Mycoplasma pneumoniae (M. pneumoniae) is a bacterium with particular characteristics that give rise to a broad clinical spectrum, being respiratory infection the most frequent presentation. Infection by M. pneumoniae occurs in cyclical epidemics, and paediatricians in Spain have noticed an increase in cases since January 2024, establishing hospital registers to collect surveillance data (as it is not a notifiable disease in Spain). The diagnosis of infection by M. pneumoniae is made through serological testing and/or the detection of genetic material by means of polymerase chain reaction (PCR). Neither methods can differentiate between colonization and active infection, so a precise diagnosis is not possible and testing should only be requested in the case of high clinical suspicion. The role of antibiotherapy in infection by M. pneumoniae in its different clinical variants is not well defined. Most infections are self-limiting and mild, and there is insufficient evidence to support the use of antibiotherapy in these cases. Antibiotic treatment is justified in patients with risk factors for the development of severe disease (Down syndrome, anatomical or functional asplenia, immunosuppression), in hospitalized patients with respiratory infection and in patients with moderate or severe extrapulmonary forms. Taking into account aspects concerning the rational use of antimicrobials, the treatment of choice would be clarithromycin, with azithromycin as an alternative, reserving the use of doxycycline and levofloxacin for cases of antimicrobial resistance and/or infections of the central nervous system.
Mycoplasma pneumoniae (MP) es una bacteria con características peculiares que produce un amplio espectro de manifestaciones clínicas siendo la infección respiratoria la más frecuente de ellas. Se presenta en epidemias cíclicas, percibiendo los pediatras en España un incremento de casos desde enero del 2024, poniéndose en marcha registros hospitalarios (no es una enfermedad con declaración obligatoria en nuestro medio). El diagnóstico de la infección por MP se realiza mediante la determinación de serologías y/o la detección de material genético mediante reacción en cadena de polimerasa (PCR). Ambas son incapaces de diferenciar la colonización de la infección activa, por lo cual el diagnóstico preciso no es posible y deben solicitarse sólo en caso de alta sospecha clínica. El papel del tratamiento antibiótico en la infección por MP en sus diferentes variantes clínicas no está bien definido. La mayoría de las infecciones son autolimitadas y leves, sin evidencia suficiente en favor de la antibioterapia en estos casos. El tratamiento antibiótico estaría justificado en pacientes con factores de riesgo para desarrollo de formas graves (Síndrome de Down, asplenia anatómica o funcional, inmunodepresión), en pacientes hospitalizados con infección respiratoria y en pacientes con formas extrapulmonares moderadas o graves. Considerando aspectos referentes a la optimización del uso de antimicrobianos, el tratamiento de elección sería claritromicina, siendo alternativo el uso de azitromicina; y reservando el uso de doxiciclina y levofloxacino para los casos de resistencias antimicrobianas y/o infecciones del sistema nervioso central.
This document broaches the rational use of diagnostic tests and antimicrobials in the management of infection by Mycoplasma pneumoniae (M. pneumoniae) in children, based on the current scientific evidence.
What is M. pneumoniae?M. pneumoniae is a species of bacteria without a cell wall that belongs to the class Mollicutes.1 Infection by M. pneumoniae spreads through respiratory droplets and the incubation period is of 2 to 4 weeks. The immunity generated by infection is temporary, and reinfection is frequent.1 Asymptomatic carriage status can persist for a few months, even in patients treated with antibiotics.1
M. pneumoniae has virulence factors which gives it a high affinity to respiratory epithelial cells, the capacity to cause direct damage, extracellular and intracellular survival and diverse interactions with the immune system. The pathogenesis of M. pneumoniae involves direct damage caused by the bacterium and indirect damage mediated by the host immune response and vasculitis or thrombosis.
What is the current epidemiological framework?M. pneumoniae follows a cyclic epidemic pattern that recurs every 3 to 5 years, with outbreaks usually occurring in summer or early autumn.2 During the COVID-19 pandemic, the measures implemented to control coronavirus disease were associated with a significant reduction in the incidence of M. pneumoniae infection.3 Contrary to the trends observed in other respiratory pathogens, the incidence of M. pneumoniae infection did not immediately surge back after the COVID containment measures were lifted,3 and instead an increasing trend was observed starting in January 2023, continuing through the second semester of 2023 in various countries in America, Europe, Oceania and Asia.2
China has reported a significant increase in new cases since June 2023, with a positive detection rate as high as 61% in the samples of children with respiratory illness.4 The current epidemic in China is characterised by an increased rate of macrolide resistance (MR), greater disease severity and an increased incidence in children aged less than 3 years compared to previous years.4
In Spain, accurate data are not available, as this is not a notifiable disease. However, since January 2024, paediatricians across the country have noticed an increase in cases, hospital admissions and extrapulmonary forms of disease, some of them severe. It is still unclear whether the latter increase is proportional to the overall increase in cases or due to the circulation of more virulent strains. Hospital-based registers have been set up to collect descriptive and epidemiological surveillance data on M. pneumoniae infections.
Case definition of infection by M. pneumoniae and associated risk factorsThe executive summary can be found in Table 1.
