Alterations in thyroid hormones during critical illness, known as non-thyroidal illness syndrome (NTIS), were suggested to have a prognostic value. However, paediatric data is limited. The aim of this study was to assess prevalence and prognostic value of NTIS among critically ill children.
Materials and methodsA prospective observational study conducted on 70 critically ill children admitted into paediatric intensive care unit (PICU). Free triiodothyronine (FT3), free thyroxine (FT4), and thyroid stimulating hormone (TSH) were measured within 24h of PICU admission. Primary outcome was 30-day mortality.
ResultsNTIS occurred in 62.9% of patients but it took several forms. The commonest pattern was low FT3 with normal FT4 and TSH (25.7% of patients). Combined decrease in FT3, FT4, and TSH levels occurred in 7.1% of patients. An unusual finding of elevated TSH was noted in three patients, which might be related to disease severity. Low FT4 was significantly more prevalent among non-survivors compared with survivors (50% versus 19.2%, P=.028). NTIS independently predicted mortality (OR=3.91; 95% CI=1.006–15.19; P=.0491). Concomitant decrease in FT3, FT4, and TSH was the best independent predictor of mortality (OR=16.9; 95% CI=1.40–203.04; P=.026). TSH was negatively correlated with length of PICU stay (rs=—0.35, P=.011). FT3 level was significantly lower among patients who received dopamine infusion compared with those who did not receive it (2.1±0.66 versus 2.76±0.91pg/mL, P=.011).
ConclusionNTIS is common among critically ill children and appears to be associated with mortality and illness severity.
Se ha sugerido que las alteraciones en las hormonas tiroideas ocurridas en pacientes con enfermedad crítica, fenómeno conocido como síndrome del enfermo eutiroideo (SEE), pueden tener valor pronóstico. No obstante, los datos en población pediátrica son escasos. El objetivo del estudio fue evaluar la prevalencia y el valor pronóstico del SEE en niños críticos.
Materiales y métodosEstudio prospectivo observacional en 70 niños críticos ingresados en la unidad de cuidados intensivos pediátricos (UCIP). Se determinaron los niveles de triyodotironina libre (T3L), tiroxina libre (T4L) y tirotropina (TSH) en las primeras 24 horas de ingreso. La variable de resultado principal fue la mortalidad a los 30 días.
ResultadosSe observó SEE en el 62,9% de los pacientes, aunque adoptó formas diversas. El patrón más frecuente fue un nivel bajo de T3L con niveles normales de T4L y TSH (25,7% de los pacientes). La combinación de valores bajos de T3L, T4L, y TSH ocurrió en el 7,1% de los pacientes. Hubo un hallazgo inusual de TSH elevada en 3 pacientes que podría estar asociado a la gravedad de la enfermedad. Los valores bajos de T4L se observaron con una frecuencia significativamente mayor en pacientes fallecidos en comparación con supervivientes (50% versus 19,2%, p=0,028). El SEE predijo la mortalidad de manera independiente (OR=3,91; IC 95%=10,06–15,19; p=0,0491). La combinación de niveles bajos de T3L, T4L, y TSH fue el mejor factor pronóstico independiente de mortalidad (OR=16,9; IC 95%=1,40–203,04; p=0,026). Se observó una correlación negativa entre la TSH y la duración de la estancia en la UCIP (rs=—0,35; p=0,011). El valor de T3L fue significativamente menor en pacientes tratados con perfusión de dopamina, comparados con pacientes que no la recibieron (2,1±0,66 versus 2,76±0,91pg/ml, p=0,011).
ConclusiónEl SEE es común en niños críticos y parece estar asociado a la mortalidad y la gravedad de la enfermedad.
