Nutritional rickets is an emergent disease in Spain, and occurs particularly in black and dark-skinned infants and children from immigrant populations. The aim of this study was to ascertain the vitamin D reserve in a population of native and immigrant children under the age of 6 years.
Population and methodsA prospective study was conducted at a Primary Healthcare Centre in Salt (Girona). Patients: 307 children with the following origin and race distribution: Caucasian (n=85; 28%), Sub-Saharan (n=101; 32.5%); Maghrebí (n=87, 28.0%); Central-American (n=20; 6.4%) and Indo-Pakistani (n=14; 4.5%). The biochemistry blood parameters studied were calcium, phosphorus, alkaline phosphatase, 25-hydroxivitamin D, and parathormone. A nutritional survey was used to estimate calcium and vitamin D intake and degree of sun exposure.
ResultsVitamin D deficiency (<20ng/ml) was detected in Caucasians (8%), Sub-Saharans (18%), Central-Americans (20%), Maghrebís (34.5%), and Indo-Pakistanis (64%). Of the children studied (n=9), 2.9% had serious vitamin D deficiency (<10ng/ml); only one child of Sub-Saharan origin met the biochemical criteria for classical rickets. The prevalence of vitamin D deficiency was significantly higher in children not receiving vitamin D supplements in the first year of life.
ConclusionsPlasma vitamin D concentrations were deficient in 22.5% of children under the age of six, being more prevalent in children of Indo-Pakistani and Maghrebí origin.
El raquitismo carencial es una enfermedad emergente en nuestro medio y se describe especialmente en lactantes y niños inmigrantes de raza negra o piel oscura procedentes de países en vías de desarrollo. El objetivo de este trabajo está dirigido a conocer el estado nutricional de vitamina D en lactantes y niños inmigrantes de diferentes etnias de edad inferior a 6 años y compararlo con una población infantil autóctona.
Población y métodosEstudio prospectivo efectuado en un Centro de Asistencia Primaria de la localidad de Salt (Girona). Población: 307 niños con la siguiente distribución por origen y etnia: caucásicos (n=85; 28%), subsaharianos (n=101; 32,5%); magrebíes (n=87, 28,0%); centroamericanos (n=20; 6,4%) e indopakistaníes (n=14; 4,5%). Parámetros bioquímicos evaluados: calcemia, fosforemia, fosfatasa alcalina, 25-hidroxivitamina D y parathormona. Encuesta nutricional para estimar la ingesta de calcio, el aporte de vitamina D y el grado de exposición solar.
ResultadosPresentan déficit de vitamina D (< 20ng/ml) el 8% de los niños de origen caucásico, el 18% de los subsaharianos, el 20% de los centroamericanos, el 34,5% de los magrebíes y el 64% de los niños de origen indopakistaní. El 2,9% de los niños estudiados (n=9) presentan déficit grave de vitamina D (< 10ng/ml), de los que tan solo un niño de origen subsahariano reúne criterios bioquímicos de raquitismo clásico. La prevalencia de la deficiencia de vitamina D es significativamente más elevada en los niños sin suplementación con vitamina D durante el primer año de vida.
ConclusionesEl 22,5% de los niños menores de 6 años de edad presenta concentraciones plasmáticas en rango deficitario de vitamina D, siendo más prevalente en los niños de origen indopakistaní y magrebí.