Case definition of infection by M. pneumoniae and associated risk factors.
| [0,1–2]A. What is the clinical presentation of infection by M. pneumoniae? | |
| [0,1–2]Asymptomatic infection/colonization | |
| [0,1–2]• Variable frequency estimated to range from ≤ 3% to 50%,4 can persist for months after infection. | |
| [0,1–2]• Antibiotherapy is not indicated, as it does not reduce either shedding or transmission. | |
| [0,1–2] | |
| [0,1–2]Symptomatic infection | |
| [0,1–2]• Respiratory tract infection (Table 2). | |
| [0,1–2]Most frequent presentation. Chiefly caused by direct effects of the bacterium. The course is benign in most cases. | |
| [0,1–2]• Extrapulmonary manifestations (Table 2). | |
| [0,1–2]Occur in 25% of cases. Most are indirect effects of infection mediated by the host immune response, vasculitis or thrombosis. Although the benefits of antibiotherapy are debatable, its initiation will be contingent on the severity and the morbidity/mortality associated with each manifestation, and supportive care or specific treatment for the particular manifestation is equally or possibly more important (see Treatment section). | |
| [0,1–2] | |
| [0,1–2]B. What is the definition of confirmed case of infection by M. pneumoniae? | |
| [0,1–2]Compatible clinical manifestations + one of the followinga: | |
| • Direct detection of M. pneumoniae: | - PCR in respiratory/blood/cerebrospinal fluid sample |
| - Antigen testb | |
| - Cultureb | |
| [0,1–2] | |
| • Contact with M. pneumoniae: | - Serology: serological conversion with detection of specific IgMc or > 4-fold increase in IgG titre in 2 samples collected at least 2 weeks apart |
| - Detection of antibody-secreting cell responseb | |
| [0,1–2] | |
| [0,1–2]C. What are the risk factors for severe forms of disease? | |
| [0,1–2]• The factors currently considered to increase the risk of severe pneumonia caused by M. pneumoniae include anatomical or functional asplenia, sickle cell anaemia, Down syndrome and immunocompromised status. | |
| [0,1–2]• Impairment of the humoral immunity increases the risk of severe and prolonged respiratory disease and the development of extrapulmonary forms of disease. | |
The definition of confirmed case is based on the practical application of these criteria, taking into account the challenges involved in the microbiological diagnosis of infection by M. pneumoniae (see Diagnostic methods section).
Diagnostic methods that are not generally available in clinical practice outside a research framework.
Isolated detection of IgM does not confirm the diagnosis with certainty, although it makes the diagnosis highly probable in the presence of compatible clinical manifestations if alternative diagnoses have been ruled out. In practice, if a previous IgM measurement is not available, diagnosis requires detection of IgG seroconversion (if initial IgG was negative) or an increase in the Ig titre (in patients with a positive IgM and IgG result) at 2 weeks.
The most frequent presentation is respiratory infection including upper respiratory tract infection, tracheobronchitis and pneumonia. Extrapulmonary manifestations develop in approximately 1 in 4 patients (Table 2).5
Pulmonary and extrapulmonary manifestations of infection by M. pneumoniae.
| Pulmonary manifestations/Pneumonia caused by M. pneumoniae | |||
|---|---|---|---|
| Most frequent findings | Increase probability of MP (but do not confirm it) | Decrease probability of MP (but do not rule it out) | |
| Clinical manifestations | • Fever• Persistent dry cough• Fatigue• Dyspnoea• Headache• Sore throat | • Age > 5 years• MP in household • Extrapulmonary manifestations (especially cutaneous and gastrointestinal)• Chest pain• Rales• Duration > 6 days• Lack of response to beta-lactam antibioticsa | • Age < 5 years• Wheezing• Rhinorrhoea |
| [0,1–4] | |||
| Imaging testsb(radiography/ultrasound) | • Consolidation (59%)• Infiltration involving 1 lobe (32%)• Unilateral infiltrates involving >2 lobes (11%)• Bilateral infiltrates involving >2 lobes (12%)• Pleural effusion (26%) | [0,3–4]• There are no pathognomonic imaging findings that can be used to differentiate the aetiological agent of pneumonia | |
| Laboratory tests | • Complete blood count: mild leukocyte and neutrophil elevation • Acute phase reactants: mild to moderate elevation of CPR (20-80 mg/L) and PCT | • Mild-moderate elevation of leukocytes, neutrophils, CPR and PCT• Frequently, findings suggestive of haemolysis, usually mild | • Marked elevation of CPR and PCT |
| [0,1–4] | |||
| [0,1–4]Extrapulmonary manifestations of infection by M. pneumoniae | |
|---|---|
| Site | [0,2–4]Manifestations |
| CutaneousMost frequent manifestations | [0,2–4]• Maculopapular rash, urticaria (most frequent manifestations)• Erythema multiforme lesions• Reactive infectious mucocutaneous eruption (RIME): mucositis ± cutaneous lesions |
| NeurologicalMost severe manifestations(0.1% of infected, 6% of hospitalized) | [0,2–4]• Meningoencephalitis, acute disseminated encephalomyelitis (ADEM), transverse myelitis, cerebellar ataxia, Guillain-Barré syndrome, cerebellar stroke, peripheral neuropathy, cranial nerve palsy• Cerebrospinal fluid: lymphocytic pleocytosis, increased protein level with normal glucose level |
| Haematological | [0,2–4]• Mild haemolysis (except in patients with underlying haematological disease, in whom it may be severe) |
| Gastrointestinal | [0,2–4]• Abdominal pain, vomiting, diarrhoea• Abnormal transaminase levels |
| Other | [0,2–4]• Cardiac (myocarditis), musculoskeletal (arthralgia, arthritis), renal (glomerulonephritis) |
CRP, C-reactive protein; MP, Mycoplasma pneumoniae; PCT, procalcitonin.
