There is no current data on the prevalence of urinary tract infection (UTI) in infants and toddlers with high fever. We conducted this study to assess the point prevalence of UTI in children aged less than 2 years presenting with high fever to the emergency department.
MethodsWe conducted a prospective, multicentre, observational study including febrile children aged less than 2 years in whom urinalysis was performed to rule out UTI over a 1-year period in seven paediatric emergency departments in Spain. Boys younger than 1 year and girls younger than 2 years of age were eligible for the study if they had a rectal temperature greater than 39°C, were not taking antibiotics, and there was no identifiable source of fever. The diagnosis of UTI was based on the presence of leukocyturia and positive urine culture results.
ResultsWe included a total of 1675 patients. Two hundred sixty infants (15.5%; 95% CI, 13.8–17.3%) received a diagnosis of UTI. The point prevalence of UTI was 32.9% (95% CI, 26.6–39.9%) in febrile boys aged less than 6 months and 19.3% (95% CI, 16.1–22.9%) in febrile girls aged less than 12 months. The point prevalence of UTI was 13% (95% CI, 10.8–15.6%) in children with a duration of fever of less than 24h, compared to 17.5% (95% CI, 15.2–20.1%) in those with a longer duration of fever (P=.014).
ConclusionsThe point prevalence of UTI in infants and toddlers with fever without source greater than 39°C was higher in our study compared to previous studies of UTI prevalence, especially in male infants aged less than 6 months and female infants aged less than 12 months. Our findings suggest that clinicians need to carefully assess for UTI in infants with unexplained fever greater than 39°C.
No existen datos actuales de prevalencia de infección de orina (ITU) en lactantes con fiebre elevada en nuestro medio. Se realizó este estudio para evaluar la prevalencia puntual de ITU en lactantes con fiebre elevada en urgencias.
MétodoEstudio prospectivo, multicéntrico, observacional incluyendo lactantes con fiebre a los que se realizó análisis de orina para descartar ITU en un periodo de un año en siete servicios de urgencia pediátricos españoles. Se incluyeron niños menores de un año y niñas menores de 2 años con fiebre sin focalidad > 39°C y sin antibioterapia previa. El diagnóstico de ITU se basó en la presencia de leucocituria y urocultivo positivo.
ResultadosUn total de 1675 pacientes fueron incluidos. Doscientos sesenta lactantes (15,5%, 95% IC 13,8-17,3) fueron diagnosticados de ITU. La prevalencia puntual de ITU fue 32,9% (95% IC 26,6-39,9) en niños febriles menores de 6 meses y 19,3% (95% IC 16,1-22,9) en niñas febriles menores de 12 meses. La prevalencia puntual de ITU fue 13% (95% IC 10,8-15,6) en lactantes con fiebre de menos de 24 horas de evolución versus 17,5% (95% IC 15,2-20,1) en aquellos con más horas de evolución de fiebre (p < 0,014).
ConclusionesLa prevalencia puntual de ITU en lactantes con fiebre sin focalidad > 39°C en nuestro medio es superior a la referida previamente, especialmente en niños<6 meses y niñas<12 meses. Nuestros resultados sugieren que los pediatras deben buscar de forma estrecha ITU en lactantes con fiebre sin causa >39°C.
Urinary tract infection (UTI) is the most common type of severe bacterial infection (SBI) in infants presenting with fever without a source (FWS). Several management protocols have been developed to assess children with FWS and identify those with a UTI.1–3 The overall prevalence of UTI in infants with FWS is around 5%.4–9 However, this prevalence is not homogeneous and can vary with age, sex, race, body temperature or circumcision status. For example, a UTI is found in a large proportion of febrile girls aged less than 24 months and febrile boys aged less than 12 months.
Infants that receive a diagnosis of UTI require special care. Most febrile infants with this diagnosis exhibit renal parenchymal involvement. This can lead to long-term health problems, including hypertension and diminished renal function. The risk of renal impairment is greater in infants, and the diagnosis may be challenging to clinicians. The signs and symptoms of a UTI are often nonspecific, and the definitive diagnosis requires testing of a noncontaminated urine sample collected by catheterization or suprapubic aspiration.10,11 An accurate diagnosis is important to identify, evaluate and treat children at risk of UTI and renal scarring and to avoid overdiagnosis and overtreatment in children who are not at risk.11,12 Nevertheless, there are still differences between clinical guidelines on how to identify infants with FWS in whom UTI has to be ruled out in the paediatric emergency department (PED).1,2,13–18 Most clinical guidelines recommend performing urinalysis in infants with a rectal temperature of 39°C or higher.