Vitamin D plays an important role in bone phosphorus and calcium homeostasis, and therefore its deficiency determines the development of rickets and osteomalacia. Besides these functions, growing evidence has been found in recent years on its importance in other functions of phosphorus and calcium metabolism. Various epidemiological studies have demonstrated the association of low plasma concentrations of 25-hydroxyvitamin D (25(OH)D) and the development of several chronic diseases, like osteoporosis, and a greater risk of bone fractures, asthma, respiratory infections, type 1 diabetes, and obesity among others.1–5
Reference values that mark the threshold of vitamin D sufficiency have yet to be properly established, although the recommendations of the Institute of Medicine of the National Academies and the American Academy of Paediatrics in the United States provide an estimated threshold of 20ng/mL (50nmol/L) of plasma 25(OH)D for meeting the requirements of almost all healthy individuals.6–9
The main source of vitamin D is skin exposure to sunlight. Only a small portion of vitamin D comes from nutritional sources. Thus, factors that influence skin sun exposure, such as unfavourable weather conditions or geographical locations, clothing, and racial factors (increased skin pigmentation), and factors associated with an insufficient nutritional intake of vitamin D, carry a risk of developing hypovitaminosis D.10–13
At present, severe nutritional vitamin D deficiency is rare in most developed countries, although it is estimated that a considerable percentage of the population, including children and adults, have varying degrees of hypovitaminosis D. In Spain, nutritional rickets in the native population is extremely rare, but clinical and epidemiological data show a re-emergence of this disease in dark-skinned or black immigrant infants and children from developing countries that have been breastfed exclusively for long periods of time without receiving vitamin D supplementation and with limited exposure to sunlight.14–20
Our study was motivated by the growing population in Spain of children born in, or to families from, developing countries, with dietary, cultural and lifestyle habits that differ from those of the native Spanish population and that may carry a higher risk of developing vitamin D deficiency. The aim of the study was to determine the nutritional status and reserves of vitamin D in immigrant infants and children of different ethnicities younger than 6 years, and to compare them to those of a native Spanish population of children.
Population and methodsWe conducted a prospective study between 2008 and 2010 in a primary care centre in the town of Salt (Girona). The study included 307 infants and children younger than 5 years (139 girls and 168 boys) that received care regularly or sporadically in the outpatient paediatric clinic, selected at random. The sample had the following distribution by ethnic origin: Caucasian, n=85 (32 girls and 53 boys); Maghrebi, n=87 (41 girls and 46 boys); Sub-Saharan, n=101 (48 girls and 53 boys); and the distribution by nationality was: Gambia (n=52), Senegal (n=13), Nigeria (n=10), Mali (n=10), Guinea-Conakry (n=6), Ghana (n=4), Ivory Coast (n=2), Mauritania (n=1), Angola (n=1), Mozambique (n=1) and Cameroon (n=1); Central American, n=20 (13 girls and 7 boys), mostly from Honduras, and Indo-Pakistani, n=14 (5 girls and 9 boys).
In children younger than 2 years, length was measured in a horizontal position on a stretcher with an aluminium stadiometer accurate to 1mm. The patient was placed in the supine position, with the head held in a fixed position with the infant looking vertically upward, and the legs fully extended with the toes pointing up; the length was measured 3 consecutive times and recorded as the mean of the 3 measurements. The height of children older than 2 years was measured with a stadiometer accurate to 1mm vertically attached to the wall. The patient stood unclothed and barefoot with the feet together. The body and head were against the board, traversed by an imaginary plane (known as the Frankfurt plane) that extends from the lower margin of the orbit to the upper margin of the auditory meatus. The height was measured 3 times and recorded as the mean of these measurements.
The weight of children younger than 2 years was measured by a paediatric scale accurate to 10g, placing the naked child on the middle of the scale. An adult scale accurate to 100g was used in older children.
We compared the anthropometric measurements of children in our sample to the weight, length or height, and BMI (kg/m2) values obtained in the nationwide Spanish cross-sectional study on growth, and calculated age- and sex-specific z-scores.21,22
We assessed the daily intake of calcium and vitamin D by means of the Food Frequency Questionnaire published by Garabédian, which estimates the frequency with which the foods that contain them are consumed on a weekly basis. To assess exposure to sunlight, we took into account body surface area, time range, weather, use of sunscreen, and season of the year, rating these parameters from 0 to 3 to obtain a maximum of 9 points, following the tables of Garabédian, depending on the colour of the skin.23 We also collected data on vitamin D supplementation (dosage and route of administration) received in the first year of life.