The most frequent symptoms of pneumonia caused by M. pneumoniae are: fever (86–96%), persistent dry cough (85–96%), fatigue (78%), dyspnoea (67%), headache (11–48%) and sore throat (12%–47%), while rales are the most frequently detected sign on auscultation.5 These features are nonspecific and overlap with those of other pathogens. Table 2 presents the features associated with an increased or decreased probability of infection by M. pneumoniae, although none of them, together or combined, is sufficient to establish a certain diagnosis.2,5,6
What is the definition of confirmed case of infection by M. pneumoniae?Confirmation of a suspected case of M. pneumoniae infection requires direct detection of the pathogen or evidence of a compatible serologic response (Table 1B),2 as clinical, radiological and laboratory criteria are insufficient to make the diagnosis. At the same time, microbiological and serological criteria also have limitations and must always be interpreted taking into account the clinical presentation and context, as it is not always possible to differentiate between infection and asymptomatic carriage or coinfection with another pathogen.7
What are the risk factors for severe forms of disease?Infection by M. pneumoniae tends to be mild and many patients do not require hospitalization, but it may cause severe pneumonia or extrapulmonary manifestations.
The risk of severe pneumonia is greater in children with anatomical or functional asplenia (e.g., children with sickle-cell anaemia), Down syndrome or those who are immunocompromised (Table 1C).8
In addition, although the virulence determinants have only been partially elucidated, the severity of extrapulmonary disease may be determined in part by the individual strain and the toxin concentration it produces.8
Broadly speaking, it is reasonable to say that, as occurs in other types of infection, the characteristics and magnitude of the host immune response and immunocompetence can significantly affect the clinical picture of respiratory disease caused by M. pneumoniae and the wide array of possible extrapulmonary complications. For many years, research has been conducted on the role of the host immune response involving macrophages, mast cells, neutrophils and natural killer cells as well as T and B cells and humoral immune responses. Although the humoral response to infection does not provide full immunity against future infection, the importance of an intact antibody response in containing the disease cannot be overstated. Individuals with antibody deficiencies can develop severe and prolonged respiratory disease and are at increased risk of extrapulmonary complications such as meningitis or arthritis.9
Diagnostic methodsTable 3 presents the executive summary.
Diagnostic methods.
| A. What are the methods for microbiological diagnosis of M. pneumoniae available in clinical practice? |
| • The methods currently available in Spain for microbiological diagnosis of infection by M. pneumoniae are serologic tests (seroconversion with detection of specific IgM or at least a 4-fold increase in IgG titres in 2 samples collected at least 2 weeks apart) and detection of nucleic acid by polymerase chain reaction (PCR) in a respiratory specimen (usually, nasopharyngeal aspirate). |
| • Neither serologic tests nor PCR can discriminate between colonization and active infection, so an accurate diagnosis is not possible. |
| • The combination of 2 diagnostic techniques, such as PCR and serologic testing (development of IgM or increase in IgG titre) increase the precision of diagnosis, but take a long time in real-world clinical practice (3–4 weeks). |
| • The antibody-secretory cell response test could differentiate infection from colonization, but at present is only available for research purposes. |
| • Other tests, like culture or antigen tests, are not used routinely. |
| B. When should PCR testing of respiratory specimens and/or serologic tests for detection of M. pneumoniae be ordered? |
| • To maximise diagnostic yield, we recommend limiting testing to those cases in which there is a high clinical suspicion (high pre-test probability) and diagnosis would result in changes in treatment (see treatment section). |
| • Performance of diagnostic tests is not recommended in the outpatient management of pneumonia in healthy children in whom empirical therapy tends to be effective, even if it does not include antibiotics with activity against M. pneumoniae. |
| • Microbiological diagnosis would be indicated in the following cases: |
| ◦ Suspected atypical pneumonia with moderate/severe disease requiring hospitalization (PCR, serology or both). |
| ◦ Patients with atypical pneumonia with risk factors for severe disease (see Table 1C) (PCR, serology or both). |
| ◦ Patients with severe pneumonia requiring admission to intensive care (PCR, serology or both). |
| ◦ Typical or undetermined pneumonia with a poor response to beta-lactam antibiotics after 48–72 hours of treatment once complications have been ruled out (PCR, serology or both). |
| ◦ Presence of other respiratory diseases (bronchitis, asthma exacerbation) that do not respond to usual treatment (PCR, serology or both). |