In the context of acute illness, several changes in thyroid hormones occur that are collectively known as non-thyroidal illness syndrome (NTIS) or euthyroid sick syndrome.1
NTIS has been reported in the context of infection, trauma, burns, myocardial infarction, and malignancy.2 It also occurs in fasting states in otherwise healthy individuals.3
The most consistent finding in NTIS is a decrease in the serum level of triiodothyronine (T3) that occurs rapidly after the onset of stress, hence the term “low T3 syndrome”. This decrease may also be associated with an increase in the serum level of reverse T3 (rT3) and, in severe cases, a decrease in the level of thyroxine (T4).4 A salient feature of this syndrome is that the drop in the levels of T3 and T4 is not accompanied by a concomitant rise in serum thyroid-stimulating hormone (TSH) levels. Instead, serum TSH might even decrease due to inhibition of the hypothalamic–pituitary–thyroid (HPT) axis.5
It is believed that the pathogenesis of NTIS involves the induction of type III deiodinase, which catabolizes T4 to rT3 instead of T3. There is also decreased expression of type I deiodinase, which normally converts T4 to T3.1 Furthermore, the HPT axis is inhibited due a decrease in leptin production during fasting states6 or to upregulation of type II deiodinase in the third ventricle, which increases conversion of T4 to T3 in the hypothalamus and therefore results in decreased production of thyrotropin-releasing hormone.7 In addition, there is a decrease in the serum levels of thyroid hormone transport proteins, which inhibits T4 transport in T3-producing tissues.8
Non-thyroidal illness syndrome may represent an attempt by the body to decrease energy expenditure, so it may be an adaptive response that should be left untreated.9 However, some authors believe that NTIS is a maladaptive response that requires treatment, as there is evidence of its association with poor outcomes.10,11 This question remains unresolved.
Although it is well known in adult patients, NTIS has received little attention by paediatric researchers and the paediatric studies on the subject are few and of small scope. Moreover, many paediatric intensivists are unaware of the syndrome. In addition, the results from previous studies are inconsistent as regards the association of thyroid hormones with clinical outcome.
We assessed thyroid function in a cohort of critically ill children with the aim of determining the prevalence and potential prognostic value of NTIS.
Materials and methodsWe carried out a prospective observational study in the paediatric intensive care unit (PICU) of a university hospital from January to September 2017. We included 70 critically ill children admitted to the PICU.
The study protocol was approved by the Committee for Medical Research Ethics of the School of Medicine of the University of Menoufia. Critically ill patients aged 1 month to 18 years were consecutively enrolled after obtaining informed consent from the parents. The exclusion criteria were underlying primary thyroid disease, history of chemotherapy or radiotherapy in the last 6 months, suspicion of underlying hypothalamic or pituitary disease, and failure to obtain parental consent.
A complete diagnostic workup was performed in all patients on admission, including a complete blood count, measurement of C-reactive protein (CRP), blood gas test and other routine tests. Patients also underwent screening for sepsis. Sepsis was diagnosed based on the presence of systemic inflammatory response syndrome (SIRS) in association with confirmed or suspected infection.12 We also obtained the paediatric risk of mortality (PRISM) score13 and the paediatric index of mortality (PIM2)14 for each patient.
The assessment of thyroid function consisted of measurement of serum free T3 (FT3), free T4 (FT4) and TSH in a single sample obtained within 24h of PICU admission. Hormone levels were considered low if they were at or below the 2.5th percentile, and high if they were at or above the 97.5th percentile for age.15 Normal ranges for FT3 change based on sex in addition to age, while normal ranges for FT4 and TSH are not influenced by sex.
We defined NTIS as any abnormality in thyroid function tests in the presence of critical illness and absence of a pre-existent abnormality in the hypothalamic–pituitary–thyroid axis. This definition allows the identification of usual and unusual patterns of NTIS.
Patients were closely monitored, and the primary outcome was 30-day all-cause mortality.
Laboratory methodsSerum FT3, FT4, and TSH were measured by enzyme-linked fluorescent assay (ELFA), with a MINI VIDAS immunoanalyzer (bioMérieux, Marcy-l’Étoile, France). The lower limits of detection for FT3, FT4 and TSH were 0.46pg/mL, 0.076ng/dL and 0.05μIU/mL, respectively.
