12/290 | 1.15 [0.54–2.44] |
| Lin et al. (2005) | 22/180 | 36/187 | 0.63 [0.39–1.04] |
| Lin et al. (2008) | 40/217 | 24/217 | 1.67 [1.04–2.67] |
| Manzoni et al. (2009) | 7/151 | 9/153 | 0.27 [0.12–0.60] |
| Rougé et al. (2009) | 15/45 | 13/49 | 1.26 [0.67–2.34] |
| Samanta et al. (2009) | 13/91 | 28/95 | 0.48 [0.27–0.87] |
| Sari et al. (2010) | 29/110 | 26/111 | 1.13 [0.71–1.78] |
| Braga et al. (2011) | 40/119 | 42/112 | 0.90 [0.63–1.27] |
| Jacobs et al. (2013) | 72/548 | 89/551 | 0.81 [0.61–1.08] |
| Global | 252/1756 | 279/1765 | 0.91 [0.78–1.06] |
Data on all-cause mortality was recorded in 9 of the randomised controlled trials (RCTs), with a total of 3521 infants divided into a probiotic group (n=1756) and a control group (n=1765). There was also a statistically significant reduction in all-cause mortality, RR 0.70; 95%CI: 0.52–0.93. The numbers needed to treat to prevent 1 death from all-cause mortality was 53; 95% CI: 30–238.
When analysing the NEC-associated mortality, it was only reported in 4 of the 9 trials. A total of 2339 infants were studied, divided into the probiotic group (n=1170) and the control group (n=1169). In this case, the NEC-associated mortality was lower in the probiotic group and this reduction was statistically significant, RR 0.35; 95% CI: 0.14–0.89 (Table 6).
Probiotic vs control mortality.
| Studies | Death probiotic | Death control | Relative risk |
|---|---|---|---|
| All cause | |||
| Dani et al. (2002) | 0/295 | 2/290 | 0.20 [0.01–4.08] |
| Lin et al. (2005) | 7/180 | 20/187 | 0.36 [0.16–0.84] |
| Lin et al. (2008) | 2/217 | 9/217 | 0.22 [0.05–1.02] |
| Manzoni et al. (2009) | 6/151 | 4/153 | 0.56 [0.21–1.45] |
| Rougé et al. (2009) | 2/45 | 4/49 | 0.54 [0.10–2.83] |
| Samanta et al. (2009) | 4/91 | 14/95 | 0.30 [0.10–0.86] |
| Sari et al. (2010) | 3/110 | 3/111 | 0.76 [0.17–3.30] |
| Braga et al. (2011) | 26/119 | 27/112 | 0.91 [0.46–1.45] |
| Jacobs et al. (2013) | 27/548 | 28/551 | 0.97 [0.58–1.62] |
| Global | 77/1756 | 111/1765 | 0.70 [0.52–0.93] |
| NNT | 53 [30–238] | ||
| NEC mortality | |||
| Dani et al. (2002) | 0/295 | 2/290 | 0.20 [0.01–2.75] |
| Lin et al. (2008) | 2/217 | 3/217 | 0.67 [0.11–3.95] |
| Sari et al. (2010) | 0/110 | 1/111 | 0.34 [0.01–8.17] |
| Jacobs et al. (2013) | 4/548 | 11/551 | 0.37 [0.12–1.14] |
| Global | 6/1170 | 17/1169 | 0.35 [0.14–0.89] |
| NNT | 106 [58–702] | ||
Strand-associated difference was also taken into account (Table 4); the 2nd group, with 2 probiotics, had a reduction in the relative risk of mortality that was significant in comparison to the other groups, RR 0.32; 95% CI: 0.15–0.66. The newborns needed to treat for the Infloran® group were 20; 95% CI: 12–50. After equalling the numbers of cases and applying the chi-square analysis, there was a statistically significant difference between the different groups; 2 probiotics versus 1 probiotic (p=0.016) and also compared to 3 or more probiotics (p=0.004) (Fig. 2).
DiscussionAlthough there have been systematic publications and good quality reviews that sustain the correct use of probiotics for preventing NEC, probiotics have still not truly been implemented worldwide. In order to provide more data supporting the use of probiotics as a medical tool to prevent NEC, this meta-analysis was performed using only high-quality studies, based on their allocation concealments procedures and blinding of the intervention. Therefore, after making a pre-selection of 24 studies, only 9 of them where finally considered adequate for the analysis given that they had a good allocation concealment procedure, good blinding interventions and also provided information on all factors that influence the incidence of NEC.