The study protocol was approved by the Comitè Étic d’ Investigació Clinica (Committee of Ethics and Clinical Research) of the IDIAP (University Institute of Research in Primary Care) Jordi Gol i Gurina-Barcelona, and we obtained the informed consent of the parents or legal guardians.
We evaluated biochemical and hormonal markers of phosphorus and calcium metabolism. For calcium, the normal range was defined as 8.6–10.2mg/dL. For phosphorus, the normal range was 30–281IU/L. Their levels were determined by spectrophotometry, using the 4-nitrophenol-phosphate substrate method and calorimetry. We used a Cobas C 711 automated analyser. The reagents and modular system were manufactured by Roche Diagnostics. The normal range of PTH was defined as 15–65pg/mL. We determined the levels of PTH by means of the ECLIA method (Roche) using an E 170 modular analyser. For assessment of vitamin D, we used an ELISA method (Vitro) to determine the level of 25(OH)D. We stratified nutritional vitamin D status into the following categories: severe deficiency, plasma levels of 25(OH)D<10ng/mL; moderate deficiency, 25(OH)D at or above ≥10ng/mL and <20ng/mL; sufficiency, 25(OH)D≥20ng/mL; and elevated, 25(OH)D≥100ng/mL.
Statistical analysis: we have expressed the results of quantitative variables as mean±standard deviation (SD). The distribution of these variables was analysed by means of the Kolmogorov–Smirnov test. We analysed the differences between the populations for each quantitative parameter according to their distribution: ANOVA and Student's t test for normally distributed parameters (height standard deviation score [SDS]) and the nonparametric Wilcoxon or Kruskal–Wallis with a chi-square approximation for non-normally distributed parameters (all others). The differences between populations in the distribution of nominal variables were analysed by Pearson's chi-square test. We performed simple linear regression analysis to determine the statistically significant correlations between quantitative parameters. To analyse which clinical and biochemical parameters of phosphorus and calcium metabolism influence the plasma levels of 25(OH)D, we used the least squares method of multiple linear regression. We considered differences to be statistically significant in all of these analyses for P<.05. The programme used for the analyses was JMP® 7.0.1 (SAS, Cary, NC, USA).
ResultsThe mean decimal chronological age of the children at the time of inclusion in the study was 1.8±0.9 years (mean±SD; range: 0.2–5.6 years), with no statistically significant differences in age between the different ethnic groups analysed. Ninety percent of the children were younger than 3 years. Eight percent were 3–4 years of age, and the remaining two percent were 4–5.6 years of age. The mean BMI at the time of examination was 16.2±1.7kg/m2, which corresponded to a SDS of −0.50±1.1, and we found no statistically significant differences by ethnic group.
Table 1 shows the average values, expressed as mean±SD, for gestational age, anthropometric parameters at birth (weight and length), and anthropometric parameters at the time of inclusion in the study (weight, length or height [children more than 2 years of age] and BMI [kg/m2] in absolute values and SDs) by ethnic group, showing no statistically significant differences in these parameters between ethnic groups.
Gestational age, anthropometric parameters at birth (weight and length) and age and anthropometric parameters (weight, length and BMI) of the population at the time of inclusion in the study, stratified by ethnicity (n=307).