Statistical methodsWe have expressed continuous variables as mean±standard deviation if they were normally distributed, and otherwise as median and range. We have summarised qualitative variables as absolute frequencies and percentages. We assessed the association between qualitative variables by means of chi squared or Fisher exact test, as applicable. We compared the means of normally distributed continuous variables and those of non-normally distributed continuous variables with the Mann–Whitney U test. The Pearson and Spearman correlation coefficients were used to assess the correlations between normally distributed variables and non-normally distributed variables, respectively. We performed univariate logistic regression to test the association of variables with mortality. Variables with significant associations in the univariate analysis were included into a multivariate logistic regression model to identify independent predictors of mortality. We defined statistical significance as a P-value less than .05 in any of the tests. We performed the statistical analyses with the software SPSS version 20 (SPSS Inc.; Chicago, IL, USA).
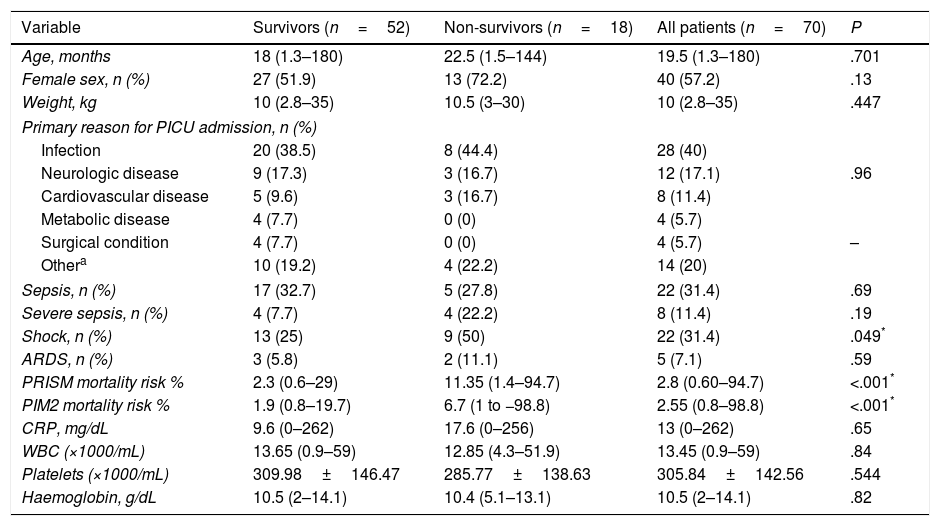
ResultsPatient characteristicsThe study included 70 patients. Table 1 shows their basic demographic and clinical characteristics. Infection was the most frequent reason for admission to the PICU.
Characteristics of the patients under study.
| Variable | Survivors (n=52) | Non-survivors (n=18) | All patients (n=70) | P |
|---|---|---|---|---|
| Age, months | 18 (1.3–180) | 22.5 (1.5–144) | 19.5 (1.3–180) | .701 |
| Female sex, n (%) | 27 (51.9) | 13 (72.2) | 40 (57.2) | .13 |
| Weight, kg | 10 (2.8–35) | 10.5 (3–30) | 10 (2.8–35) | .447 |
| Primary reason for PICU admission, n (%) | ||||
| Infection | 20 (38.5) | 8 (44.4) | 28 (40) | |
| Neurologic disease | 9 (17.3) | 3 (16.7) | 12 (17.1) | .96 |
| Cardiovascular disease | 5 (9.6) | 3 (16.7) | 8 (11.4) | |
| Metabolic disease | 4 (7.7) | 0 (0) | 4 (5.7) | |
| Surgical condition | 4 (7.7) | 0 (0) | 4 (5.7) | – |
| Othera | 10 (19.2) | 4 (22.2) | 14 (20) | |
| Sepsis, n (%) | 17 (32.7) | 5 (27.8) | 22 (31.4) | .69 |
| Severe sepsis, n (%) | 4 (7.7) | 4 (22.2) | 8 (11.4) | .19 |
| Shock, n (%) | 13 (25) | 9 (50) | 22 (31.4) | .049* |
| ARDS, n (%) | 3 (5.8) | 2 (11.1) | 5 (7.1) | .59 |
| PRISM mortality risk % | 2.3 (0.6–29) | 11.35 (1.4–94.7) | 2.8 (0.60–94.7) | <.001* |
| PIM2 mortality risk % | 1.9 (0.8–19.7) | 6.7 (1 to −98.8) | 2.55 (0.8–98.8) | <.001* |
| CRP, mg/dL | 9.6 (0–262) | 17.6 (0–256) | 13 (0–262) | .65 |
| WBC (×1000/mL) | 13.65 (0.9–59) | 12.85 (4.3–51.9) | 13.45 (0.9–59) | .84 |
| Platelets (×1000/mL) | 309.98±146.47 | 285.77±138.63 | 305.84±142.56 | .544 |
| Haemoglobin, g/dL | 10.5 (2–14.1) | 10.4 (5.1–13.1) | 10.5 (2–14.1) | .82 |
Data expressed as mean±SD, median (maximum−minimum), or absolute frequency (percentage).