| ◦ Presence of extrapulmonary manifestations of M. pneumoniae, if associated with respiratory symptoms and in patients with more severe manifestations (neurologic, cutaneous, cardiac) (PCR, serology or both). |
| ◦ Lower respiratory tract specimens obtained through bronchoalveolar lavage (PCR) if M. pneumoniae is included in the differential diagnosis. |
| ◦ Under circumstances in which patient isolation and cohorting is necessary (PCR). |
| ◦ Epidemiological/outbreak study (PCR and serology). |
| C. When should PCR testing of non-respiratory specimens for detection of M. pneumoniae be ordered? |
| • PCR testing of non-respiratory samples should not be performed routinely and is not validated in most cases.a |
| • In sites with clinical manifestations resulting from direct damage, PCR testing can be used to attempt to identify the aetiological agent, but PCR testing cannot be used for this purpose in the case of immune-mediated manifestations (Table 6). |
| • The detection by PCR of genetic material of M. pneumoniae in a non-respiratory specimen has no prognostic value, cannot guide changes to treatment and could be considered as complementary to the diagnosis made by PCR detection in a respiratory sample or serological testing. |
| D. When should imaging tests be ordered, and which are the tests of choice? |
| • In Spain, the gold standard for diagnosis of community-acquired pneumonia, including pneumonia caused by M. pneumoniae, is the plain chest radiograph. |
| • There are no features in the chest radiograph that allow differentiation of the aetiological agent in pneumonia, so the added value of this test for this purpose is limited. |
| • A chest radiograph is also indicated if complications of pneumonia are suspected, if a clinical diagnosis of pneumonia cannot be made and its presence must be assessed as part of the differential diagnosis, to rule out other possible diagnoses and in cases requiring hospital admission. |
| • A pulmonary ultrasound is an appropriate option that does not entail irradiation for the diagnosis of pneumonia and the detection of complications such as parapneumonic effusion if performed by adequately trained staff. |
At present, there are no laboratory tests that can be used in isolation for diagnosis of pulmonary infection by M. pneumoniae. Although there are some characteristic clinical and imaging features in children, it is not possible to differentiate community-acquired pneumonia caused by M. pneumoniae from pneumonia caused by other pathogens.2,10
On the other hand, the microbiological tests that are currently available generally offer information that is difficult to interpret.
The ideal specimens for aetiological diagnosis of pulmonary infection include bronchoalveolar lavage, pleural fluid, lung biopsy or tracheal aspiration samples, all techniques that are not used routinely. In real-world clinical practice, specimens tend to be collected from a site that is far from the primary site of infection (like the upper respiratory tract) or from tissues that offer indirect evidence of infection (serological tests), thus offering suboptimal results in terms of sensitivity and positive predictive value.11
Several studies demonstrate that a positive result in any of these tests does not necessarily entail active infection by M. pneumoniae, which severely compromises their positive predictive value.2,10,12 On the other hand, further complicating matters, a negative result in any one test does not rule out the involvement of M. pneumoniae with certainty.13
Due to all these challenges in the interpretation of results, it is strongly recommended that testing for aetiological diagnosis is reserved for patients in whom there is a strong clinical suspicion based on the characteristics discussed in the previous section. Combining tests to try to improve the precision of the aetiological diagnosis (paired serology testing + PCR) is not cost-effective in everyday clinical practice and is not recommended other than for research purposes.
Table 4 summarises the available diagnostic tests.
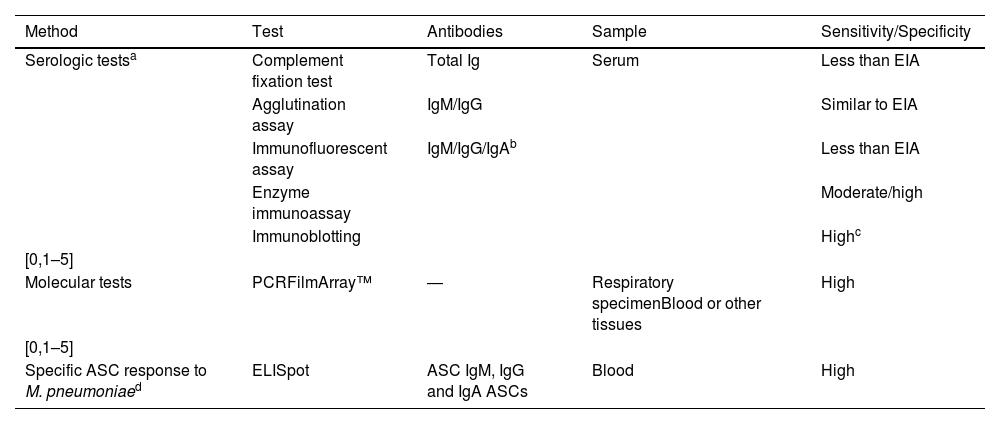
Tests available for microbiological diagnosis of M. pneumoniae.
| Method | Test | Antibodies | Sample | Sensitivity/Specificity |
|---|---|---|---|---|
| Serologic testsa | Complement fixation test | Total Ig | Serum | Less than EIA |
| Agglutination assay | IgM/IgG | Similar to EIA | ||
| Immunofluorescent assay | IgM/IgG/IgAb | Less than EIA | ||
| Enzyme immunoassay | Moderate/high | |||
| Immunoblotting | Highc | |||
| [0,1–5] | ||||
| Molecular tests | PCRFilmArray™ | ― | Respiratory specimenBlood or other tissues | High |
| [0,1–5] | ||||
| Specific ASC response to M. pneumoniaed | ELISpot | ASC IgM, IgG and IgA ASCs | Blood | High |
ASC, antibody-secreting cells; EIA, enzyme immunoassay; PCR, polymerase chain reaction.
If serologic tests are used, we recommend enzyme immunoassay for determination of antibodies due to its higher sensitivity and specificity.
IgA levels increase and then decrease before IgM levels, but are hardly ever measured due to their low sensitivity and specificity.