| Population | Gestational age | Birth length (cm) | Birth length±SD | Birth weight (kg) | Birth weight±SD | Decimal age at assessment (years) | Weight at assessment (kg) | Weight SDS at assessment (kg) | Length at assessment (cm) | Length SDS at assessment | BMI at assessment (kg/m2) | BMI SDS at assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total population | ||||||||||||
| Mean±SD | 39.0±2.0 | 49.3±2.7 | −0.02±1.17 | 3.22±0.59 | 0.09±1.11 | 1.8±1.0 | 11.36±3.04 | −0.75±1.05 | 83.2±11.3 | −0.38±1.22 | 16.2±1.7 | −0.50±1.12 |
| n=307 | 281 | 275 | 274 | 278 | 277 | 307 | 307 | 307 | 307 | 307 | 307 | 307 |
| Range | 28–42 | 38–55 | −3.85 to 3.37 | 1.34–5.25 | −2.57 to 3.27 | 0.2–5.6 | 5.33–21.00 | −3.05 to 2.84 | 57.0–117.0 | −3.73 to 3.42 | 12.2–25.6 | −3.01 to 5.04 |
| Caucasian population | ||||||||||||
| Mean±SD | 39.0±1.8 | 49.1±2.4 | −0.16±1.18 | 3.18±0.54 | 0.00 ± 1.01 | 1.7±0.9 | 10.80±2.44 | −0.83±0.80 | 80.9±9.5 | −0.66±1.11 | 16.4±1.6 | −0.49±1.10 |
| n=85 | 84 | 83 | 83 | 84 | 84 | 85 | 85 | 85 | 85 | 85a,b | 85 | 85 |
| Range | 33–42 | 40.5–54 | −3.85 to 3.37 | 1.6–4.18 | −2.3 to 1.96 | 0.3–3.3 | 5.58–16.50 | −2.88 to 0.9 | 58.5–99.0 | −3.73 to 1.31 | 12.6–20.1 | −2.96 to 2.94 |
| Maghrebi population | ||||||||||||
| Mean±SD | 39.3±2.4 | 49.4±2.7 | −0.06±1.10 | 3.25±0.59 | 0.13±1.03 | 1.9±1.1 | 11.82±3.13 | 0.57±1.05 | 84.8±12.4 | −0.16±1.17 | 16.3±1.5 | −0.40±0.92 |
| n=87 | 80 | 78 | 78 | 79 | 79 | 87 | 87 | 87 | 87 | 87a | 87 | 87 |
| Range | 28–42 | 40–55 | −3.57 to 2.24 | 1.34–4.34 | −2.57 to 2.14 | 0.3–5.6 | 5.33–19.40 | −2.99 to 2.2 | 57.0–117.0 | −3.63 to 3.08 | 13.3–21.0 | −2.04 to 1.75 |
| Sub-Saharan population | ||||||||||||
| Mean±SD | 38.9±1.9 | 49.5±3.0 | 0.09±1.24 | 3.23±0.64 | 0.11±1.31 | 1.9±1.0 | 11.64±3.19 | −0.72±1.21 | 84.3±11.3 | −0.26±1.30b | 16.2±1.7 | −0.53±1.12 |
| n=101 | 87 | 86 | 85 | 86 | 85 | 101 | 101 | 101 | 101 | 101 | 101 | 101 |
| Range | 30–41 | 38–54 | −3.71 to 2.75 | 1.36–5.25 | −2.3 to 3.27 | 0.4–5.0 | 5.72–21.00 | −3.05 to 2.84 | 62.0–109.5 | −3.00 to 3.29 | 12.2–21.6 | −3.01 to 2.75 |
| Central American population | ||||||||||||
| Mean±SD | 38.6±2.3 | 49.5±2.9 | 0.26±1.22 | 3.20±0.61 | 0.28±0.94 | 1.6±1.0 | 10.13±3.33 | −1.16±0.89 | 78.4±11.0 | −0.71±0.92 | 16.2±2.7 | −0.52±1.76 |
| n=20 | 19 | 19 | 19 | 19 | 19 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Range | 33–41 | 44–55 | −1.7 to 2.45 | 2.00–4.43 | −1.22 to 2.36 | 0.2–4.0 | 5.50–17.50 | −2.77 to 0.36 | 59.0–105.0 | −3.08 to 1.07 | 13.1–25.6 | −2.76 to 5.04 |
| Indo-Pakistani population | ||||||||||||
| Mean±SD | 38.8±1.1 | 49.6±1.6 | −0.08±0.65 | 3.23±0.59 | 0.04±1.23 | 2.1±0.9 | 11.74±3.56 | −1.05±1.13 | 86.6±11.8 | −0.41±1.62 | 15.4±1.8 | −0.94±1.20 |
| n=14 | 11 | 9 | 9 | 10 | 10 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| Range | 37–41 | 46–52 | −1.33 to 0.7 | 2.23–4.24 | −1.61 to 1.89 | 0.7–3.5 | 6.38–20.00 | −2.34 to 1.88 | 67.7–103.5 | −3.14 to 3.42 | 13.5–20.4 | −2.09 to 2.40 |
Table 2 shows the results of the study in terms of the age (in years) at which the study was conducted, the duration of breastfeeding (in months), the results of Garabédian's Food Frequency Questionnaire that estimates the daily intake of calcium (mg/day), the vitamin D input (U/day), the sun exposure index, and finally the results of the laboratory analysis of biochemical markers of phosphorus and calcium metabolism, analysed for the total sample and the different ethnic groups.