Including trauma, anaphylaxis, drowning, poisoning, autoimmune, haematological, and oncological diseases.
ARDS, acute respiratory distress syndrome; CRP, C-reactive protein; PIM2, paediatric index of mortality 2; PRISM, paediatric risk of mortality; PICU, paediatric intensive care unit; WBC, white blood cell count.
The mean FT3 level in our patients was 2.619±0.9pg/mL. The median FT4 level was 0.99ng/dL (range, 0.44–2.2ng/dL). The median TSH level was 1.99μIU/mL (range, 0.08–32μIU/mL).
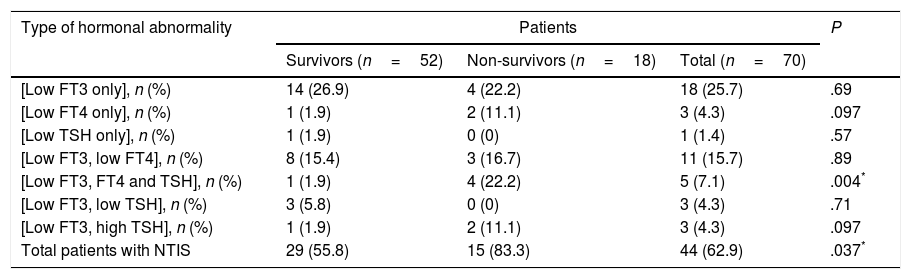
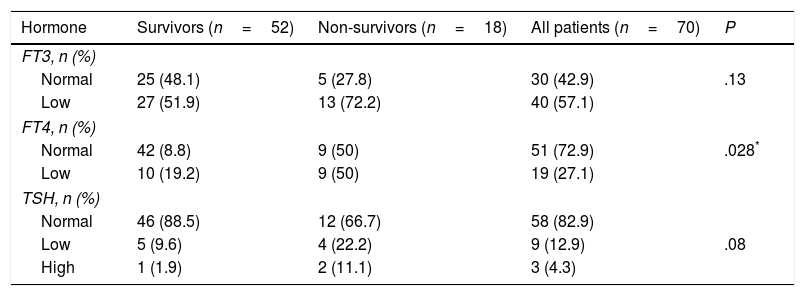
Prevalence and patterns of NTISNon-thyroidal illness syndrome was identified in 62.9% of patients. Some of them had abnormal levels in only one hormone, while others had various combinations of hormone abnormalities. The most frequent pattern was an isolated decrease in the level of FT3, and the least frequent was an isolated decrease in the level of TSH (Table 2). Table 3 shows the distribution of FT3, FT4 and TSH abnormalities in the patients under study.