Currently, there are no data available on the prevalence of UTI in infants with high fever in the paediatric emergency department (PED) setting. Such data could help physicians in deciding which children require further diagnostic testing.13
The aim of our study was to determine the point prevalence of UTI in febrile boys aged less than 12 months and girls less aged less than 24 months with FWS greater than 39°C admitted to PEDs in Spain.
Materials and methodsStudy design and patientsWe conducted a prospective, multicentre, observational study in febrile infants in whom urinalysis was performed to rule out UTI over a 1-year period (October 2013–September 2014) in 7 Spanish PEDs. To calculate the point prevalence in each age group, we subdivided participants into 5 smaller subgroups: infants aged less than 3 months, 3–6 months, 6–12 months, and children aged 12–18 months and 18–24 months of age.
Inclusion criteriaThe study included:
- •
Boys aged less than 12 months with FWS greater than 39°C in whom urinalysis was performed to rule out UTI.
- •
Girls aged less than 24 months with FWS greater than 39°C in whom urinalysis was performed to rule out UTI.
Before including patients in the study, we obtained the informed consent of the parents or caregivers.
Exclusion criteriaWe excluded patients for any of the following reasons:
- •
Patients with fever and a high white blood cell (WBC) count in urine referred to the PED by paediatricians to rule out UTI.
- •
Patients who took antibiotics in the 72h preceding the visit.
- •
Parental refusal to participate.
All infants enrolled in the study underwent a standard evaluation by the managing physician at the PED, with a history taking including the Paediatric Assessment Triangle and a physical examination. Standard laboratory tests were also performed, including urinalysis and urine culture of samples obtained by catheter or suprapubic aspiration. An initial screening of UTI was performed with urine test strips.
DefinitionsWe defined fever without source as an axillary or rectal temperature of 38°C (100.4°F) or greater measured either at home or at the PED, in the absence of cold or other respiratory signs/symptoms (such as tachypnoea) or diarrhoea in infants with a normal physical examination. We defined positive urinalysis as detection of traces or higher levels of leucocyte esterase and/or nitrite in the test strips. We defined urinary tract infection as the growth of a single known pathogen in a urine culture from a sample collected by suprapubic aspiration, or more than 10000 colony-forming units (CFU)/mL in a urine culture from a sample collected by urethral catheterization, or growth of more than 100000CFU/mL in a urine culture from samples collected by clean-catch or midstream methods combined with abnormal urinalysis results. The following were considered true pathogens: Citrobacter freundii, Citrobacter koseri, Enterobacter aerogenes, Enterobacter cloacae, Enterococcus faecalis, Enterococcus faecium Escherichia coli, extended-spectrum β-lactamase (ESBL)-producing Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Proteus mirabilis and Streptococcus agalactiae.
Data collectionPrior to the start of the study, a member of the core research team (MG) distributed an electronic questionnaire via email to the site investigators at the participating emergency departments (EDs) to confirm their understanding of the text and the feasibility of the data collection process at all sites, and to ensure the clarity of the documentation. All queries regarding data collection were dealt with by the principal investigator in order to maintain the accuracy of the recorded data. Eligible infants were identified by ED physicians who collected demographic, clinical and management data: age, sex, personal history, any treatment administered before arrival to the ED, duration of fever, symptoms, physical examination, tests, diagnosis, administered treatment, length of stay in the hospital and patient outcomes. For each included patient, site investigators completed an electronic questionnaire on Google Drive and submitted it to the principal investigator. In addition, each month each site investigator completed another electronic questionnaire summarising the total number of infants managed, the number of febrile infants managed, and the number of febrile infants with fever greater than 39°C assessed with urinalysis, as well as the number of infants excluded and the reasons for their exclusion. In order to obtain good quality data, we needed to recruit at least 80% of the infants that met the inclusion criteria.
Statistical analysisThe sample size calculation was based on UTI prevalence data reported in the meta-analysis published by Shaik et al.13 To achieve a precision of 2.0% in the estimation of a proportion with a normal two-tailed asymptotic confidence interval (CI) of 95.0%, assuming an overall prevalence of UTI of 7.3% in infants with FWS and a prevalence of 8.0% in infants aged less than 24 months with FWS, we estimated that we needed to include at least 650 infants aged less than 12 months and 707 children aged less than 24 months in the study. Based on this calculation, the actual numbers of each age group that we needed to recruit to allow for an expected dropout rate of 10.0% were 723 and 786, respectively.