The specific antibody-secretory cell (ASC) response to stimulation with M. pneumoniae antigens with ELISpot techniques, similar to testing methods used in other diseases like tuberculosis, has proven quick and reliable in differentiating active infection by M. pneumoniae from asymptomatic carriage. However, these techniques are still under development and not yet available outside their use in research.
Customarily, the development of specific IgM or an increase in the IgG titre by at least 4-fold in 2 samples collected at least 2 to 4 weeks apart is diagnostic of acute infection by M. pneumoniae.
This diagnostic criterion only allows retrospective diagnosis and has little impact on care delivery. IgM levels can remain elevated for months after the initial infection and can also, on the contrary, increase only mildly in future reinfections in older children or adults, who may respond only with a transient increase in IgG levels.
A positive IgM result in the first week from onset of symptoms is unlikely.10 The seroprevalence of IgM in the healthy paediatric population can be as high as 40% and some studies have found IgG, IgM and IgA antibodies in healthy children with M. pneumoniae carriage, so the validity and sensitivity of these tests are questionable.10. In addition, serologic tests for detection of IgM can yield false positives on account of: (1) cross-reactivity with other antibodies, (2) the presence of substances that can interfere with the technique, or (3) the limitations of the technique used.
Polymerase chain reaction (PCR)A positive result in molecular diagnostic tests indicates the presence of genetic material of the bacterium in the sample, but there is strong evidence that this does not necessarily prove a causative role in the ongoing infection.2,10,12 In well-selected patients in whom samples are collected correctly, the specificity is greater than 90%, and gradually declines from the first week from onset of symptoms.
The frequency of positive PCR detection in nasopharyngeal samples in the general population can be as high as 45% to 56%10 on account of either asymptomatic carriage or the prolonged presence of the pathogen in the oropharynx after active infection, which may persist for several months.
The near universal availability of PCR techniques allows rapid testing for detection of multiple respiratory pathogens, but the results have to be interpreted correctly to determine whether the detected pathogen is the causative agent.14
When should PCR testing of respiratory specimens and/or serologic tests for detection of detection of M. pneumoniae be ordered?Testing for identification of M. pneumoniae should be individualised and only performed when the pre-test probability is high and test results are likely to change the management of the patient (Table 3B).11
In general, performance of diagnostic tests is not recommended in the outpatient management of pneumonia in healthy children in whom empirical therapy tends to be effective, even if it does not include antibiotics with activity against M. pneumoniae.11 The reported positivity rate of PCR testing for M. pneumoniae in respiratory specimens of children with community-acquired pneumonia varies widely in the scientific literature (4%–39%), and a positive result does not allow differentiation between active infection and asymptomatic carrier status.12 The prevalence of M. pneumonia carriage in the upper respiratory tract varies considerably in different years and seasons, and is higher after an epidemic season (given the cyclic pattern of M. pneumoniae). In patients appropriately selected based on clinical suspicion, the positive predictive value and especially the negative predictive value of testing increase and testing results contribute significantly to increasing the appropriate use of antibiotherapy.15
Despite the limitations noted above, PCR testing of respiratory specimens is the quickest method currently available (1–2 h) and is sensitive and specific compared to other microbiological tests, although the value ranges have not been clearly established.16 In the most recent meta-analysis on the usefulness of point-of-care and rapid tests for aetiological diagnosis in paediatric respiratory infections, out of the 57 included studies, only one focused specifically on M. pneumoniae, and it found a substantial increase in appropriate antibiotic prescribing with the use of the test.16 In Spain, molecular tests for detection of MR are not widely available.
Ordering tests for microbiological diagnosis is indicated in the circumstances detailed in Table 3B.2,17,18 The technique to be used will be selected based on availability, cost, the characteristics of the patient and the clinical status (taking into account how quickly results need to be available).
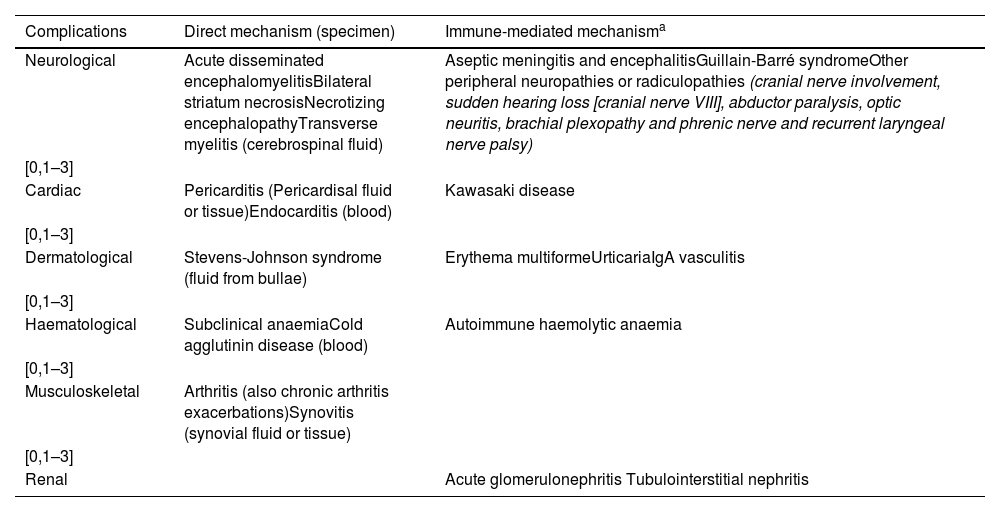
When should PCR testing of non-respiratory specimens for M. pneumoniae be ordered?In addition to respiratory infection, neurologic, cutaneous, cardiac, renal, haematological, immunological, muscular, osteoarticular and gastrointestinal involvement in M. pneumonia infection have been described.18 Testing for detection of M. pneumoniae in these systems is not performed routinely, and extrapulmonary testing is most frequently performed in cerebrospinal fluid samples. Although not conducted on a routine basis, testing for detection of the bacterium can be performed in specimens from sites with clinical manifestations resulting from direct damage, but is not indicated in the case of immune-mediated manifestations (Table 5).