Prevalence and patterns of non-thyroidal illness syndrome in the study sample.
| Type of hormonal abnormality | Patients | P | ||
|---|---|---|---|---|
| Survivors (n=52) | Non-survivors (n=18) | Total (n=70) | ||
| [Low FT3 only], n (%) | 14 (26.9) | 4 (22.2) | 18 (25.7) | .69 |
| [Low FT4 only], n (%) | 1 (1.9) | 2 (11.1) | 3 (4.3) | .097 |
| [Low TSH only], n (%) | 1 (1.9) | 0 (0) | 1 (1.4) | .57 |
| [Low FT3, low FT4], n (%) | 8 (15.4) | 3 (16.7) | 11 (15.7) | .89 |
| [Low FT3, FT4 and TSH], n (%) | 1 (1.9) | 4 (22.2) | 5 (7.1) | .004* |
| [Low FT3, low TSH], n (%) | 3 (5.8) | 0 (0) | 3 (4.3) | .71 |
| [Low FT3, high TSH], n (%) | 1 (1.9) | 2 (11.1) | 3 (4.3) | .097 |
| Total patients with NTIS | 29 (55.8) | 15 (83.3) | 44 (62.9) | .037* |
FT3, free triiodothyronine; FT4, free thyroxine; NTIS, non-thyroidal illness syndrome; TSH, thyroid-stimulating hormone.
Distribution of FT3, FT4 and TSH abnormalities in the patient sample.
| Hormone | Survivors (n=52) | Non-survivors (n=18) | All patients (n=70) | P |
|---|---|---|---|---|
| FT3, n (%) | ||||
| Normal | 25 (48.1) | 5 (27.8) | 30 (42.9) | .13 |
| Low | 27 (51.9) | 13 (72.2) | 40 (57.1) | |
| FT4, n (%) | ||||
| Normal | 42 (8.8) | 9 (50) | 51 (72.9) | .028* |
| Low | 10 (19.2) | 9 (50) | 19 (27.1) | |
| TSH, n (%) | ||||
| Normal | 46 (88.5) | 12 (66.7) | 58 (82.9) | |
| Low | 5 (9.6) | 4 (22.2) | 9 (12.9) | .08 |
| High | 1 (1.9) | 2 (11.1) | 3 (4.3) | |
Non-thyroidal illness syndrome was significantly more prevalent in non-survivors compared with survivors (Table 2). The prevalence of low FT4 levels was significantly higher in non-survivors compared to survivors (Table 3).
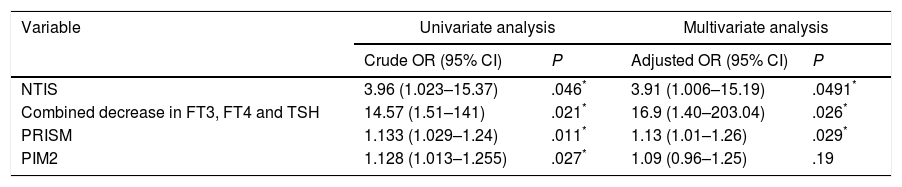
In the univariate logistic regression analysis (Table 4), the odds ratio for predicting mortality was higher for NTIS than for the PRISM score or the PIM2. The highest odds ratio corresponded to a combined decrease in FT3, FT4 and TSH levels.
Association of NTIS, combined decrease in FT3, FT4 and TSH, PRISM and PIM2 with mortality.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Crude OR (95% CI) | P | Adjusted OR (95% CI) | P | |
| NTIS | 3.96 (1.023–15.37) | .046* | 3.91 (1.006–15.19) | .0491* |
| Combined decrease in FT3, FT4 and TSH | 14.57 (1.51–141) | .021* | 16.9 (1.40–203.04) | .026* |
| PRISM | 1.133 (1.029–1.24) | .011* | 1.13 (1.01–1.26) | .029* |
| PIM2 | 1.128 (1.013–1.255) | .027* | 1.09 (0.96–1.25) | .19 |
In the multivariate analysis (Table 4), NTIS remained an independent predictor of mortality. A combined decrease in FT3, FT4 and TSH levels was the best independent predictor of mortality.
NTIS and indicators of disease severityWe found a significant negative correlation between TSH and the length of PICU stay (Spearman r=−0.35; P=.011).