Duration of breastfeeding (months), sun exposure index, estimated calcium intake (mg/day), estimated vitamin D intake (IU/day) and biochemical parameters of phosphorus and calcium metabolism.
| Populations | Breastfeeding duration* (months) | Sun exposure index* | Ca intake (mg/day | Vit D intake** (IU/day) | 25(OH)D* (ng/mL) | PTH*** (pg/mL) | AP (U/L) | Ca (mg/dL) | P*** (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|
| Total population | |||||||||
| Mean±SD | 6.4±6.1 | 2.9±2.2 | 1097.2±161.3 | 694.8±342.0 | 35.8±22.9 | 31.7±26.5 | 292.8±437.8 | 10.1±0.5 | 5.6±0.6 |
| Range | 0.0–32.0 | 0.0–8.0 | 325.7–1491.4 | 225.0–1725.0 | 2.0–150.0 | 7.8–407.4 | 120.0–5160.0 | 7.9–11.5 | 2.3–7.4 |
| n=307 | |||||||||
| Caucasian population | |||||||||
| Mean±SD | 4.7±6.0 | 4.8±1.8 | 1108.0±189.2 | 743.8±164.3 | 40.0±19.7 | 27.3±10.8 | 352.0±742.4 | 10.1±0.5 | 5.5±0.6 |
| Range | 0.0–32.0 | 0.0–8.0 | 497.1–1491.4 | 225.0–1096.4 | 9.1–150.0 | 7.8–65.6 | 120.0–5160.0 | 9.0–11.4 | 3.5–7.4 |
| n=85 | |||||||||
| Maghrebi population | |||||||||
| Mean±SD | 5.7±6.1 | 1.8±1.6 | 1117.6±143.6 | 637.5±226.8 | 28.5±19.1 | 33.0±16.0 | 282.1±328.9 | 10.1±0.4 | 5.6±0.6 |
| Range | 0.0–24.0 | 1.0–7.0 | 720.0–1422.9 | 225.0–996.4 | 9.0–150.0 | 8.4–120.5 | 129.0–2910.0 | 9.2–11.3 | 3.0–7.0 |
| n=87 | |||||||||
| Sub–Saharan population | |||||||||
| Mean±SD | 8.0±5.6 | 2.3±2.1 | 1066.8±153.9 | 734.8±514.5 | 40.4±27.7 | 33.6±41.4 | 269.7±166.5 | 10.1±0.5 | 5.7±0.6 |
| Range | 0.0–24.0 | 0.0–8.0 | 325.7–1302.9 | 225.0–1725.0 | 2.0–150.0 | 8.0–407.4 | 134.0–1272.0 | 7.9–11.5 | 2.3–7.1 |
| n=101 | |||||||||
| Central American population | |||||||||
| Mean±SD | 6.5±6.5 | 3.1±2.3 | 1121.6±162.9 | 705.7±177.1 | 38.5±16.0 | 29.9±13.5 | 230.3±50.7 | 10.1±0.5 | 5.6±0.6 |
| Range | 0.0–21.0 | 0.0–7.0 | 720.0–1388.6 | 310.7–1025.0 | 13.5–68.9 | 11.0–58.2 | 151.0–315.0 | 9.1–11.2 | 4.4–7.0 |
| n=20 | |||||||||
| Indo–Pakistani population | |||||||||
| Mean±SD | 5.8±5.5 | 1.5±0.9 | 1088.6±110.9 | 449.0±201.2 | 19.7±13.5 | 39.3±18.2 | 258.4±59.0 | 10.1±0.5 | 5.9±0.5 |
| Range | 0.0–15.0 | 1.0–4.0 | 925.7–1337.1 | 225.0–825.0 | 6.3–50.7 | 18.2–89.3 | 193.0–389.0 | 9.4–11.1 | 4.6–6.5 |
| n=14 | |||||||||
Comparison between the different populations.