We performed the statistical analysis with the software SPSS Statistics for Windows version 22 (IBM; Armonk, New York, USA). We have summarised quantitative data as means, CIs and standard deviations, and categorical data as absolute frequencies and percentages. We compared continuous variables using the Student's t test and categorical variables with the χ2 test and the Fisher exact test. We defined statistical significance as a P-value of less than 0.05. We expressed point prevalences as percentages with their corresponding CIs. We analysed the data with the software Stata 12 (Stata Corp., College Station, Texas, USA).
The study was approved by the Ethics Committee of the Basque Country. We obtained informed consent from the parents of legal guardians of the children. The study was endorsed by the Research Network of the Sociedad Española de Urgencias en Pediatría (Spanish Society of Paediatric Emergency Medicine) (RISEUP-SPERG).
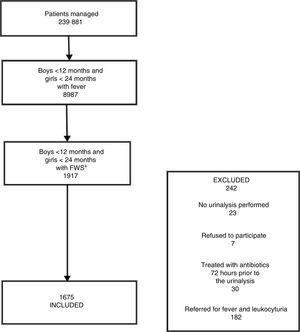
ResultsDescription of the study sampleOver the 1-year study period, 239881 episodes were documented in the 7 PEDs, including 8987 (3.7%) in febrile boys aged less than 12 months and febrile girls aged less than 24 months. In 1917 of these cases, the patient presented with FWS greater than 39°C. The final sample included 1675 children aged less than 24 months. Fig. 1 presents a flow chart of the sample selection.
The mean age of the 1675 children included in the study was 10±5.5 months, and the median age was 9 months (boys, 7 months; girls, 12 months); 1086 were girls (64.8%). A total of 1625 (97%) children were previously healthy, 42 (2.5%) had pre-existing renal or urologic disorders and 8 (0.5%) had a previous history of disease not involving the urinary system. Only 20 (1.2%) patients were not well-appearing (5 [25%] received a diagnosis of UTI). The presenting signs and symptoms were isolated fever in 1107 cases (66%; median, 39.6°C; range, 39.1–41°C) and fever combined with another sign or symptom in the rest (n=568): vomiting (259; 15.4%), poor feeding (127; 7.5%), irritability (70; 4.1%), malodorous urine (6; 0.3%), abdominal or side pain (4; 0.2%) and other (102; 6%). The duration of fever was 24h or fewer in 750 cases (44.8%).
A total of 301 children (17.9%; 95% CI, 16.2–19.9%) received an initial diagnosis of UTI. All of them had abnormal urinalysis results, testing positive for leucocyte esterase (198; 65.7%); nitrite (13, 4.3%) or both (90; 29.9%). Only 2 urine samples were obtained by suprapubic aspiration, and the rest being were collected by urethral catheterization. The urine culture was positive in 279 children, with diagnosis of UTI in 260 (15.5%; 95% CI, 13.8–17.3%). In 19 children with positive urine cultures, the results of urinalysis had been normal.
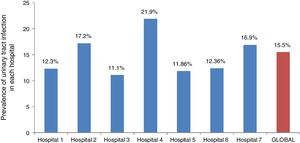
Point prevalence of UTIThe point prevalence of UTI was 15.7% (95% CI, 13.1–18.9%) in the subset of febrile boys aged less than 12 months and 15.37% (95% CI, 13.3–17.6) in the subset of febrile girls aged less than 24 months. Table 1 summarises the prevalence of UTI by age and sex. The point prevalence of UTI was 15% (95% CI, 13.4–16.9%) in previously healthy children compared to 33.3% (95% CI, 20.9–48.5%) in febrile infants with pre-existing renal or urological diseases (P=.002). Table 2 presents the point prevalence of UTI in children by duration of fever, and Fig. 2 presents the point prevalence by hospital.
Point prevalence of urinary tract infection (UTI) in febrile children stratified by age and sex.
| Age in months | Boys | Girls | P | ||
|---|---|---|---|---|---|
| n | UTI | n | UTI | ||
| 0–3 | 60 | 2643.3% (31.5–55.9) | 35 | 822.8% (11.8–39.2) | .07 |
| 3–6 | 128 | 3628.1% (21–36.4) | 94 | 2021.2% (14.1–30.6) | .31 |
| 6–12 | 401 | 317.7% (5.4–10.7) | 404 | 7518.5% (15–22.6) | <.001 |
| 12–18 | – | – | 331 | 3711.1% (8.1–15) | – |
| 18–24 | – | – | 222 | 2712.1% (8.4–17.1) | – |
Data expressed as absolute frequencies (n) and percentages (%) with their 95% confidence intervals.