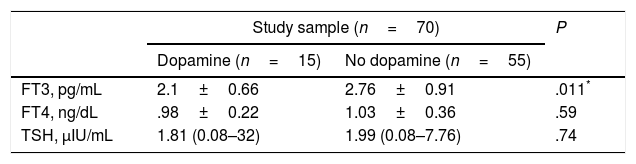
We found decreased FT3 levels in 12 (80%) of the 15 patients who received dopamine compared to 50.9% of the patients who did not receive it (P=.044). The mean FT3 level was significantly lower in the patients who received dopamine compared with those who did not receive it. We did not find significant differences in FT4 or TSH between the two subgroups (Table 5).
Comparison of hormone levels in patients who received dopamine versus patients who did not receive it.
| Study sample (n=70) | P | ||
|---|---|---|---|
| Dopamine (n=15) | No dopamine (n=55) | ||
| FT3, pg/mL | 2.1±0.66 | 2.76±0.91 | .011* |
| FT4, ng/dL | .98±0.22 | 1.03±0.36 | .59 |
| TSH, μIU/mL | 1.81 (0.08–32) | 1.99 (0.08–7.76) | .74 |
Data expressed as mean±SD or median (range).
FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
We also found a significant negative correlation between the white blood cell count and both FT3 (Spearman r=−0.36; P=.002) and FT4 (Spearman r=−0.34; P=.005), but there were no significant correlations between thyroid hormones and CRP.
DiscussionIn our study, about 63% of critically ill children had hormone abnormalities consistent with NTIS. There is previous evidence on this syndrome in critically ill adults and, to a much lesser extent, in children, although there is wide variability between studies in the reported prevalence. Thus, NTIS was diagnosed in 57% of children with diabetic ketoacidosis,16 60.7% of full term neonates with sepsis,17 all children with meningococcal sepsis18 and all children that had undergone cardiac bypass surgery.19
This wide variability could be explained by differences between the studies in the underlying critical illness, sample size, assay technique, or other factors like ethnicity or salt iodination. Furthermore, the effects of drugs commonly used in PICU should not be ignored. For example, dopamine and glucocorticoids suppress TSH secretion. Phenytoin and phenobarbital increase hepatic metabolism of thyroid hormones, decreasing their serum levels. Amiodarone and beta adrenergic blocking agents inhibit deiodinase activity and consequently T3 production. Conversely, furosemide, heparin, and non-steroidal anti-inflammatory drugs transiently increase free thyroid hormone levels through inhibition of their binding to plasma transport proteins.20
FT3 was the hormone most frequently in our patients, followed by FT4 and then TSH, which was consistent with the prevalent notion that NTIS is a state affecting primarily T3—hence the name “low T3 syndrome.”5 Similarly, a previous study found decreased levels of total T3, total T4 and TSH in 80%, 50% and 6.7% of the included critically ill children, respectively.21
Unexpectedly, we found increased levels of TSH in 3 of our patients, even though it is thought that the HPT axis is inhibited during critical illness. Elevation of TSH levels in the context of NTIS is usually attributed to recovery of the HPT axis,5 but this is an unlikely explanation in our case, since TSH was measured at admission and therefore before recovery. Our finding can be interpreted in light of previous reports that refer to a similar elevation of TSH in the most severely ill patients with significant thyroid failure.22,23 In support of this, 2 of our 3 patients with elevated TSH died. Alternatively, TSH elevation may occur for a brief period early on in stress states.24
Interestingly, although the characteristic finding in NTIS is a decreased T3 level, four out of the 44 patients with abnormal thyroid function had FT3 within the normal range. This might have occurred because binding of T3 to plasma proteins is almost always inhibited in NTIS, so that the level of total T3 is low but the level of free T3 is normal.23
In our study, we also assessed the potential prognostic value of NTIS. We found that the prevalence of NTIS was significantly higher in non-survivors compared with survivors. Furthermore, the risk of mortality was 3.91 greater in patients with NTIS, and 16.9 times greater in patients with a combined decrease in FT3, FT4 and TSH. The latter finding is consistent with a previous study that reported that out of 8 critically ill newborns with a combined deficiency of these three hormones, 5 died.25
It is worth noting that in our patients, thyroid function abnormalities were a better predictor of mortality than the PRISM score or the PIM2.