Regarding the study selection criteria, infants less than 34 weeks or weighing less than 1500g were selected due to their increased risk of suffering from NEC. In addition, the majority of studies also contemplated these age/weight groups for the aforementioned reasons. Moreover, preterm infants will experiment a growth in number, especially in those countries where maternal age increases and with the upsurge in artificial methods of conception. Under such circumstances, it seems important to pay special attention to the group with a higher risk of NEC for which a prevention method could be very important. In term infants, or late preterm infants, the incidence of NEC is significantly lower, meaning that the costs of preventing a NEC case could be much higher than the possible risks associated with NEC. Furthermore, the use of probiotics for prevention could render no positive results. For the bias risk assessment, the Jadad score was used because it has been recognised as a good method for reducing the blinding bias, in conjunction with others, and increases the statistical quality of systematic reviews and meta-analysis.24 In addition to the use of the Jadad score, the standard methods of the Cochrane Collaboration and Neonatal Review Group were also taken into account since this institution is very well regarded for its scientific and research rigour.25
When considering the actual effect of probiotics in NEC prevention, it is clear that there is a reduction of severe NEC, consistent with many other existing meta-analyses5,26–31 and studies, as well as a reduction in mortality without any effect on late-onset sepsis: neither reduction nor increase (late-onset sepsis is the principal feared risk of giving such prophylactic measures). Although the numbers needed to treat to prevent a NEC case are relatively high, 33, and the numbers to prevent death in neonates are even higher, 53, these have to be taken into account when facing the fact that premature births are increasing, the costs of a NEC episode are high and probiotics are easy to handle in addition to having a low cost. However, since NEC has a high morbimortality, would it not be unethical to keep this inexpensive measure from newborns? In future trials, it would seem better to focus on the effect of different probiotic strands in order to identify the best combination possible for these patients. Following this direction, in the current meta-analysis, strand-associated difference was studied and noted. Although there was no difference between probiotics when it came to reducing NEC incidence, there was a clear benefit in death reduction when giving a two probiotic combination (L. acidophilus and B. bifidum), compared to three or more probiotics (B. infantis, S. termophilus, B. lactis, B. bifidum, B. longum and L. acidophilus) or just one probiotic (L. rhamnosus GG). When focusing on late-onset-sepsis, the combination of 3 or more probiotics proved to be beneficial when compared to the rest. Therefore, both the combination of 2 probiotics and 3 or more probiotics would be beneficial for the newborns, one especially for mortality and the other for late-onset sepsis.
As for the dose of probiotics, it is important to achieve an optimal mass of probiotic in order to survive and colonise the gut, proliferating in adequate amounts, to confer a health benefit. The evidence suggests this dose should be minimally 106–107 colony-forming units in each gram of product.32–34
Finally, concerning the strengths and weaknesses, this meta-analysis is the first to study the different probiotic compounds and mixtures existing and the first to find out if there is a probiotic mix or compound with superior performance when compared with the rest. However, neither the mix of probiotics nor the dose nor the duration of their administration are always the same, leading to a reduction in the certainty of the results. Moreover, in some cases, although concealment and blinding of intervention were correct, there was a substantial difference in the age at birth between groups, a fundamental factor for the risk of NEC, making it difficult to know if the reduction of relative risk was due to probiotics or simply because the maturity was different between groups. Other studies, although still correct, had poor data analysis and lacked information concerning the kind of feeding infants received, or lacked information about some risk or prevention factors concerning NEC. It is also important to note that no studies have assessed the effect of probiotics in extremely low birth weight neonates (<1000g) where the risk of late-onset sepsis could be higher.
To conclude, the use of probiotics as a method to prevent severe necrotising enterocolitis in preterm neonates has been proven, with the combination of L. acidophilus and B. bifidum emerging as the best in reducing overall cause mortality. Therefore, probiotics should be given routinely to all high-risk neonates. Where there are safety concerns, probiotics could be continued within a research framework that does not necessarily need studies with a placebo comparison.
Conflict of interestsAuthors declare no conflict of interest.
Please cite this article as: Baucells BJ, Mercadal Hally M, Álvarez Sánchez AT, Figueras Aloy J. Asociaciones de probióticos para la prevención de la enterocolitis necrosante y la reducción de la sepsis tardía y la mortalidad neonatal en recién nacidos pretérmino de menos de 1500g: Una revisión sistemática. An Pediatr (Barc). 2016;85:247–255.