Point prevalence of urinary tract infection (UTI) in febrile children stratified by duration of fever.
| Age | <24h of fever | >24h of fever | P | ||
|---|---|---|---|---|---|
| n | UTI | n | UTI | ||
| All infants | 751 | 9813% (10.8–15.6) | 924 | 16217.5% (15.2–20.1) | .014 |
| <12 months | 523 | 8416% (13.1–19.4) | 599 | 11218.6% (15.7–22) | .27 |
| >12 months | 228 | 146.1% (3.6–10.1) | 325 | 5015.3% (11.8–19.7) | .001 |
Data expressed as absolute frequencies (n) and percentages (%) with their 95% confidence intervals.
The pathogen isolated most frequently was Escherichia coli (256; 91%). Other isolated pathogens were: Enterococcus species (8; 3%), Klebsiella pneumoniae (6; 2%), Proteus mirabilis (4; 1.4%), Citrobacter species (2; 1%), Enterobacter species (2; 1%) and Pseudomonas species (2; 1%). There were 8 cases of bacteraemia secondary to UTI (all due to Escherichia coli), all of them in well-appearing, previously healthy children.
Two-thirds of the children (172; 66.2%) that received a diagnosis of UTI were managed at the outpatient level. Nearly one fifth (18.8%; 95% CI, 13.6–24.7%) of previously healthy children aged more than 3 months with normal urinalysis results given a diagnosis of UTI were admitted to a ward. Nine of the patients (5.2%) returned to the PED due to persistence of the symptoms; none exhibited clinical deterioration, but 2 were admitted to hospital.
DiscussionIn this multicentre prospective study of febrile infants evaluated in 7 PEDs for FWS greater than 39°C, we found a higher point prevalence of UTI, 15.5%, compared to the prevalence reported in previous studies, especially in boys aged less than 6 months and girls aged less than 12 months. One difference between our study and previous studies is that we only included children with a high temperature. Other epidemiological patterns, such as differences in point prevalence by age, sex, signs and symptoms of disease and bacteria isolated in urine or blood culture, were similar to those previously reported in infants and young children diagnosed with UTI.
Several previous studies have assessed the prevalence of UTI. They have reported an overall prevalence of UTI in infants presenting with fever without an evident source based on the medical history and physical examination of approximately 5%.7,19,20 In a review and meta-analysis of 14 studies, Shaikh et al.13 found a slightly higher pooled prevalence of UTI of 7% (95% CI, 5.5–8.4%) in febrile children aged less than 24 months of both sexes, with or without additional symptoms of UTI. In boys, the prevalence was highest in the first 3 months of life and then exhibited a declining trend, whereas in girls the prevalence was highest in the first 12 months of life.9,13 Although the differences based on sex and age found in our study were consistent with data from previous epidemiological studies of UTI, the point prevalences we found in each age group were higher compared to those reported in the literature.8,21 We found the highest point prevalence in febrile male infants aged less than 3 months, with UTI diagnosed in 43% of them. Our findings are consistent with a series of studies performed by Bachur,21 Zorc22 and colleagues, which showed that high fever (rectal temperature>39°C) and uncircumcised status were associated with a greater probability of UTI. Uncircumcised status22 has been identified as an important risk factor for UTI and seems to have a greater impact on younger children.23–27 Although we did not document circumcision status in our study, due to cultural customs the population we serve is mostly uncircumcised, which might explain the high point prevalence found in male infants.
Previous studies13 have proposed criteria for clinical prediction rules that clinicians can use to select patients from whom they should collect urine samples for analysis or culture. Our findings suggests that clinicians need to carefully investigate the possibility of UTI in all boys aged less than 12 months and girls aged less than 24 months with unexplained fever higher than 39°C. Given the high point prevalences found in our study, it would be reasonable to consider obtaining a urine specimen from infants with FWS and temperatures below this value. Factors other than the temperature, such as sex, duration of fever, history of UTI or pre-existing renal or urological conditions, must be considered to rule out UTI in febrile infants. The duration of fever has not been previously reported as a factor to be considered to rule out UTI in febrile infants seen in the PED. In the present study, the UTI point prevalence was higher in children with a longer duration of fever, especially in those aged more than 1 year. Although additional studies are needed to confirm this finding, we believe that this factor should be taken into account in the evaluation of a febrile infant.