The mean duration of breastfeeding for the total sample was 6.4±6.1 months, with statistically significant differences (P<.0001) between ethnic groups, with the longest duration found in children of Sub-Saharan origin (8.0±5.6 months) and the shortest in Caucasian children (4.7±6.0 months). The estimated daily calcium intake for the total sample was 1.100±155mg/day, with no statistically significant differences by ethnic group, and the estimated daily vitamin D input was 124±105IU/day, with statistically significant differences found between children of Indo-Pakistani descent (57±97IU/day) and the remaining ethnic groups (P<.001). When it came to the degree of sun exposure for the total sample, the mean value was 2.9±2.2, which is considered deficient. There were statistically significant differences (P<.0001) between ethnic groups, with Caucasian children having the highest mean value (4.8±1.8), corresponding to a medium degree of sun exposure, while children of Indo-Pakistani descent had the lowest values (1.5±0.9), followed by Maghrebi children (1.8±1.6), degrees of exposure that are considered deficient.
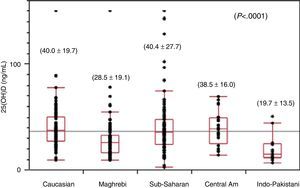
The mean value of the plasma levels of 25(OH)D for the total sample was 35.8±22.9ng/mL (range, 2.0–150.0). We found statistically significant differences (P<.0001) between the various ethnic groups, with the highest mean levels found in Caucasian (40.0±19.7ng/mL) and Sub-Saharan children (40.4±27.7ng/mL), followed by Central American children (38.5±16.0ng/ml), and the lowest levels found in Maghrebi (28.5±19.1ng/mL) and Indo-Pakistani (19.7±13.5ng/mL) children (Fig. 1). Out of the total sample, 24.5% had deficient levels of 25(OH)D (<20ng/mL), with statistically significant differences (P<.0001) between ethnic groups (Caucasian: 8.2%; Sub-Saharan: 17.8%; Central American: 20%; Maghrebi: 34.4%; and Indo-Pakistani 64.2%). One child of Sub-Saharan descent had a level of 25(OH)D of 3.7ng/mL and biochemical values consistent with classical rickets (PTH, 407pg/ml; alkaline phosphatase, 1204IU/L; blood calcium, 9.3mg/dL; blood phosphorus: 2.3mg/dL); 5 Maghrebi children, 2 Indo-Pakistani children and 1 Caucasian child had plasma levels corresponding to severe vitamin D deficiency (<10ng/mL) without meeting the biochemical criteria for rickets, as we only found moderately elevated PTH and alkaline phosphatase levels in 3 of them. Winter and spring, considered to be the seasons associated with the lowest degrees of sun exposure, were when 68.7% of the laboratory samples were collected, and the remaining 31.3% were obtained in summer and autumn. We did not observe statistically significant differences in 25(OH)D levels between samples collected in the two different periods mentioned above. We must mention that all blood samples with results that indicated severe vitamin D deficiency were collected during winter or spring.
The mean PTH plasma concentration for the entire sample was 31.7±26.5pg/mL (range, 7.8–407.4pg/mL). There were statistically significant differences between ethnic groups (P<.05), with the lowest mean values found in Caucasian (27.3±10.8pg/mL) and Central American children (29.9±13.5pg/mL), followed by Maghrebi (33.0±16.0pg/mL) and Sub-Saharan children (33.6±41.4pg/mL), while children of Indo-Pakistani descent had the highest values (39.3±18.2ng/mL). There was a statistically significant inverse correlation between plasma 25(OH)D and PTH levels (adjusted r2=−0.19; P=.001).