Samples for molecular testing based on clinical manifestation and pathophysiological mechanism.
| Complications | Direct mechanism (specimen) | Immune-mediated mechanisma |
|---|---|---|
| Neurological | Acute disseminated encephalomyelitisBilateral striatum necrosisNecrotizing encephalopathyTransverse myelitis (cerebrospinal fluid) | Aseptic meningitis and encephalitisGuillain-Barré syndromeOther peripheral neuropathies or radiculopathies (cranial nerve involvement, sudden hearing loss [cranial nerve VIII], abductor paralysis, optic neuritis, brachial plexopathy and phrenic nerve and recurrent laryngeal nerve palsy) |
| [0,1–3] | ||
| Cardiac | Pericarditis (Pericardisal fluid or tissue)Endocarditis (blood) | Kawasaki disease |
| [0,1–3] | ||
| Dermatological | Stevens-Johnson syndrome (fluid from bullae) | Erythema multiformeUrticariaIgA vasculitis |
| [0,1–3] | ||
| Haematological | Subclinical anaemiaCold agglutinin disease (blood) | Autoimmune haemolytic anaemia |
| [0,1–3] | ||
| Musculoskeletal | Arthritis (also chronic arthritis exacerbations)Synovitis (synovial fluid or tissue) | |
| [0,1–3] | ||
| Renal | Acute glomerulonephritis Tubulointerstitial nephritis | |
It is important to take into account that in many instances, these techniques have not been validated for use in non-respiratory specimens, so their diagnostic value must be interpreted with caution. In light of this, it is more reliable to assess whether the mechanism of damage is direct or indirect based on the form of disease, and the detection of genetic material of M. pneumoniae and the detection by PCR of genetic material of M. pneumoniae in a non-respiratory specimen would simply support the diagnosis, and would not be used to select or modify treatment.19
When should imaging tests be ordered, and which are the tests of choice?The recommendations and indications of imaging tests in patients with suspected infection by M. pneumoniae are the same as those that apply to any case of community-acquired pneumonia. In Spain, the gold standard of diagnosis is the plain chest radiograph. A chest radiograph is also indicated if the course of disease differs from what is expected, if complications are suspected or if there is diagnostic uncertainty. The chest radiograph is no more useful for diagnosis than it is for any other cause of pneumonia, and its role in differential diagnosis remains unclear.2
When performed by adequately trained paediatricians, the point-of-care lung ultrasound is an adequate screening technique, as it does not involve exposure to radiation and its sensibility is equal or even superior to that of the chest radiograph if performed 48 to 72 hours from the onset of fever, and is also the imaging test of choice for diagnosis of parapneumonic pleural effusion.
Some authors suggest the use of imaging tests to support the diagnosis in patients with reactive infectious mucocutaneous eruption (RIME) preceded by respiratory symptoms.6
TreatmentTable 6 presents the executive summary.
Treatment.
| A. Is treatment necessary in every case? Which cases can be managed with symptomatic treatment and watchful waiting? |
| • Most infections by M. pneumoniae are self-limited and mild. |
| • The role of antibiotherapy in the management of infections by M. pneumoniae is still unclear. There is not sufficient evidence that antibiotherapy is superior to placebo in mild forms of disease or of its effectiveness in reducing transmission or preventing progression to severe forms of disease independently of the epidemiological context. |
| • In infants, infection by M. pneumoniae is less frequent than viral infection, so empirical treatment against M. pneumoniae is not justified. In cases in which there is a strong suspicion due to the presence of infection in the family, confirmation of the infection is recommended prior to treatment initiation. |
| • In the outpatient management patients with suspected respiratory infection by M. pneumoniae without risk factors for severe disease (see Table 1C) watchful waiting without treatment is a possible option, as long as close monitoring of the patient is guaranteed. |
| • In both outpatients and inpatients in whom treatment with a beta-lactam antibiotic for community-acquired pneumonia has been initiated and achieved a good clinical response, the detection of M. pneumoniae genetic material through molecular tests is not an indication to initiate targeted antibiotherapy in the absence of risk factors for severe disease (see Table 1C). |
| B. When is initiation of antibiotherapy indicated? |
| Outpatient care (primary and emergency care) |
| • At the outpatient level, antibiotherapy may be indicated if there is strong clinical suspicion of infection by M. pneumoniae in patients with risk factors for severe disease (see Table 1C). |
| • An antibiotic with activity against M. pneumoniae can be added in patients with community-acquired pneumonia who do not improve after 48–72 hours of treatment with a beta-lactam antibiotic after ruling out possible complications (consult the clinical scenarios detailed in Table 7). |
| Inpatient care |
| • In the hospital setting, antibiotherapy for confirmed infection by M. pneumoniae is indicated in patients with risk factors (Table 1C) or with moderate to severe respiratory or extrapulmonary disease (pneumonia with hypoxaemia requiring supplemental oxygen or ventilatory support, encephalitis or severe cutaneous/mucosal involvement). |
| C. Which treatments are available? |
| • First-line agents: |
| ◦ Oral clarithromycin: 15 mg/kg/day split in 2 doses for 5–7 days. |