The definition of UTI in febrile infants is controversial and differences in the definition used may explain the variability in the prevalences reported in different studies. In our study, the diagnosis of UTI was based on the quantitative results of urine culture in addition to evidence of pyuria and/or bacteriuria. In this sense, our UTI definition was more restrictive than those used in most previous studies, which have generally only required a positive urine culture.7,8,10 Our approach is in line with the most recent guidelines from the American Academy of Pediatrics, which define UTI as the growth of more than 50000CFU/mL of a single bacterium in a urine culture together with positive urine test strip or microscopic examination results. These criteria reduce the probability of overdiagnosis of UTI in infants with asymptomatic bacteriuria or contaminated specimens. Contrary to the guidelines proposed by the American Academy of Pediatrics guidelines for UTI, but in line with several other authors,14,18,28,29 we opted for a conservative threshold of 10000CFU/mL to consider a urine culture positive in order to avoid the risk of misdiagnosing real UTIs. Some factors, such as the urine collection method, can lead to a low bacterial count. A substantial proportion of infants with bacterial growth in urine culture of between 10000 and 50000CFU/mL and with a normal urinalysis results may have asymptomatic bacteriuria; we therefore considered urinalysis results to attempt to distinguish between acute infection and asymptomatic bacteriuria in infants with low bacteria counts.14,30,31
Quantitative urine culture is the gold standard for diagnosis of UTI, but unfortunately this method usually requires a period of up to 48h for results to become available. Dipstick urinalysis is a more attractive option in the ED setting as it is cheaper, quicker and easier to perform and interpret than microscopy or culture. However, urinalysis cannot just be substituted for urine culture to document the presence of UTI, but has to be used in conjunction with culture. Although dipstick urinalysis allows rapid testing, there is some doubt about its use in infants and young children.32–34 In our study, 19 children with positive urine cultures from samples collected with sterile technique and without pyuria were classified as possible cases of UTI. These patients were not included, therefore, in the global UTI point prevalence. In Spain, there is still a wide variability in the management of patients with normal urine test strip and positive urine culture results. Some authors suggest that patients with normal urine test strip results and a positive urine culture might have asymptomatic bacteriuria, and the source of the fever could be a viral infection.35 Once the results of urine culture are available, the management of these patients should be individualised.
There are several limitations to this study. First, we did not reach the initially estimated sample size for male infants; however, we were able to recruit 85% of the required number of males, so the results may be representative of the entire population. Second, by including only infants with FWS greater than 39°C we may have overestimated the point prevalence of UTI in the overall population of febrile infants. Nevertheless, we consider that the point prevalence of UTI reported by our study may be useful to emergency paediatricians who routinely use this temperature as the cut-off point to rule out this type of infection. Third, we did not recruit all the infants with FWS greater than 39°C managed in the participating hospitals, as we only needed a minimum of 80% of the total for the study. It is possible that our sample included patients at greater risk of having an UTI, which would have led to an overestimation of the actual point prevalence of UTI. Still, we believe our response rate of 80% of children eligible for the study minimises the effect of sampling bias and that the high point prevalence rate found in our study is attributable to other factors already discussed. Finally, although there is a specific definition for the concept of FWS, it is still a subjective one that can therefore vary between hospitals. This could limit the applicability of our results to other health settings where different diagnostic criteria are applied. Nevertheless, we ought to note that the point prevalences observed in the different hospitals were all similar and close to the overall point prevalence.
The point prevalence found in infants with FWS of more than 39°C was higher in our study compared to previous studies on the prevalence of UTI, especially in male infants aged less than 6 months and female infants aged less than 12 months. Our findings suggest that clinicians need to make a thorough assessment of UTI in all boys aged less than 12 months and girls aged less than 24 months with unexplained fever of more than 39°C.
The point prevalence in infants aged less than 12 months was highest in girls. On the other hand, the point prevalence in febrile male infants aged less than 3 months is twice that in female infants of the same age.
Ana Isabel Fernández Lorente. Hospital Universitario Basurto.
Laura Minguell Domingo. Hospital Universitari Arnau de Vilanova.
María Amalia Pérez Sáez. Hospital Universitario Zumarraga.
Mireya Orio Hernández. Hospital del Tajo.
Verónica García González. Hospital Universitario Cabueñes.
Victoria Trenchs Sainz de la Maza. Hospital Universitari Sant Joan de Deu.
Please cite this article as: Gonzalez M, Salmon A, Garcia S, Arana E, Mintegi S, Benito J, et al. Prevalencia de las infecciones del tracto urinario en lactantes con fiebre alta en los servicios de urgencias. An Pediatr (Barc). 2019;91:386–393.