The mean plasma levels of calcium (mg/dL), phosphorus (mg/dL) and alkaline phosphatase (IU/L) were within normal ranges, with no statistically significant differences between the ethnic groups under study, and were not useful in identifying patients with hypovitaminosis D.
To study vitamin D supplementation, we collected data on the recommendations made by paediatricians in the first year of life. Sixty-four percent of the sample received prophylactic treatment with oral vitamin D3 (400IU/day), with the following percentages in each ethnic group: Caucasian children, 92%; Central American children, 85%; Maghrebi children, 69%; Indo-Pakistani children, 29%; and Sub-Saharan children, 5%. Thirty-two percent of Sub-Saharan children also received a massive oral dose of 100,000IU every 3 months in the first year of life in order to ensure adherence. The percentage of children with plasma levels of 25(OH)D below 20ng/mL was significantly higher (chi-square test, P<.0001) in children that did not receive supplementation (44%) versus those that did receive 400IU/day of supplementary vitamin D (23%), while we did not observe vitamin D deficiency at the time of the assessment in any of the children that received massive oral doses of 100,000IU every 3 months, although 4 children had elevated 25(OH)D levels (<150ng/ml), with no hypercalcaemia detected at the time of assessment (Table 3).
Classification and distribution of children in relation to the vitamin D supplementation received and the plasma levels of 25(OH)D (ng/mL).
| <20 | 20–100 | >100 | % 25(OH)D<20 | |
|---|---|---|---|---|
| No vitamin D supplementation | 39 | 66 | 1 | 44.0% |
| 400U/day vitamin D supplement | 26 | 135 | 2 | 23.0% |
| 100,000U/3 months | 0 | 29 | 4 | 0% |
P<.0001.
Finally, the multiple regression analysis of the plasma levels of 25(OH)D and the different clinical and biochemical parameters of phosphorus and calcium metabolism showed that 43.9% (adjusted r2=0.439; P<.0001) of the former parameter could be explained by vitamin D intake (P<.0001), degree of sun exposure (P=.0004), daily calcium intake (P=.0005), PTH levels (P=.006) and age at the time of assessment (P=.02).
DiscussionThe determination of 25(OH)D plasma levels is the best available measure of vitamin D stores, and thus should be the preferred method to be used in their assessment. This consensus is based on the following facts: (a) most vitamin D, either nutritional or produced in the skin, is converted into 25(OH)D in the liver, although only some of it is converted to the active metabolite. Thus, 25(OH)D reflects both nutritional intake and endogenous production through the skin; (b) the production of 25(OH)D is not regulated in any significant way and fundamentally depends on the availability of vitamin D; and (c) the half life of 25(OH)D is 3–4 weeks, making it a stable metabolite and the one found in largest quantities in serum. However, we must keep in mind that assessing vitamin D status by the determination of 25(OH)D provides a measure of the vitamin D input, and not of its function. However, there is controversy around the plasma levels of 25(OH)D that are associated with deficiency (risk of developing rickets), adequate bone health or optimal health, and thresholds have not been established by a specific consensus process. Many recent publications have proposed a level of 30ng/mL as the cut-off point for sufficiency, and 25(OH)D values between 21 and 29ng/mL for mild deficiency.8 However, in 2011, after a systematic review of the existing literature, the Institute of Medicine (IOM) of the United States concluded that 25(OH)D plasma levels of 20ng/mL met the requirements of 97.5% of the population, and that the cut-off point of 20ng/mL would be most useful to health providers in the management of patients in clinical practice.24–29
In Spain, severe vitamin D deficiency or vitamin deficient rickets is extremely rare in native children; however, clinical and epidemiological data show that the disease is re-emerging, albeit with very specific features. A previous study that analysed the clinical and epidemiological characteristics of 62 infants and children diagnosed with classical rickets revealed that this condition was most prevalent in infants between 3 and 12 months of age, with black or dark skin and with little diversification in their diets past 6 months of age, with the diet consisting mostly of breast milk. Younger infants had the most severe clinical features, seeking care in hospitals for tetany and hypocalcaemic seizures, while bone and skeletal deformities became more evident once children had started to walk.20
The town of Salt (Girona), where the primary care clinic in which this study was conducted is located, has an immigrant population from 71 different countries that amounts to 38% of the total population. Most of the immigrant population comes from Morocco, Sub-Saharan Africa, Central America and India. When the study started, the town census included 6643 children younger than 15 years, of whom 1586 were less than 2 years of age. Our study evinced that the nutritional status of the populations of native Spanish children and immigrant children younger than 6 years living in Salt is similar, showing no statistically significant differences and no malnutrition. The assessment of daily calcium intakes for the different ethnic groups under study showed that intakes were within the recommended range for this age group, while the amounts of vitamin D obtained through the diet and sun exposure were particularly deficient in immigrant populations.