We also tried to assess the prognostic value of individual thyroid hormones. We found that the proportion of children with low FT4 was significantly greater in non-survivors compared with survivors, although the prevalence of low FT3 or low TSH in non-survivors was not significantly greater. The results of previous studies regarding the association of thyroid hormones with mortality have been inconsistent. Some have found significantly lower FT3, FT4 and TSH levels in non-survivors compared to survivors,26 while others have found a significant decrease in total T418 or total T3 and total T427 in non-survivors. Other authors, however, have not found significant differences in any of the hormones between both groups.28
A possible explanation for these contradictory findings could be differences in the timing of sample collection for hormone testing. For example, Peeters et al.10 found significantly lower levels of T3, T4 and TSH in non-survivors only after 5 days from admission. It is also possible that total T3 and total T4 are better prognostic factors than the free hormone fractions, as the latter may increase to some extent during critical illness due to decreased binding to plasma transport proteins.23
In addition to assessing their value in predicting mortality, we explored the potential association of FT3, FT4 and TSH with indicators of disease severity. We found that the level of TSH was negatively correlated with the length of PICU stay. Furthermore, the mean FT3 level was significantly lower in patients treated with dopamine infusion compared to those who did not receive dopamine. It is likely that the lower concentration of FT3 in the dopamine subgroup is due to the severity of the illness rather than the inhibitory effect of dopamine on the pituitary secretion of TSH,29 as the levels of TSH were not significantly lower in the patients treated with dopamine. On the other hand, the reason that we failed to detect decreased levels of TSH in the dopamine subgroup may be that the TSH level was measured before initiation of dopamine infusion in some of these patients.
Thus, our data suggest an association of NTIS with two indicators of illness severity, namely the length of PICU stay and the presence of shock severe enough to warrant dopamine infusion. Similarly, previous studies have found an association of thyroid hormones with some factors of disease severity. One study found that FT3 and FT4 were negatively correlated with the anion gap and positively correlated with the serum level of bicarbonate in children with diabetic ketoacidosis.16 Another paediatric study reported an association of total T3 with the duration of mechanical ventilation and of the ICU stay after cardiac surgery.19
We also found that FT3 and FT4 were significantly and negatively correlated to WBC but not to CRP. It is possible that the onset and peak of CRP level changes are not synchronous with those of thyroid hormones. A previous paediatric study also failed to find a correlation between thyroid hormones and CRP.18 Conversely, other studies have found a negative correlation between FT3 and CRP.26,30
The association of NTIS with mortality and indicators of disease severity suggests that it is a maladaptive response, but there is still no conclusive answer to the question of whether or not NTIS should be treated.
The limitations of our study include the small sample size, which is a generalised characteristic of previous studies of NTIS in paediatric patients. This is not to say that a small study is useless, since it can later be included in a meta-analysis from which firmer conclusions can be drawn. Another limitation is the lack of repeat assessments of thyroid function. We also did not measure total T3, total T4 or rT3. Lastly, we did not exclude patients receiving medications that may affect thyroid function, as these are used very frequently in PICUs and are difficult to avoid in clinical practice.
ConclusionNon-thyroidal illness syndrome is common among critically ill children, but it has a variable presentation. The presence of NTIS predicted mortality independently. Specifically, a combined decrease in FT3, FT4 and TSH levels was the best independent predictor of mortality. Non-thyroidal illness syndrome was also correlated to 2 indicators of disease severity, namely the length of PICU stay and the need for dopamine infusion. Larger studies are needed to better evaluate the prognostic value of NTIS in critically ill children.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: El-Ella SS, El-Mekkawy MS, El-Dihemey MA. Prevalencia y valor pronóstico del síndrome del enfermo eutiroideo en niños críticos. An Pediatr (Barc). 2019;90:237–243.