| ◼ If there is a favourable response within 48-72 h, a 5-day course suffices. |
| ◼ First-line treatment recommended as the best possible option from an antimicrobial treatment policy perspective (antibiotic stewardship) as it significantly decreases the exposure of the host to the antibiotic agent due to its short half-life. |
| ◦ Oral azithromycin: 3-day course (10 mg/kg to a maximum of 500 mg for 3 days). |
| ◼ Its prolonged half-life entails a prolonged period of exposure to the antibiotic, which can promote the development of drug resistance. |
| • Second-line agents: only indicated in the case of suspected drug resistance, central nervous system involvement or immunocompromised status. |
| ◦ Doxycycline: 2 mg/kg every 12 hours on day 1 (maximum of 200 mg) and every 24 hours thereafter via the oral or intravenous route (in patients with severe disease, maintain dose of 2 mg/kg every 12 hours [maximum of 100 mg/12 h]) for 7 days. |
| ◼ Doxycycline has a narrower spectrum compared to levofloxacin and offers adequate activity in both the respiratory system and the central nervous system. Given that the contraindication for the use of tetracyclines in children under 8 years has been lifted for courses of treatment lasting less than 3 weeks, it has a better safety profile, with a lower risk of serious adverse events and less impact on the development of antimicrobial resistance. |
| ◦ Levofloxacin: (only if it is not possible to use a macrolide or doxycycline). Dose of 10 mg/kg every 12 hours, oral or IV, in children aged 6 months to 5 years and 10 mg/kg/24 hours to a maximum of 750 mg/day, oral or IV, in children aged more than 5 years, for 7–10 days. |
| • Azithromycin, clarithromycin and levofloxacin are available in intravenous formulations that should be reserved for patients with severe disease or oral intolerance. |
| • Given the absence of reliable data on the concentration and efficacy of macrolides in the central nervous system, doxycycline would be the drug of choice in the case of central nervous system involvement. |
| • Levofloxacin would be the alternative option in the case of suspected macrolide resistance or in immunocompromised patients requiring empirical broad-spectrum antibiotherapy covering other possible pathogens (e.g., Pseudomonas aeruginosa) until the definitive aetiological diagnosis is made. |
The role of antibiotherapy in the management of M. pneumoniae infections is not well established.20–22 In Spain, most of these infections are self-limiting and mild.23,24
Although some studies conducted in epidemiological contexts different from ours suggest that complications, extrapulmonary manifestations or severe or prolonged disease can develop if diagnosis is delayed or an ineffective antibiotic agent is prescribed,23,25–27 several studies, including a Cochrane review, have concluded that the evidence on the efficacy of antibiotherapy for treatment of respiratory infections by M. pneumoniae is insufficient.22–24
Recent observational studies have found no differences in the course and outcomes of respiratory tract infection by M. pneumoniae in patients with community-acquired pneumonia who received empirical treatment with beta-lactam antibiotics including vs excluding a macrolide, supporting the hypothesis that routine initiation of treatment is not indicated.22,28
Given the lack of scientific evidence in support or against antibiotherapy, it seems reasonable to apply the benefit of the doubt criterion and prescribe antibiotherapy in patients with severe illness that could be caused by M. pneumoniae. In most cases, a watchful waiting approach can be considered, even in patients with community-acquired pneumonia, in the absence of warning signs, as long as it is possible to guarantee close outpatient follow-up.2,29,30 In such cases, the decision whether to perform tests for aetiological diagnosis should be made based on the statements regarding their indication and interpretation presented in Table 3B.
A key factor is the age of the patient. Young children are less likely to have infection by M. pneumoniae compared to school-aged children. However, the incidence of symptomatic infection has increased significantly in infants and preschool-aged children in Asia and Europe, although the impact in terms of the clinical picture is not yet clear.31 In children under 3–5 years, complicated disease with development of pneumonia is infrequent if additional aetiological agents are not concurrently involved.25,26 In other age groups, it is estimated that 10% to 40% may develop pneumonia.25
When is initiation of antibiotherapy indicated?Considering what has been discussed thus far, prescribing of targeted antibiotherapy against M. pneumoniae is indicated when there is a high clinical suspicion of this aetiology or microbiological tests evince the presence of the bacterium and the patient has risk factors for severe disease (Table 1C) or presents with severe respiratory or extrapulmonary manifestations. Pneumonia with hypoxaemia requiring supplemental oxygen or ventilatory support, encephalitis and certain severe mucosal or cutaneous manifestations are considered indications for antibiotic treatment.2,32,33
In outpatient care settings (primary and emergency care), where molecular tests may not be immediately available, the epidemiological context, age and disease severity must be considered to identify patients with a high probability of infection by M. pneumoniae requiring empirical antibiotherapy (Table 7).2,26
Clinical situations at the outpatient care level (primary and emergency care) in which treatment initiation should or should not be considered.