When it came to vitamin D status assessed by means of plasma levels of vitamin D, 24.5% of the studied population had levels within the deficit range, that is, lower than 20ng/dL, especially children of Indo-Pakistani (64%), Maghrebi (34.5%) and Sub-Saharan descent (18%), while only 8% of Caucasian children had vitamin D deficiency. It must be noted that 2.9% of the population under study had severe vitamin D deficiency (<10ng/dL), among whom one child of Sub-Saharan descent met the biochemical criteria for classical rickets, and 5 Maghrebi children, 2 Indo-Pakistani children and one Caucasian child had plasma levels corresponding to severe vitamin D deficiency but did not meet the biochemical criteria for vitamin D deficiency rickets.
The absence of clinical manifestations and abnormal biochemical markers of phosphorus and calcium metabolism in infants and children with severe vitamin D deficiency leads us to consider the existence in these populations of mechanisms to adapt to vitamin D deficiency, with the adequate or high calcium intake of these populations and the potential activation of local intestinal regulatory mechanisms that do not depend on vitamin D (intestinal calcistat) or PTH in the kidneys potentially playing a relevant role.
We believe that the most important factors that may contribute to the greater and considerable prevalence of hypovitaminosis D in immigrant children populations compared to native Spanish children are the habitual use of traditional garments that cover most of the body, a social lifestyle that unfolds mostly inside homes and with little outdoor activity, and the promotion of breastfeeding, which is usually exclusive and of very long duration and lacking adequate vitamin D supplementation.17–19
Our study gathered data on the dosage of supplemental vitamin D recommended or prescribed by the paediatricians that cared for these children during the first year of life, and demonstrated its efficacy in achieving adequate plasma levels of 25(OH)D in a high proportion of the children receiving it, especially when massive doses of vitamin D were administered, although it must be taken into account that the latter can lead to subclinical hypervitaminosis D. At any rate, we believe that before generalising and recommending these interventions, randomised controlled trials should be performed in Spain to determine whether it is necessary to routinely prescribe supplemental vitamin D to children, and under which circumstances (season, latitude, ethnic origin, skin phototype, and exclusive breastfeeding).9,20,30–32
To summarise, our study showed that 22.5% of children younger than 6 years of age have deficient vitamin D plasma concentrations, a status that is more prevalent in children of Indo-Pakistani and Maghrebi descent that are not receiving vitamin D supplements. It would be useful to conduct a systematic review of phosphorus and calcium metabolism with particular emphasis on determining the plasma levels of PTH and 25(OH)D in dark-skinned immigrant infants and children that are exclusively breastfed and do not receive vitamin D supplementation.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sánchez Muro JM, Yeste Fernández D, Marín Muñoz A, Fernández Cancio M, Audí Parera L, Carrascosa Lezcano A. Niveles plasmáticos de vitamina D en población autóctona y en poblaciones inmigrantes de diferentes etnias menores de 6 años de edad. An Pediatr (Barc). 2015;82:316–324.