| Clinical scenario | Recommended approach |
|---|---|
| • Patients with suspected infection by M. pneumoniae and risk factors for severe disease (see Table 1C). | • Treatment initiation would be rational. |
| [0,1–2] | |
| • Infants with wheezing, bronchiolitis and tracheobronchitis of varying severity. | • Initiation of empirical therapy against M. pneumoniae or other bacterial agents is not justified. |
| [0,1–2] | |
| • Infants and children aged up to 5 years with non-severe community-acquired pneumonia. | • An antibiotic with activity against M. pneumoniae may be added if there is no improvement after 48–72 hours of treatment with a beta-lactam antibiotic, taking into account the epidemiological context and the potential exposure at home or in school. In these cases, it is important to rule out complications of pneumonia (empyema, necrotising pneumonia) and the possible involvement of a different bacterial agent not covered by the first-line treatment, like Staphylococcus aureus. |
| [0,1–2] | |
| • Children > 5 years and adolescents with mild to moderate community-acquired pneumonia confirmed by imaging and a radiographic pattern consistent with lobar or atypical pneumonia. | • Targeted antibiotherapy against M. pneumoniae can be considered. If any warning sign or symptom develops or the patient does not improve within 72 hours, consider broadening the antimicrobial spectrum to cover Streptococcus pneumoniae and the performance of laboratory and testing for aetiological diagnosis. |
| [0,1–2] | |
| • Uncomplicated but prolonged cases of community-acquired pneumonia and tracheobronchitis. | • Consider treatment against M. pneumoniae, especially in school-aged children and adolescents or patients with prodromal manifestations lasting > 6 days, with extrapulmonary manifestations (chiefly cutaneous), with household contacts with respiratory symptoms and with normal or mildly elevated C-reactive protein levels (if the test is available in the primary care setting). Although the acute fever can last a week or even less, the cough and fatigue can last longer than 2 weeks. |
Table 6C presents the different treatment options with the recommended doses and duration of treatment. Azithromycin and clarithromycin are equivalent in terms of efficacy, antimicrobial spectrum, safety profile (especially regarding QT prolongation) and antimicrobial drug resistance mechanisms. Both interact with multiple drugs (they inhibit cytochrome P450 enzymes).
Traditionally, azithromycin has been considered the first-line treatment due to the ease of use and excellent tolerability (gastrointestinal intolerance is reported more frequently with the use of clarithromycin). However, there is growing concern about the induction of antimicrobial resistance due to its long half-life, which results in a prolonged persistence of the drug in the body.2 Thus, from an antimicrobial stewardship standpoint, the use of clarithromycin is prefer, as it reduces the duration of antimicrobial exposure.
In patients with central nervous system involvement, since the evidence on the efficacy of macrolides is insufficient,34 doxycycline is considered the treatment of choice. In real-world clinical practice, macrolides are widely used with the aim of reducing the bacterial load in the body to avoid indirect immune-mediated pathophysiological mechanisms. However, considering the severity of extrapulmonary presentations involving the central nervous system and the possibility that some neurologic manifestations may result from direct damage, tetracyclines are a more judicious option, especially since their use is no longer considered contraindicated in children younger than 8 years as long as the treatment duration is less than 3 weeks.35
Fluoroquinolones are the antimicrobials of choice if a broader spectrum is required to cover other pathogens in empirical antibiotherapy, in immunocompromised patients and if macrolide resistance is suspected.
Macrolide resistance has been increasing significantly, especially in Asia.36,37 In Europe, the reported rates of MR range from 1% to 25%,38 with an approximate prevalence of 8% in Spain.39 Testing for MR is not performed in Spain outside of research projects. Few studies offer data on the clinical impact of MR, which could increase the length of stay or the incidence of complications.40 Since tests for MR are not available in clinical practice, antibiotic selection must be guided by local data on drug resistance, disease severity or the patient’s response to treatment with macrolides.
FundingNo funding was received for this work.
Conflicts of interestThe authors have no conflicts of interest to declare in relation to this article’s contents.
Alfredo Tagarro García (Hospital Infanta Sofía, Madrid), Marta Cruz Cañete (Hospital de Montilla, Córdoba), Cristian Launes (Hospital Sant Joan de Déu, Barcelona), Cristina Calvo Rey (Hospital la Paz, Madrid, CIBERINFEC), José Antonio Couceiro Gianzo (Complejo Hospitalario de Pontevedra, Pontevedra), Enrique Otheo de Tejada (Hospital Universitario Ramón y Cajal, Madrid), Laura Francisco González (Centro de Salud, Madrid), Carlos Pérez (Hospital de Cabueñez, Gijón) and Marta Llorente (Hospital Universitario del Sureste, Madrid).
David López Martín (Hospital Costa del Sol, Marbella), Roi Piñeiro Pérez (Hospital General de Villalba, Madrid), Alicia Berghezan Suárez (Centro de Salud De Xaló, Alicante), Marta Cruz Cañete (Hospital de Montilla, Córdoba), María Belén Hernández Rupérez (Centro de Salud Mirasierra, Madrid), Antonio Iofrío de Arce (Centro de Salud El Ranero, Murcia) and Nerea Cardelo Autero (Hospital Costa del Sol, Marbella, Malaga).